Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.520
Peer-review started: December 15, 2022
First decision: January 3, 2023
Revised: January 4, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 27, 2023
Processing time: 129 Days and 0.9 Hours
Although the incidence and mortality of gastric cancer (GC) have been decreasing steadily worldwide, especially in East Asia, the disease burden of this malignancy is still very heavy. Except for tremendous progress in the management of GC by multidisciplinary treatment, surgical excision of the primary tumor is still the cornerstone intervention in the curative-intent treatment of GC. During the relatively short perioperative period, patients undergoing radical gastrectomy will suffer from at least part of the following perioperative events: Surgery, anesthesia, pain, intraoperative blood loss, allogeneic blood transfusion, post
Core Tip: During the relatively short perioperative period, patients undergoing radical gastrectomy will suffer from various perioperative events, which have been shown to affect long-term outcomes. Therefore, in recent years, studies have been carried out to identify and test interventions during the perioperative period to improve the long-term survival of patients following radical gastrectomy. As the majority of these interventions are already safely applied clinically for other indications, are cost-effective and can be administered conveniently, if the desired survival benefits are prospectively confirmed, considerable economic and social improvements can be achieved at little financial cost.
- Citation: Liu LB, Li J, Lai JX, Shi S. Harnessing interventions during the immediate perioperative period to improve the long-term survival of patients following radical gastrectomy. World J Gastrointest Surg 2023; 15(4): 520-533
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/520.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.520
Although the incidence and mortality of gastric cancer (GC) have been declining gradually in recent decades, its survival improvement is relatively poorer than that of other common malignancies, such as colorectal and breast cancer[1]. In 2020, there were an estimated 768793 GC-related deaths[2]. Therefore, strategies aiming to decrease the burden of GC are being extensively explored globally. Currently, radical surgical removal of the primary GC is the preferred choice for patients whose disease is still locally resectable[1]. Unfortunately, postoperative development of recurrence and metastasis, the main cause of morbidity and mortality of GC, is inevitable in some patients, especially in those with advanced disease[3]. As no visible evidence of metastasis is the prerequisite for radical gastrectomy, postoperative relapses mainly result from occult cancer cells whose spreading has already occurred or been induced during the perioperative period by the surgery itself or its related events, including anesthesia, pain, intraoperative blood loss, allogeneic blood transfusion, postoperative complications (POCs), and their related anxiety, depression and stress response.
The notion that surgical trauma may enhance the risk of cancer metastasis was already noticed by the ancient Greeks, who cautioned against disturbing cancers[4]. In the 1960s, surgeons found that long-term survival was only moderately improved compared to historical nonoperated controls, and even rapid recurrence and progression were found in patients with cancer following radical resection, indicating the promoting effects of surgery on the spread of cancer cells[4]. However, these negative effects were largely ignored; only when perioperative adjuvant therapies have gained success in survival improvement has interest in this theory reemerged. Typically, perioperative adjuvant therapies for metastasis prevention are administered at least one month before or after surgery for cancer, including GC. The immediate perioperative period is rarely exploited for such interventions, largely owing to concerns over contraindications to surgery[5]. Such a concept has changed in recent years, as the significance of this timeframe in determining long-term oncological outcomes is widely recognized[5]. Therefore, various interventions have been explored during the perioperative timeframe, and some of them show great promise. As the recurrence and metastasis rates are higher and radical gastrectomy is relatively more extensive than surgeries for other malignancies, the immediate perioperative period of radical gastrectomy may be a critical timeframe to improve the prognosis of GC.
In this review, we briefly discuss the mechanisms underpinning the negative effects of radical gastrectomy and its related events and then describe the measures that could be harnessed to mitigate their cancer-promoting effects while improving the long-term survival of patients following radical gastrectomy, with the hope of transforming the perioperative period from a prominent facilitator of cancer recurrence to a window of opportunity for improving oncological outcomes in patients with GC.
In clinical settings, extensive surgery for GC always provides no additional survival benefits and even leads to poor survival in some patients[6,7]. Other necessary events during the perioperative period, including anesthesia and analgesia, can shorten the long-term survival of patients with GC if the modality or agents are administered inappropriately[8]. Adverse events during gastrectomy or postoperative recovery, such as intraoperative blood loss, allogeneic blood transfusion, and POCs, were all proven to be independent negative prognostic factors for patients following curative gastrectomy[8]. In addition, concomitant anxiety, depression and stress response can aggravate the cancer-promoting effects of gastrectomy and its related events. The perioperative physiological responses that underpin the cancer-promoting effects of radical surgery and its related events have been extensively studied in surgical oncology and have been excellently reviewed in previous publications[5]. Conclusions relevant to GC have also been discussed in our previous review[8]. Briefly, gastrectomy and its related events activate the sympathetic nervous system (SNS) and inflammatory response, also referred to as the surgical stress response, leading to enhanced growth of residual cancer cells, which may be preexisting micrometastases, incompletely resected fractions of tumor cells or disseminated from the primary tumor during the operation. In addition, following the activation of the surgical stress response, antitumor immunity is suppressed and fails to eliminate these residual cancer cells[5]. Therefore, measures that can alleviate the acute stress response to surgery and liberate the suppression of antitumor immunity are the focus of studies aiming to harness the immediate perioperative period for improving the long-term survival of patients with GC.
