Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.440
Peer-review started: December 6, 2022
First decision: December 27, 2022
Revised: January 9, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 27, 2023
Processing time: 110 Days and 22.7 Hours
Endoscopic resection remains an effective method for the treatment of small rectal neuroendocrine tumors (NETs) (≤ 10 mm). Moreover, endoscopic mucosal resection (EMR) with double band ligation (EMR-dB), a simplified modification of EMR with band ligation, is an alternative strategy to remove small rectal NETs.
To evaluate the feasibility and safety of EMR-dB for the treatment of small rectal NETs (≤ 10 mm).
A total of 50 patients with small rectal NETs, without regional lymph node enlargement or distant metastasis confirmed by endoscopic ultrasound, computerized tomography scan, or magnetic resonance imaging, were enrolled in the study from March 2021 to June 2022. These patients were randomly assigned into the EMR-dB (n = 25) group or endoscopic submucosal dissection (ESD) group (n = 25). The characteristics of the patients and tumors, procedure time, devices cost, complete resection rate, complications, and recurrence outcomes were analyzed.
There were 25 patients (13 males, 12 females; age range 28-68 years old) in the EMR-dB group, and the ESD group contained 25 patients (15 males, 10 females; age range 25-70 years old). Both groups had similar lesion sizes (EMR-dB 4.53 ± 1.02 mm, ESD 5.140 ± 1.74 mm; P = 0.141) and resected lesion sizes(1.32 ± 0.52 cm vs 1.58 ± 0.84 cm; P = 0.269). Furthermore, the histological complete resection and en bloc resection rates were achieved in all patients (100% for each). In addition, there was no significant difference in the complication rate between the two groups. However, the procedure time was significantly shorter and the devices cost was significantly lower in the EMR-dB group. Besides, there was no recurrence in both groups during the follow-up period.
The procedure time of EMR-dB was shorter compared with ESD, and both approaches showed a similar curative effect. Taken together, EMR-dB was a feasible and safe option for the treatment of small rectal NETs.
Core Tip: Endoscopic mucosal resection (EMR) with double band ligation (EMR-dB), a simplified modification of EMR with band ligation, is an alternative strategy to remove small rectal neuroendocrine tumors (NETs). Our study first evaluates the feasibility and safety of EMR-dB and endoscopic submucosal dissection (ESD) for the treatment of small rectal NETs (≤ 10 mm). We discovered that the EMR-dB technique took less time than ESD, and displayed a similar curative effect to ESD. If no lymph nodes and distant metastases are revealed by either endoscopic ultrasound or computerized tomography, EMR-dB is a feasible and safe option for the treatment of small rectal NETs.
- Citation: Huang JL, Gan RY, Chen ZH, Gao RY, Li DF, Wang LS, Yao J. Endoscopic mucosal resection with double band ligation versus endoscopic submucosal dissection for small rectal neuroendocrine tumors. World J Gastrointest Surg 2023; 15(3): 440-449
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/440.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.440
With the wide application of screening colonoscopy, the incidence of neuroendocrine tumors (NETs) has increased in the past few decades. The gastrointestinal (GI) tract is the most frequent site for NETs[1]. Rectal NETs represent 34% of all diagnosed GI NETs and are the most common NETs behind small bowel NETs[2]. Most GI NETs do not cause clinical symptoms. Therefore, they are only found by colonoscopy accidentally[3]. Rectal tumors of 10-19 mm in diameter have a metastatic rate of 4%-30%[4,5], whereas over 80% of tumors measuring more than 20 mm in diameter are associated with lymph nodes or liver metastases. Well-differentiated NETs ≤ 10.0 mm in diameter and limited to the submucosal layer are reported to be associated with a low frequency of lymph nodes and distant metastases. These NETs are good candidates for endoscopic resection (ER)[6,7], because ER can achieve high resection(R0) resection rates like many minimally invasive techniques[8], and it has reduced costs, morbidity, and mortality[9] compared with conventional surgery. However, the consensus about the optimal endoscopic treatment modality for rectal NETs has not been established yet.