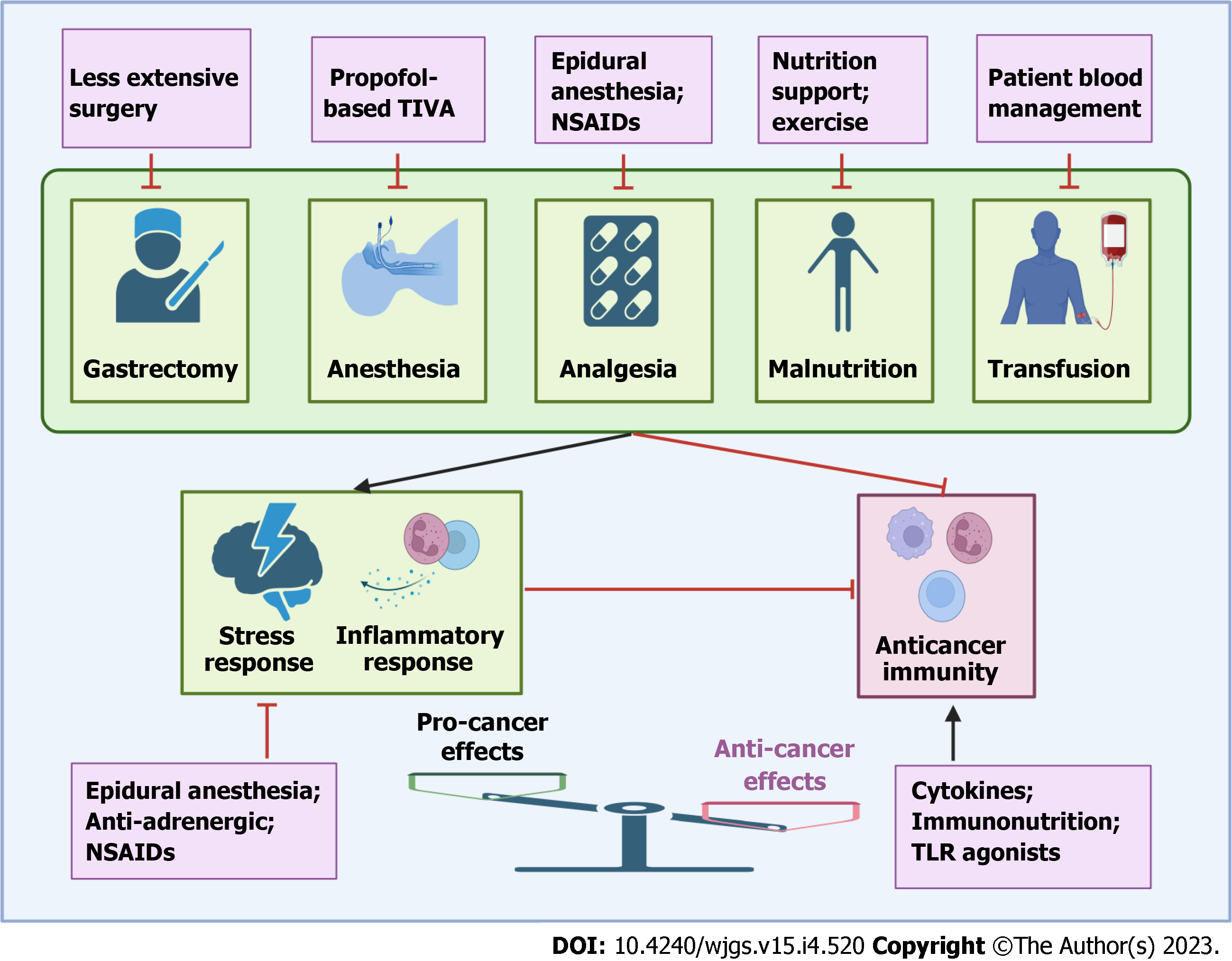
For a long time, surgeons did everything just for operation and believed that the side effects of surgery must be borne. Therefore, it is not necessary to complicate the perioperative timeframe by additional interventions due to the relatively short time span of tumor evolution- or justified and/or speculated concerns over contraindications to surgery[9]. However, this concept has been challenged by three foundations. First, after curative resection, all visible cancer cells are removed, and the probability of future recurrence or metastasis mainly originates from minimal residual cancer cells, whose metastatic progression can be efficiently arrested or prevented by relatively minimal effort and innocuous therapies. In contrast, when these residual cancer cells evolve into larger and more self-sustaining diseases, this goal becomes more difficult or even impossible. Second, some existing interventions have been demonstrated to be tolerable or circumventable contraindications to surgery and can be administered safely during the perioperative period. Third, a robust biological rationale supports the likely antimetastatic efficacy of various interventions during the perioperative period, including appropriate operation, anesthesia and analgesia selection, approaches to limit stress-inflammatory responses and to preserve or activate anticancer immunity (Figure 1).
Historically, a series of randomized controlled trials (RCTs) have been conducted to compare the safety and survival benefit between radical gastrectomy with different intensities[10]. The primary principle for surgical management and clinical trial design of curatively resected GC is a balance between resection of tissues with possible cancer cell colonization to achieve long-term survival and acceptable postoperative early death. This high-quality evidence suggests that extensive gastrectomy has few advantages in improving long-term survival compared to less extensive surgery, and some extended resections even lead to increased recurrence or metastasis, indicating that the material benefit conferred by extended surgery may be offset by higher early mortality and increased recurrence resulting from an extensive surgical stress response[6,7]. Therefore, decision-making on surgical approaches for GC should be guided by the latest scientific evidence, and extended gastrectomy without survival benefit should be avoided. Within the modern knowledge of GC management, even in the West, the most appropriate surgery for GC is gastrectomy with D2 lymphadenectomy transabdominally but avoids inevitable paraaortic lymph node dissection, splenectomy, pancreatectomy, and bursectomy[10]. Minimally invasive surgery, such as endoscopic resection and laparoscopic or robotic gastrectomy for early or even advanced GC, is widely adopted as an alternative to traditional open gastrectomy. Although improved long-term survival has not been observed in these studies, less blood loss, fewer POCs, faster recovery, and reduced surgical stress have been found in patients undergoing minimally invasive surgery[11,12]. Therefore, minimally invasive surgery for GC is recommended whenever the conditions of surgeons and patients are feasible. Theoretically, the reduced surgical stress during minimally invasive surgery may translate into a long-term survival benefit when other antimetastatic interventions are coadministered perioperatively.
Nevertheless, both minimally invasive and traditional surgical approaches for GC are highly technically demanding, with a high incidence of intraoperative blood loss and POCs in less experienced hands. Numerous data support the negative effects of intraoperative blood loss, transfusion and POCs on prognosis in patients following radical gastrectomy[8]. In addition, the inferior short-term and long-term outcomes following radical gastrectomy in the East compared with the West were not only determined by more advanced stage and comorbidities but also by the low incidence of GC and uncommon regional specialization. Several other studies have also found that the outcomes of patients with GC were better in experienced and high-volume hospitals[13,14]. Therefore, from the viewpoint of survival benefit, gastrectomy should be centralized in high-throughput centers with the ability to provide standardized perioperative management for GC.
Patients diagnosed with locally resectable GC will require anesthesia for endoscopic examination or gastrectomy, and analgesics are commonly prescribed for pain relief following surgery. Currently, several modalities and agents are widely applied for general anesthesia and postoperative analgesia, while total intravenous anesthesia (TIVA), inhalation anesthesia and neuraxial anesthesia are the most studied in cancer surgery. Evidence from studies in GC suggests that these modalities and agents have distinct effects on the stress response, inflammation, anticancer immunity, cancer progression and long-term survival. Inhalational anesthetics, including isoflurane and sevoflurane, have been observed to provide some degree of cytoprotection to organs, which may also support the survival of cancer cells[15]. Although data in GC are lacking, in vivo data in other cancer types demonstrate that volatile anesthetics might promote immunosuppression and support tumor cell growth and spreading[16,17]. In contrast, propofol-based TIVA, an alternative to inhalational anesthesia, has appealing anticancer properties and has been extensively studied in GC. Administration of propofol has been shown to inhibit GC cell proliferation, migration and invasion in vitro while preserving the cellular immune function of patients undergoing curative gastrectomy[18,19]. Consistent with this evidence, the findings of several retrospective studies or meta-analyses demonstrated that in patients undergoing gastrectomy, long-term survival is better in patients anesthetized with propofol-based TIVA than in those with volatile anesthesia[20]. However, what calls for special attention is that all these studies were retrospectively designed, and selection bias cannot be neglected, providing a limited reference for the choice of anesthesia type during gastrectomy. Currently, a number of prospective trials elucidating the effects of propofol on cancer recurrence and patient survival are ongoing (NCT01975064, NCT03034096, NCT02660411, NCT02840227), and their results may raise the possibility that propofol-based TIVA could become the standard anesthesia approach for cancer surgery, including gastrectomy.
Epidural anesthesia has been widely used jointly with or as an alternative to general anesthesia or for postoperative analgesia in patients undergoing gastrointestinal cancer surgery. Limited clinical evidence suggests the survival benefit of epidural anesthesia for patients with GC[21,22]. The association between epidural anesthesia and decreased cancer recurrence following surgery might reflect the multifaceted effects of this anesthetic and analgesic modality. The addition of epidural anesthesia to general anesthesia significantly decreased the expression of various inflammatory mediators and increased the proportion and activity of antitumor immune cells in patients undergoing GC surgery[23,24]. SNS blockade and inhibition of perioperative lymph flow both contribute to the anticancer effect of epidural anesthesia, although they have not been validated in GC[25,26]. Furthermore, as a widely adopted postoperative analgesic technique, epidural anesthesia significantly decreases the prescription of opioids for postoperative pain relief[22]. Opioids have been found to promote the growth of cancer and inhibit the antimetastatic activity of immune cells, including natural killer (NK) cells, cytotoxic T lymphocytes, dendritic cells and macrophages[27,28]. Nevertheless, the results from two RCTs including lung or unspecified cancer types did not support the positive effect of epidural anesthesia on overall or cancer-specific survival[29,30]. Therefore, determining whether epidural anesthesia provides a recurrence-preventing effect for patients with GC requires further research.
Activation of the SNS is one of the responses to surgical stress, resulting in increased levels of circulating catecholamines, including adrenaline and noradrenaline. Consistent with this mechanism, the levels of circulating catecholamines were higher in patients undergoing more extended gastrectomy or with an eventful postoperative recovery[5]. Beta 2-adrenergic receptor (β2-AR), the main type of receptor that mediates the biological functions of catecholamines, was found to be overexpressed in cancer tissues from patients with GC and correlated with metastasis and poor prognosis[31]. The activation of β2-AR by catecholamines leads to increased formation of liver and lung metastases by primary GC cells[31]. Mechanisms underlying the cancer-promoting effect of SNS signaling have also been elucidated in various studies. Activation of β2-AR was shown to induce epithelial to mesenchymal transition and cancer stem cell attributes of GC cells[32,33]. Sympathetic nerves can also help establish premetastatic niches in bone by stimulating host bone marrow stromal cells[34]. On the other hand, SNS activation promotes the establishment of an immune-privileged microenvironment, which is beneficial to tumor growth and metastasis formation[35,36]. Therefore, increasing data reported in recent years indicate that perioperative interventions to attenuate SNS activity are promising for reducing the recurrence risk of GC, which can be achieved by pharmaceutical inhibition (β-AR antagonists) or by epidural anesthesia.
Population-based cohort studies have shown that β-AR antagonists (also known as β-blockers), which are widely used in the clinic for hypertension, can significantly decrease the risk for GC[37]. Therefore, as effective strategies to inhibit sympathetic signaling, which is activated by perioperative events, β-blockers may be used as an effective adjunctive strategy to reduce the risk of cancer recurrence. In vitro studies, propranolol, the most commonly used nonselective-adrenergic antagonist, showed the ability to induce apoptosis, repress growth, suppress the expression of matrix metalloproteinases and vascular endothelial growth factor, and inhibit the migration of GC cells[38,39]. In a xenograft mouse model, propranolol decreased the levels of phosphorylated AKT, MEK, and ERK proteins and blocked depression-promoted neuroendocrine phenotypic transformation and lung metastasis of GC[31,40,41]. In patients with GC, treatment with propranolol for one week before surgery significantly inhibited the proliferation of cancer, as measured by Ki-67[40]. In other cancer types, the anticancer ability of propranolol has also been found to be associated with enhanced antitumor immunity[42,43]. However, this effect was not observed in GC animal models or patients[40]. Based on the findings of preclinical studies, several clinical trials have been carried out to assess the effects of perioperative β-blockers on oncological outcomes, but none of them have been designed to focus on survival. Only one small RCT in colorectal cancer collected data on the 3-year recurrence rate and revealed a favorable trend toward reduced cancer recurrence in patients receiving β-blockers[44]. Compared with surgery for breast and colorectal cancer, gastrectomy for GC has a relatively higher intensity. It is reasonable to speculate that the intensity of activation of sympathetic responses in radical gastrectomy will be stronger, and β-blockers do have the ability to decrease the surgical responses and have the probability of decreasing the recurrence in GC patients.
One of the explanations for the lack of effect on long-term outcomes by SNS blockade is that in addition to increased levels of catecholamine, various inflammatory mediators are also abundantly released into circulation during cancer surgery. The levels of inflammatory mediators, including C-reactive protein (CRP), procalcitonin, prostaglandin E2 and plasma cortisol, were elevated significantly after gastrectomy[45,46]. Activation of the inflammatory response plays an important role in residual cancer cell survival and progression through humoral factor release or premetastatic niche establishment[47-49]. A preoperative elevated neutrophil-to-lymphocyte ratio, a systemic inflammation index frequently used in the literature, was shown to have a significant negative effect on survival[50]. Therefore, anti-inflammatory therapy might provide antimetastatic benefits. Selective or nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) for cyclooxygenase (COX) (for example, ibuprofen, celecoxib, and etodolac) are commonly used analgesics following radical gastrectomy, and several studies have shown their anticancer properties. Currently, the most tested anti-inflammatory therapies are selective COX2 inhibitors. In cancer types other than GC, perioperative use of NSAIDs has been shown to attenuate the inflammatory response, enhance the number and function of antimetastatic immune cells and prevent the formation of metastases in mouse models[51,52]. In the clinical setting, studies have reported that perioperative administration of COX2 antagonists decreases the circulating levels of prostaglandins and catecholamines while preserving anticancer immunity by buffering both the elevation of regulatory T cells and the decline in NK cell counts[5]. The anticancer effect of NSAIDs, especially celecoxib, has also been studied extensively in GC, while data are limited to preclinical or GC prevention settings. For example, a population-based intervention trial revealed that celecoxib treatment alone had beneficial effects on the regression of advanced gastric lesions[53]. Selective COX-2 inhibitors were found to suppress GC cell dissemination through apoptosis induction and migration suppression[54,55]. In a mouse model bearing orthotopic xenografts or with carcinomatous peritonitis induced with a highly metastatic human diffuse-type GC cell line, etodolac, a COX-2 inhibitor, significantly decreased tumor lymphangiogenesis and the total weight of metastatic lymph nodes[56]. In the perioperative setting, only one study reported that preoperative treatment with celecoxib significantly promotes necrosis in GC through the induction of apoptosis and the reduction of microvessel density[57].