ER, including conventional endoscopic mucosal resection (EMR), modified EMR (m-EMR), and endoscopic submucosal dissection (ESD), is a safe and effective modality for the treatment of small and localized early rectal NETs[2]. However, although conventional EMR can remove small rectal NETs in a minimally invasive manner, it is difficult to achieve deep resection margins because most rectal NETs invade the submucosal layer[10]. Therefore, various modified methods of EMR have been developed. m-EMR includes EMR with cap[11], EMR with band ligation (EMR-B)[12], (EMR-L)[13], EMR with circumferential incision[14], and so on. These strategies have all been proven to be safe and effective for removing rectal NETs. However, according to previous reports, EMR-B and EMR-L show a histological complete R0 rate that varies from 82.8% to 95.5% in treating rectal NETs[12,13,15]. The positive basal margins may be attributed to the insufficient distance from lesion to the resection margin. To overcome the shortcomings of the EMR-B and EMR-L, we presented a new EMR technique. EMR with double band ligation (EMR-dB), a simplified modification of EMR-B, could achieve a deeper vertical resection margin compared with EMR-B. However, the safety and efficacy of such m-EMR technique in treating small rectal NETs has not been determined. Therefore, in the present study, we compared the safety and efficacy of EMR-dB and ESD in the treatment of rectal NETs. Moreover, we aimed to evaluate the feasibility of EMR-dB for the treatment of small rectal NETs (≤ 10 mm) in comparison to ESD.
EMR-dB and ESD were performed in 50 patients with rectal NETs in the Gastroenterology Unit of Shenzhen People's Hospital from March 2021 to June 2022. These patients were randomly assigned into the EMR-dB (n = 25) group or ESD group (n = 25). The inclusion criteria were as follows: (1) Rectal NETs confirmed by histological diagnosis; (2) Tumors were ≤ 10 mm in diameter by endoscopic ultrasound (EUS); and (3) EUS and computerized tomography(CT) of the thorax/abdomen/pelvis were negative for lymph node and distant metastases. The study protocol was approved by the ethics committee of the hospital, and all patients gave their informed consent before the procedures (Clinical trial registration number: ChiCTR2200063871).
A researcher who was unaffiliated with this trial created a randomization list. Specific software (www.randomizer.org) was used, and the participants were randomly allocated at a 1:1 ratio to the EMR-dB group or the ESD group. Outcomes assessor was blinded after assignment to interventions.
A wide (14.9 mm in diameter), soft, straight, transparent cap with an inside rim (D-201-11802, Olympus) was fitted onto the tip of a standard single-channel endoscope (GIF-260, Olympus).
A ligating device with a 110-cm maximum Multiple Band Ligator (M00542251, Boston Scientific) was inserted into the accessory channel of the endoscope.
Other devices included a dual knife, injection needles, snares, hot biopsy forceps from Olympus, and a high-frequency generator (ICC-200, ERBE).