Data from these preclinical studies and clinical studies on inflammatory biomarker alterations point to an anticancer effect of NSAIDs. Therefore, several clinical trials have also been performed to investigate the long-term outcome impact of NSAIDs on cancer. A retrospective analysis suggested that intraoperative administration of ketorolac decreases the risk of breast cancer relapse compared with other analgesics[58]. A nationwide cohort study involving 15574 patients revealed that the use of NSAIDs can be associated with a reduced risk of early hepatocellular carcinoma recurrence within 2 years after curative liver resection[59]. However, these studies were retrospectively designed and have inherent limitations. Thus, several RCTs aimed at investigating the effect of perioperative administration of NSAIDs on improving cancer-specific survival are currently ongoing. One of these studies was completed and reported that preoperative administration of ketorolac tromethamine does not increase disease-free survival (DFS) or overall survival (OS) in high-risk breast cancer patients[60]. However, because of the relatively low surgical trauma and low recurrence rate in breast cancer, the results cannot be extrapolated to other cancer types. In addition, as mentioned above, activation of the SNS and inflammatory response have redundant roles in promoting the growth and recurrence of cancer, and anticipated survival improvement cannot be achieved by COX2 inhibitors alone. Therefore, the combination of β-blockers and COX2 inhibitors is being investigated in several RCTs. Primary results revealed that perioperative administration of β-blockers and COX2 inhibitors not only improves immune competence and metastatic biomarkers but also shows a favorable trend toward reduced cancer recurrence in treated patients[44,61]. To date, no such RCTs recruiting patients with GC have been reported or registered. As radical gastrectomy activates a relatively more severe surgical stress response, whether pharmacological blockade of these responses will provide a survival benefit deserves further clinical trials.
Immunosuppression is a widely recognized phenomenon in patients with cancer, especially during the perioperative period[9]. The total lymphocyte count decreased rapidly from preoperative levels, while the expression of lymphocyte activation gene 3 and programmed cell death 1 on lymphocytes was upregulated, indicating impaired cell-mediated immunity after surgery for GC[62]. Potential contributors to perioperative immunosuppression include the stress-inflammatory response, anxiety, hypothermia, blood loss and transfusion, and the direct and indirect effects of anesthetic and analgesic agents[9]. As immunosurveillance critically determines the fate of minimal residual cancer cells, manipulation of anticancer immunity may provide a promising opportunity to improve the long-term survival of patients following curative surgery[63]. To date, various interventions to activate host anticancer immunity have joined the therapeutic armamentarium for the treatment of many advanced-stage cancers; however, few of them have been tested during the perioperative timeframe, owing to several established and theoretical risks pertinent around the time of surgery[9]. Nevertheless, perioperative immune preservation or stimulation could hold various advantages if the interventions meet the following desired attributes: A rapid immunological response, avoidance of tumor-promoting effects, minimal contraindications to surgery, resilience to perioperative stress and a limited capacity to induce stress responses[9]. Potential strategies have been investigated in various cancers, including GC.
Cytokines are essential for an effective anticancer immune response. Treatment with recombinant interleukin-2 (IL-2), a crucial cytokine for various leukocytes, has long been tested in perioperative settings. For example, preoperative treatment with low-dose IL-2 can be safely given in patients with GC and was revealed to prevent postoperative lymphocytopenia and activate peripheral and peritumoral lymphocytes[64]. Furthermore, IL-2 seems to have an impact on the clinical course, reducing the morbidity of surgery and ameliorating OS and DFS[65]. Other cytokines, such as type I interferons and granulocyte colony-stimulating factor, have also been investigated in the perioperative period and were shown to improve anticancer immunity; however, the survival benefits were somewhat heterogeneous[66-68]. Except for these primary promising results, perioperative administration of cytokines has been discontinued in recent years, largely owing to their severe systemic adverse effects, including the induction of fever, weakness and headaches, which cannot be distinguished from signs of infection and might result in surgery being delayed.
In contrast to other strategies to improve anticancer immunity, data on the beneficial effects of immunonutrients administered perioperatively are accumulating in GC[69]. Immunonutrient interventions are nutrition support that is rich in elements beneficial for the homeostasis of immunity, including ω-3-fatty acids, glutamine, arginine, and nucleotides[70]. Although not all studies found similar clinical effects and some conflicting results have been reported, the influence of immunonutrition on immunological levels, nutrition status and postoperative course was convincing. For example, an early 5-d postoperative enteral immunonutrition supplement significantly improved immune function and the inflammatory response in GC patients following gastrectomy[71]. When immunonutrition was supplied before surgery, the abundance of tumor-infiltrating lymphocytes was upregulated in gastrectomy samples[72]. Several meta-analyses also demonstrated the efficacy of perioperative immunonutrition for improving various immunological indices and decreasing the incidence of POCs[69,73,74]. Although the benefit of improving immune function has been consistently reported, few studies have collected survival data. One study concluded that the 60-d mortality was lower in patients receiving immunomodulating enteral nutrition in the perioperative period, but no improvement in 6-mo and 1-year survival was found[75]. The explanations may be that only a small number of patients were included, and in some studies, prolonged use of immunonutrition increased tumor angiogenesis, which may offset the survival benefit of immunonutrition[72,75]. Therefore, whether perioperative immunonutrition support can provide a survival benefit needs further large prospective studies, and the ingredients and duration of immunonutrition should be determined carefully.
Some immunotherapeutic strategies, such as immune checkpoint inhibitors, require repeated administration for weeks or months to induce the desired response, making them inappropriate for perioperative settings. However, other approaches, such as some Toll-like receptor (TLR) agonists, can induce rapid activation of immune responses, which was shown to be effective when administered perioperatively. For example, preoperative treatment with CpG-C oligodeoxynucleotide, a TLR9 agonist, can synergize with propranolol and etodolac to improve cell-mediated immunity and limit metastatic progression in a mouse model[76]. A fully synthetic TLR4 agonist, glucopyranosyl lipid-A, can be safely administered perioperatively and significantly elevates both innate and adaptive immunity, leading to reduced metastatic development[77]. The TLR3 agonist polyinosinic-polycytidylic acid [poly(I:C)] significantly enhanced NK cell activity in preclinical tumor models, healthy human donors and cancer surgery patients[78]. To date, evidence is limited to preclinical models, none of them have been pursued in clinical trials, and the perioperative application potential is not clear in GC. However, as suggested by promising preclinical rationale, research into perioperative immune stimulation is warranted in the future.
GC patients always present with malnutrition, largely owing to digestion and absorption dysfunction, obstruction attributed to cancer, and anorexia caused by cancer-released cytokines. Numerous studies have reported that malnutrition is associated with poor prognosis, which may be the result of interference with treatment implementation and impaired anticancer immunity[79-81]. Therefore, perioperative nutrition support was proven to improve immune function, weaken the surgical stress response and decrease POCs, thereby theoretically prolonging the survival of GC[82]. However, no survival data are available to date. On the other hand, preclinical studies have suggested that excessive parenteral nutrition support could potentially promote the proliferation of cancer cells[83]. Accordingly, determining the patients who need nutritional support and how to carry out nutritional support optimally are the focus of future studies.
Physical fitness plays an important role in the successful administration of various cancer therapies, including surgery, and thus may determine the long-term survival of patients postoperatively. Low physical performance, partially determined by the loss of muscle mass plus low muscle strength, is associated with POCs and poor prognosis in GC patients[84-86]. Correspondingly, increasing the physical activity of patients through perioperative exercise, always administered simultaneously with nutrition support, decreased the incidence of POCs and enhanced the recovery course following gastrectomy[87]. However, although prolongation of survival has been achieved by exercise in patients with colorectal cancer, whether perioperative exercise programs have clinical benefits with regard to long-term oncological outcomes in GC patients is unclear[88,89]. Mechanisms underlying the protective effect of exercise on cancer mortality are multifarious. For example, exercise was found to decrease the inflammatory marker CRP, indicating an anti-inflammatory effect[90]. Moderate exercise is known to enhance cellular immunity and to decrease the levels of insulin and insulin-like growth factor[91,92]. In addition, activity-induced changes in the body and mental health also support improved tolerance for and the resultant effectiveness of cancer treatment[93]. Therefore, as a simple and convenient intervention that can be safely implemented during the perioperative period, exercise may provide the desired survival improvement in patients following gastrectomy.
Similar to malnutrition, anemia is another most common presentation in GC patients, and the incidence was reported to range from 27% to 44%[94]. Pretreatment anemia predicts increased POCs and decreased long-term survival, including DFS[94]. Therefore, perioperative allogeneic blood transfusion is frequently administered in GC patients. Paradoxically, abundant data suggest that transfusion is intimately associated with cancer recurrence and cancer-related deaths following radical gastrectomy, mainly owing to the inhibition of host immunity and increased risk of POCs[95].
Therefore, in the consideration of long-term survival, the optimization of preoperative anemia treatment is critical for patients with GC. For this purpose, a patient blood management (PBM) strategy was established, which includes different evidence-based interventions, aiming to maintain patients' own blood volume and avoid unnecessary blood transfusion. PBM consists of three parts: Management of anemia through early detection and use of iron preparations to stimulate erythropoiesis; mini
In the past decade, the effects of numerous perioperative interventions, including nutrition, minimally invasive surgery, nasogastric/nasojejunal decompression, early postoperative diet and mobilization, on immediate postsurgical outcomes have been studied extensively in radical gastrectomy, and these interventions have been integrated into enhanced recovery after surgery (ERAS), an evidence-based, comprehensive, multimodal approach designed to achieve early recovery for patients undergoing radical gastrectomy[100,101]. Published studies on ERAS mainly reported short-term outcomes, with similar POC incidences but reduced postoperative hospitalization and costs[102]. The ERAS approach was also found to improve the postoperative inflammatory response and surgical stress[103,104]. However, no study has yet reported the long-term survival of patients experiencing an enhanced recovery after radical gastrectomy. As the interventions comprising the ERAS approach often overlap with the principles presented herein to limit the deleterious effects of gastrectomy on surgical stress, which may induce the recurrence of GC, it is our recommendation to incorporate them in conjunction with studying oncological outcomes.
Globally, most of the one million newly diagnosed GC patients require gastrectomy each year. Gastrectomy and its related events, including anesthesia, analgesia, transfusion, POCs and malnutrition, will expose these patients to various stress responses during the immediate perioperative period. Pathophysiological alterations, such as activation of the SNS and inflammatory response and suppression of anticancer immunity, can support the survival and growth of residual cancer cells and promote cancer recurrence. Therefore, exploiting perioperative interventions to reduce the risk of recurrence and metastasis has attracted more attention in recent years. Various approaches, including appropriate operation and anesthesia selection, anti-adrenergic, anti-inflammatory, perioperative immune stimulation, nutrition support, exercise, and PBM, have been widely explored in preclinical or clinical settings, and promising results have been reported. Although data on some approaches are limited or lacking in GC at present, some of them did show the potential to improve the long-term survival of patients with various cancers. However, the majority of evidence was provided by retrospective analysis, and conflicting results have also been observed in clinical trials, perhaps owing to the complex pathophysiological alterations and heterogeneity among patients, leading to the lack of consensus on the optimal approach to perioperative care. In addition, along with the accumulating knowledge of the mechanisms underpinning the invasion-metastasis cascade, the concept of drugging metastasis has attracted more attention[105]. The immediate perioperative period represents a critical timeframe for residual cancer cells to complete the invasion-metastasis cascade, providing an important window for enhancing the efficacy of drugs targeting metastasis. Therefore, a detailed understanding of the changes that occur after surgery in each patient is pivotal for the development of new therapeutic strategies and personalized health care to prevent tumor recurrence. Large-cohort prospective RCTs are required to definitively demonstrate the effects of various perioperative interventions on oncological outcomes after radical gastrectomy. As the majority of these interventions are already safely applied clinically for other indications, are cost-effective and can be administered conveniently, if the desired survival benefits are prospectively confirmed, considerable economic and social improvements can be achieved at little financial cost.