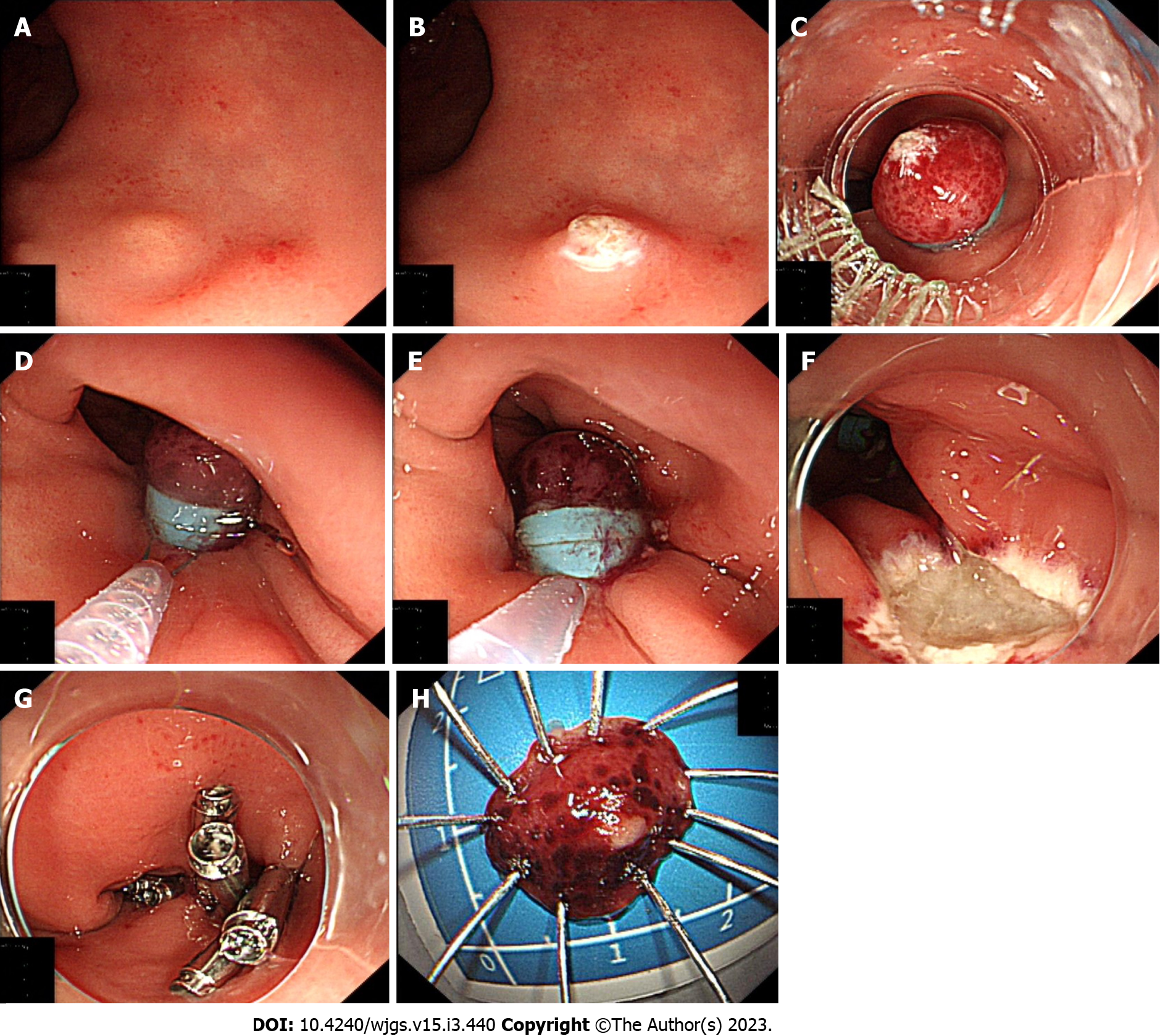
EMR-dB procedure (Figure 1): (1) Endoscopy showing a rectal carcinoid (Figure 1A); (2) Marking dots were on the lesion with an electric snare tip(KD-650Q, Olympus, Tokyo, Japan) (Figure 1B); (3) When the lesion was suctioned into the ligating device, the first band was deployed to ligate the lesion and increase luminal protuberance (Figure 1C); Then the second band was deployed below the first one after endoscopic suctioning of the tumor into the cap (Figure 1D); (4) The lesion resection was performed via electrocautery below the second band (Figure 1E); (5) Wound after resection (Figure 1F); and (6) The wound was closed with clips (Figure 1G); Subsequently, the resection specimen was entirely flattened (Figure 1H).
ESD procedure: ESD was performed using a single-channel endoscope with a short transparent cap attached to the tip of the endoscope. (1) Submucosal solution was injected as described above, and the circumferential mucosa of the lesion was incised using a dual knife. The mucosal incisions were placed at least 2-3 mm from the lesion periphery to create a sufficient tumor-free lateral resection margin; (2) Circumferential incision and submucosal dissection were carried out as previously described[16]; and (3) The wound was treated as described above.