The author thanks the Health Commission of Mianyang City and the Science and Education Department of the Third Hospital of Mianyang for their support. The space limitations of this review have unfortunately meant that we have not been able to separately cite many of the original publications that have contributed substantially to the literature. We sincerely apologize to the authors of these publications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kanaoujiya R, India; Zhi X, China S-Editor: Fan JR L-Editor: A P-Editor: Zhao S
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2855] [Article Influence: 571.0] [Reference Citation Analysis (5)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64619] [Article Influence: 16154.8] [Reference Citation Analysis (176)] |
| 3. | Salati M, Orsi G, Smyth E, Aprile G, Beretta G, De Vita F, Di Bartolomeo M, Fanotto V, Lonardi S, Morano F, Pietrantonio F, Pinto C, Rimassa L, Vasile E, Vivaldi C, Zaniboni A, Ziranu P, Cascinu S. Gastric cancer: Translating novels concepts into clinical practice. Cancer Treat Rev. 2019;79:101889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. 2009;249:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 375] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 6. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 997] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 7. | Kurokawa Y, Sasako M, Sano T, Yoshikawa T, Iwasaki Y, Nashimoto A, Ito S, Kurita A, Mizusawa J, Nakamura K; Japan Clinical Oncology Group (JCOG9502). Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg. 2015;102:341-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Zhi X, Kuang X, Li J. The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Matzner P, Sandbank E, Neeman E, Zmora O, Gottumukkala V, Ben-Eliyahu S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat Rev Clin Oncol. 2020;17:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Kiyokawa T, Fukagawa T. Recent trends from the results of clinical trials on gastric cancer surgery. Cancer Commun (Lond). 2019;39:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hakkenbrak NAG, Jansma EP, van der Wielen N, van der Peet DL, Straatman J. Laparoscopic versus open distal gastrectomy for gastric cancer: A systematic review and meta-analysis. Surgery. 2022;171:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Kim YM, Hyung WJ. Current status of robotic gastrectomy for gastric cancer: comparison with laparoscopic gastrectomy. Updates Surg. 2021;73:853-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Meyer HJ. The influence of case load and the extent of resection on the quality of treatment outcome in gastric cancer. Eur J Surg Oncol. 2005;31:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Bachmann MO, Alderson D, Edwards D, Wotton S, Bedford C, Peters TJ, Harvey IM. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. 2002;89:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Wu L, Zhao H, Wang T, Pac-Soo C, Ma D. Cellular signaling pathways and molecular mechanisms involving inhalational anesthetics-induced organoprotection. J Anesth. 2014;28:740-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Luo X, Zhao H, Hennah L, Ning J, Liu J, Tu H, Ma D. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2015;114:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35:1311-1319. [PubMed] |
| 18. | Liu YP, Qiu ZZ, Li XH, Li EY. Propofol induces ferroptosis and inhibits malignant phenotypes of gastric cancer cells by regulating miR-125b-5p/STAT3 axis. World J Gastrointest Oncol. 2021;13:2114-2128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Liu R, Suo S, Wang Y, Wang M. Effects of Dexmedetomidine and Propofol on Postoperative Analgesia and the Cellular Immune Function of Patients Undergoing Radical Gastrectomy for Gastric Cancer. Contrast Media Mol Imaging. 2022;2022:7440015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Wu WW, Zhang WH, Zhang WY, Liu K, Chen XZ, Zhou ZG, Liu J, Zhu T, Hu JK. The long-term survival outcomes of gastric cancer patients with total intravenous anesthesia or inhalation anesthesia: a single-center retrospective cohort study. BMC Cancer. 2021;21:1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Wang Y, Wang L, Chen H, Xu Y, Zheng X, Wang G. The effects of intra- and post-operative anaesthesia and analgesia choice on outcome after gastric cancer resection: a retrospective study. Oncotarget. 2017;8:62658-62665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Hiller JG, Hacking MB, Link EK, Wessels KL, Riedel BJ. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol Scand. 2014;58:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Zhou M, Liu W, Peng J, Wang Y. Impact of propofol epidural anesthesia on immune function and inflammatory factors in patients undergoing gastric cancer surgery. Am J Transl Res. 2021;13:3064-3073. [PubMed] |
| 24. | Liu W, Wu L, Zhang M, Zhao L. Effects of general anesthesia with combined epidural anesthesia on inflammatory response in patients with early-stage gastric cancer undergoing tumor resection. Exp Ther Med. 2019;17:35-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Hiller JG, Ismail HM, Hofman MS, Narayan K, Ramdave S, Riedel BJ. Neuraxial Anesthesia Reduces Lymphatic Flow: Proof-of-Concept in First In-Human Study. Anesth Analg. 2016;123:1325-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Koltun WA, Bloomer MM, Tilberg AF, Seaton JF, Ilahi O, Rung G, Gifford RM, Kauffman GL Jr. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am J Surg. 1996;171:68-72; discussion 72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Carli M, Donnini S, Pellegrini C, Coppi E, Bocci G. Opioid receptors beyond pain control: The role in cancer pathology and the debated importance of their pharmacological modulation. Pharmacol Res. 2020;159:104938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Xu ZZ, Li HJ, Li MH, Huang SM, Li X, Liu QH, Li J, Li XY, Wang DX, Sessler DI. Epidural Anesthesia-Analgesia and Recurrence-free Survival after Lung Cancer Surgery: A Randomized Trial. Anesthesiology. 2021;135:419-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Du YT, Li YW, Zhao BJ, Guo XY, Feng Y, Zuo MZ, Fu C, Zhou WJ, Li HJ, Liu YF, Cheng T, Mu DL, Zeng Y, Liu PF, Li Y, An HY, Zhu SN, Li XY, Wu YF, Wang DX, Sessler DI; Peking University Clinical Research Program Study Group. Long-term Survival after Combined Epidural-General Anesthesia or General Anesthesia Alone: Follow-up of a Randomized Trial. Anesthesiology. 2021;135:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 2019;10:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Shan T, Cui X, Li W, Lin W, Li Y, Chen X, Wu T. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014;105:847-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Lu Y, Zhang Y, Zhao H, Li Q, Liu Y, Zuo Y, Xu Q, Zuo H, Li Y. Chronic stress model simulated by salbutamol promotes tumorigenesis of gastric cancer cells through β2-AR/ERK/EMT pathway. J Cancer. 2022;13:401-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30 Suppl:S32-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 397] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 37. | Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, Chen JH, Kao CH. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine (Baltimore). 2015;94:e1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Liao X, Che X, Zhao W, Zhang D, Bi T, Wang G. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep. 2010;24:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Koh M, Takahashi T, Kurokawa Y, Kobayashi T, Saito T, Ishida T, Serada S, Fujimoto M, Naka T, Wada N, Yamashita K, Tanaka K, Miyazaki Y, Makino T, Nakajima K, Yamasaki M, Eguchi H, Doki Y. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric Cancer. 2021;24:1037-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Hu Q, Liao P, Li W, Hu J, Chen C, Zhang Y, Wang Y, Chen L, Song K, Liu J, Zhang W, Li Q, McLeod HL, He Y. Clinical Use of Propranolol Reduces Biomarkers of Proliferation in Gastric Cancer. Front Oncol. 2021;11:628613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Pan C, Wu J, Zheng S, Sun H, Fang Y, Huang Z, Shi M, Liang L, Bin J, Liao Y, Chen J, Liao W. Depression accelerates gastric cancer invasion and metastasis by inducing a neuroendocrine phenotype via the catecholamine/β(2) -AR/MACC1 axis. Cancer Commun (Lond). 2021;41:1049-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JB, Henderson MA, Nightingale SS, Ho KM, Myles PS, Fox S, Riedel B, Sloan EK. Preoperative β-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin Cancer Res. 2020;26:1803-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 43. | Liao P, Song K, Zhu Z, Liu Z, Zhang W, Li W, Hu J, Hu Q, Chen C, Chen B, McLeod HL, Pei H, Chen L, He Y. Propranolol Suppresses the Growth of Colorectal Cancer Through Simultaneously Activating Autologous CD8(+) T Cells and Inhibiting Tumor AKT/MAPK Pathway. Clin Pharmacol Ther. 2020;108:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Haldar R, Ricon-Becker I, Radin A, Gutman M, Cole SW, Zmora O, Ben-Eliyahu S. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer. 2020;126:3991-4001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 45. | Cheng W, Liu J, Zhi M, Shen D, Shao M, Zhang C, Wang G, Jiang Z. Stress and autonomic nerve dysfunction monitoring in perioperative gastric cancer patients using a smart device. Ann Noninvasive Electrocardiol. 2022;27:e12903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Ricon I, Hanalis-Miller T, Haldar R, Jacoby R, Ben-Eliyahu S. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of β-adrenergic and cyclooxygenase 2 signaling. Cancer. 2019;125:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1236] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 48. | Carpinteri S, Sampurno S, Bernardi MP, Germann M, Malaterre J, Heriot A, Chambers BA, Mutsaers SE, Lynch AC, Ramsay RG. Peritoneal Tumorigenesis and Inflammation are Ameliorated by Humidified-Warm Carbon Dioxide Insufflation in the Mouse. Ann Surg Oncol. 2015;22 Suppl 3:S1540-S1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1500] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 50. | Gao X, Pan Y, Han W, Hu C, Wang C, Chen L, Guo Y, Shi Y, Xie H, Yao L, Yang J, Zheng J, Li X, Liu X, Hong L, Li J, Li M, Ji G, Li Z, Xia J, Zhao Q, Fan D, Wu K, Nie Y. Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas. Cancer Biol Med. 2021;18:283-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 52. | Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, Liu WD, Feng GS, Zhang XD, Li J, Lu AP, Xia HH, Lam S, You WC. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Choi SM, Cho YS, Park G, Lee SK, Chun KS. Celecoxib induces apoptosis through Akt inhibition in 5-fluorouracil-resistant gastric cancer cells. Toxicol Res. 2021;37:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Jin GH, Xu W, Shi Y, Wang LB. Celecoxib exhibits an anti-gastric cancer effect by targeting focal adhesion and leukocyte transendothelial migration-associated genes. Oncol Lett. 2016;12:2345-2350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M, Miyazono K. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181-10189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Huang MT, Chen ZX, Wei B, Zhang B, Wang CH, Huang MH, Liu R, Tang CW. Preoperative growth inhibition of human gastric adenocarcinoma treated with a combination of celecoxib and octreotide. Acta Pharmacol Sin. 2007;28:1842-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, De Kock M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? Anesth Analg. 2010;110:1630-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 59. | Yeh CC, Lin JT, Jeng LB, Ho HJ, Yang HR, Wu MS, Kuo KN, Wu CY. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Forget P, Bouche G, Duhoux FP, Coulie PG, Decloedt J, Dekleermaker A, Guillaume JE, Ledent M, Machiels JP, Mustin V, Swinnen W, van Maanen A, Vander Essen L, Verougstraete JC, De Kock M, Berliere M. Intraoperative ketorolac in high-risk breast cancer patients. A prospective, randomized, placebo-controlled clinical trial. PLoS One. 2019;14:e0225748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, Arevalo J, Ma J, Horowitz M, Cole S, Ben-Eliyahu S. Perioperative COX-2 and β-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin Cancer Res. 2017;23:4651-4661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 62. | Takaya S, Saito H, Ikeguchi M. Upregulation of Immune Checkpoint Molecules, PD-1 and LAG-3, on CD4+ and CD8+ T Cells after Gastric Cancer Surgery. Yonago Acta Med. 2015;58:39-44. [PubMed] |
| 63. | Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1222] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 64. | Cesana GC, Romano F, Piacentini G, Scotti M, Brenna A, Bovo G, Vaghi M, Aletti G, Caprotti R, Kaufman H, Uggeri F. Low-dose interleukin-2 administered pre-operatively to patients with gastric cancer activates peripheral and peritumoral lymphocytes but does not affect prognosis. Ann Surg Oncol. 2007;14:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Romano F, Cesana G, Berselli M, Gaia Piacentini M, Caprotti R, Bovo G, Uggeri F. Biological, histological, and clinical impact of preoperative IL-2 administration in radically operable gastric cancer patients. J Surg Oncol. 2004;88:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Sedman PC, Ramsden CW, Brennan TG, Giles GR, Guillou PJ. Effects of low dose perioperative interferon on the surgically induced suppression of antitumour immune responses. Br J Surg. 1988;75:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Rajala P, Kaasinen E, Raitanen M, Liukkonen T, Rintala E; Finnbladder Group. Perioperative single dose instillation of epirubicin or interferon-alpha after transurethral resection for the prophylaxis of primary superficial bladder cancer recurrence: a prospective randomized multicenter study--FinnBladder III long-term results. J Urol. 2002;168:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Schneider C, von Aulock S, Zedler S, Schinkel C, Hartung T, Faist E. Perioperative recombinant human granulocyte colony-stimulating factor (Filgrastim) treatment prevents immunoinflammatory dysfunction associated with major surgery. Ann Surg. 2004;239:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Fu H, Li B, Liang Z. Effect of enteral immunonutrition compared with enteral nutrition on surgical wound infection, immune and inflammatory factors, serum proteins, and cellular immunity in subjects with gastric cancer undergoing a total gastrectomy: A meta-analysis. Int Wound J. 2022;19:1625-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? JAMA. 2001;286:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 576] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 71. | Li K, Xu Y, Hu Y, Liu Y, Chen X, Zhou Y. Effect of Enteral Immunonutrition on Immune, Inflammatory Markers and Nutritional Status in Gastric Cancer Patients Undergoing Gastrectomy: A Randomized Double-Blinded Controlled Trial. J Invest Surg. 2020;33:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Peker KD, Ozkanli SS, Akyuz C, Uzun O, Yasar NF, Duman M, Yol S. Preoperative immunonutrition regulates tumor infiltrative lymphocytes and increases tumor angiogenesis in gastric cancer patients. Arch Med Sci. 2017;13:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Qiang H, Hang L, Shui SY. The curative effect of early use of enteral immunonutrition in postoperative gastric cancer: a meta-analysis. Minerva Gastroenterol Dietol. 