Two experienced endoscopists (Yao J and Wang LS) conducted all procedures. All patients were subjected to food deprivation for 1 d after the operation.
The efficacy was evaluated by assessing the rates of histological complete R0, en bloc resection, and operation success, and the safety was evaluated by assessing the complications.
The primary outcome was the histological complete R0 rate. Histological complete resection was defined as a complete single-piece (en bloc) resection of the lesion with a tumor-free margin in both the lateral and vertical margins.
Secondary outcomes included: En bloc resection rate: En bloc resection was defined as a complete single resection of the targeted lesion, regardless of whether the basal and lateral tumor margins were infiltrated or undetermined. Complications: The primary complications included bleeding and perforation. Immediate bleeding was defined as an evident hemorrhage during the procedure that could not be controlled by endoscopic hemostasis. Delayed bleeding was defined as bleeding that caused hemoglobin to drop ≥ 2 g/dL or hematochezia, which required endoscopic and/or radiologic hemostasis or transfusions within 14 d after the procedure. Perforation was defined as the wall defect identified by endoscopy or free air in the abdominal cavity detected by radiological examinations (such as plain abdominal X-ray and/or abdominal CT) after the procedure. Procedure time was counted from the time of submucosal injection to the end of complete resection of the targeted lesion. Devices cost was defined as the cost of the required use of clips and the ligation devices in EMR-dB or dual knife using in ESD procedures, except the cost of other endoscopic procedures. Histopathologic grade included NET grade(G) 1, NET G2, NET G3, and neuroendocrine carcinomas according to the 2019 World Health Organization classification[17].
All patients were followed up by colonoscopy at 3 mo after endoscopic treatment to detect the recovery of the surgical wound and local recurrence. The patients with vertical and/or lateral margin involvement were recommended to undergo additional treatment.
All statistical analyses were performed using Statistical Product and Service Solutions statistics version 26. Continuous data were described as mean ± standard deviation, or median and range. Categorical data were expressed as numbers (n) and percentages (%). Chi-square or Fisher's exact tests were performed for comparative analysis of categorical variables. Continuous variables were analyzed using Student's t-test. P < 0.05 was considered statistically significant.
There were 25 patients (13 males, 12 females; age range 28-68 years old) in the EMR-dB group, and the ESD group contained 25 patients (15 males, 10 females; age range 25-70 years old). The average age of the EMR-dB and ESD groups was comparable between the two groups (47.04 ± 10.58 vs 42.92 ± 10.93, P = 0.182). There was no statistical difference in the location (average distance from anus) between the EMR-dB group and the ESD group (7.96 ± 3.52 cm vs 7.36 ± 2.83 cm; P = 0.509). Sex and age distribution were similar between the two groups. Moreover, both groups had identical mean lesion sizes (4.53 ± 1.02 mm vs 5.140 ± 1.74 mm; P = 0.141) and resected lesion diameters (1.32 ± 0.52 cm vs 1.58 ± 0.84 cm; P = 0.001). Table 1 shows the characteristics and tumor sizes of the patients in the two groups.