2017;63:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Cheng Y, Zhang J, Zhang L, Wu J, Zhan Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Scislo L, Pach R, Nowak A, Walewska E, Gadek M, Brandt P, Puto G, Szczepanik AM, Kulig J. The Impact of Postoperative Enteral Immunonutrition on Postoperative Complications and Survival in Gastric Cancer Patients - Randomized Clinical Trial. Nutr Cancer. 2018;70:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 77. | Matzner P, Sorski L, Shaashua L, Elbaz E, Lavon H, Melamed R, Rosenne E, Gotlieb N, Benbenishty A, Reed SG, Ben-Eliyahu S. Perioperative treatment with the new synthetic TLR-4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int J Cancer. 2016;138:1754-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Tai LH, Zhang J, Scott KJ, de Souza CT, Alkayyal AA, Ananth AA, Sahi S, Adair RA, Mahmoud AB, Sad S, Bell JC, Makrigiannis AP, Melcher AA, Auer RC. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin Cancer Res. 2013;19:5104-5115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Li J, Xu R, Hu DM, Zhang Y, Gong TP, Wu XL. Prognostic Nutritional Index Predicts Outcomes of Patients after Gastrectomy for Cancer: A Systematic Review and Meta-Analysis of Nonrandomized Studies. Nutr Cancer. 2019;71:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Huang DD, Yu DY, Song HN, Wang WB, Luo X, Wu GF, Yu Z, Liu NX, Dong QT, Chen XL, Yan JY. The relationship between the GLIM-defined malnutrition, body composition and functional parameters, and clinical outcomes in elderly patients undergoing radical gastrectomy for gastric cancer. Eur J Surg Oncol. 2021;47:2323-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 81. | Takagi K, Domagala P, Polak WG, Buettner S, Wijnhoven BPL, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: a systematic review and meta-analysis. BMC Surg. 2019;19:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Xu R, Chen XD, Ding Z. Perioperative nutrition management for gastric cancer. Nutrition. 2022;93:111492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 83. | Huhmann MB, August DA. Perioperative nutrition support in cancer patients. Nutr Clin Pract. 2012;27:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Huang DD, Zhou CJ, Wang SL, Mao ST, Zhou XY, Lou N, Zhang Z, Yu Z, Shen X, Zhuang CL. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery. 2017;161:680-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 85. | Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore). 2016;95:e3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 86. | Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, Zhou CJ, Shen X, Yu Z. Sarcopenia Adversely Impacts Postoperative Clinical Outcomes Following Gastrectomy in Patients with Gastric Cancer: A Prospective Study. Ann Surg Oncol. 2016;23:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 87. | Wada Y, Nishi M, Yoshikawa K, Takasu C, Tokunaga T, Nakao T, Kashihara H, Yoshimoto T, Shimada M. Preoperative nutrition and exercise intervention in frailty patients with gastric cancer undergoing gastrectomy. Int J Clin Oncol. 2022;27:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 88. | Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527-3534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 588] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 89. | Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 90. | Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 91. | Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721-727. [PubMed] |
| 92. | Ho RT, Wang CW, Ng SM, Ho AH, Ziea ET, Wong VT, Chan CL. The effect of t'ai chi exercise on immunity and infections: a systematic review of controlled trials. J Altern Complement Med. 2013;19:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Choy KT, Lam K, Kong JC. Exercise and colorectal cancer survival: an updated systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, Zhang X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis Markers. 2019;2019:7606128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Agnes A, Lirosi MC, Panunzi S, Santocchi P, Persiani R, D'Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol. 2018;44:404-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 96. | Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Füllenbach C, Freedman J, Waters JH, Farmer S, Leahy MF, Zacharowski K, Meybohm P, Choorapoikayil S. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Meta-analysis. Ann Surg. 2019;269:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 97. | Osorio J, Jericó C, Miranda C, Santamaría M, Artigau E, Galofré G, Garsot E, Luna A, Puértolas N, Aldeano A, Olona C, Molinas J, Feliu J, Videla S, Tebe C, Pera M. Improved postoperative outcomes and reduced transfusion rates after implementation of a Patient Blood Management program in gastric cancer surgery. Eur J Surg Oncol. 2021;47:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Beguin Y, Aapro M, Ludwig H, Mizzen L, Osterborg A. Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis--a critical review. Crit Rev Oncol Hematol. 2014;89:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Keding V, Zacharowski K, Bechstein WO, Meybohm P, Schnitzbauer AA. Patient Blood Management improves outcome in oncologic surgery. World J Surg Oncol. 2018;16:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 100. | Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K; Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 506] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 101. | Rosa F, Longo F, Pozzo C, Strippoli A, Quero G, Fiorillo C, Mele MC, Alfieri S. Enhanced recovery after surgery (ERAS) versus standard recovery for gastric cancer patients: The evidences and the issues. Surg Oncol. 2022;41:101727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Li MZ, Wu WH, Li L, Zhou XF, Zhu HL, Li JF, He YL. Is ERAS effective and safe in laparoscopic gastrectomy for gastric carcinoma? World J Surg Oncol. 2018;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Ding J, Sun B, Song P, Liu S, Chen H, Feng M, Guan W. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:75699-75711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 104. | Wang LH, Zhu RF, Gao C, Wang SL, Shen LZ. Application of enhanced recovery after gastric cancer surgery: An updated meta-analysis. World J Gastroenterol. 2018;24:1562-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 105. | Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1077] [Article Influence: 119.7] [Reference Citation Analysis (0)] |