| EMR-dB (n = 25) | ESD (n = 25) | P value | |
| Age | 47.04 ± 10.58 | 42.92 ± 10.93 | 0.182 |
| Sex (Male/Female) | 13/12 | 15/10 | 0.569 |
| Tumor size (mm) | 4.53 ± 1.02 | 5.140 ± 1.74 | 0.141 |
| Location (distance from anus) (cm) | 7.96 ± 3.52 | 7.36 ± 2.83 | 0.509 |
| Resected lesion size (cm) | 1.32 ± 0.52 | 1.58 ± 0.84 | 0.269 |
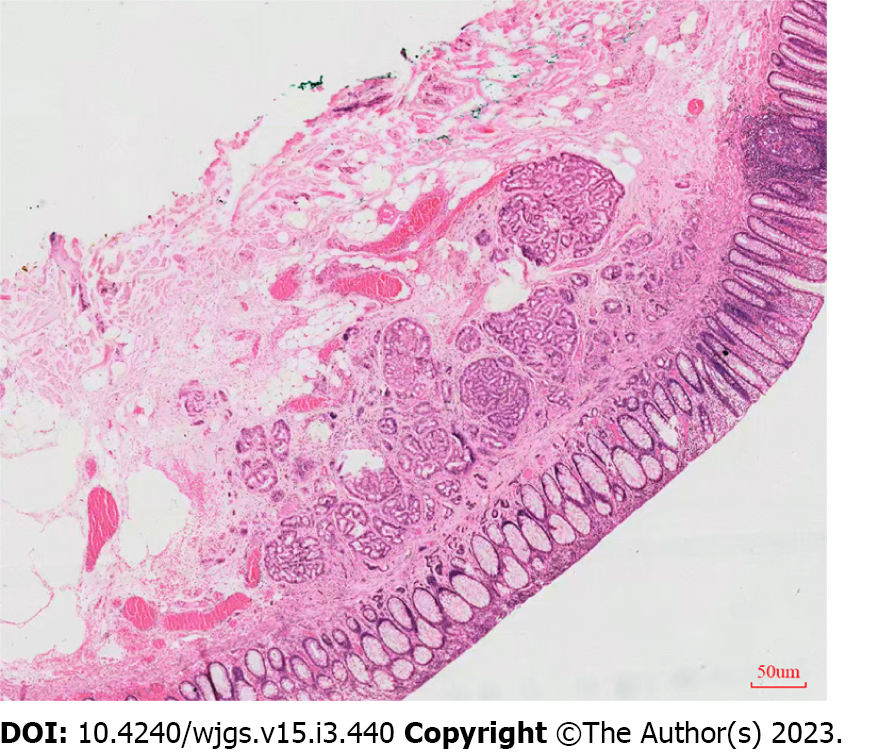
The histological complete resection and en bloc resection rates were the same in the two groups (100% for each). No significant difference in the complication rate between the two groups [delayed bleeding occurred in 0 patients in the EMR-dB group and two patients in the ESD group (8.0%) (P = 0.47), and no perforation was observed in either group]. However, the procedure time was significantly shorter in the EMR-dB group (6.28 ± 0.75 min) compared with the ESD group (14.30 ± 1.51 min) (P < 0.001) and the devices cost was significantly lower in the EMR-dB group than in the ESD group ($ 494.04 ± $ 85.47 vs $ 808.98 ± $ 143.67, P < 0.05). The pathological results were similar between the two groups (P > 0.99). All tumors were classified as NET G1 grade according to the staging system for NETs of the American Joint Committee on Cancer, absence of lymphovascular invasion, negative horizontal margin (pHM0) and negative vertical margin (pVM0) (Figure 2). Table 2 shows the therapeutic outcomes of ER in the two groups. In the two cases with delayed bleeding, bloody stool appeared on the 1st day and the 7th day after the ESD procedure, respectively. A colonoscopy revealed that the postoperative wound was bleeding, hemostasis was well managed using endoscopy, and no blood transfusion or surgical intervention was necessary. All patients were followed up after 3 mo of the treatment. Again, a colonoscopy was performed, and a postoperative scar was seen.
| EMR-dB (n = 25) | ESD (n = 25) | P value | |
| Histological complete resection, n (%) | 25 (100) | 25 (100) | > 0.99 |
| En bloc resection, n (%) | 25 (100) | 25 (100) | > 0.99 |
| Resection time (min) | 6.28 ± 0.75 | 14.30 ± 1.51 | < 0.001 |
| Delayed bleeding perforation | 0 | 2 | 0.470 |
| Perforation | 0 | 0 | > 0.99 |
| Devices cost | $ 494.04 ± $ 85.47 | $ 808.98 ± $ 143.67 | < 0.001 |
| Histopathological classification | > 0.99 | ||
| NET G1, n (%) | 25 (100) | 25 (100) | |
| NET G2 | 0 | 0 | |
| NET G3 | 0 | 0 | |
| NEC | 0 | 0 | |
| Recurrence follow-up | 0 | 0 | > 0.99 |
No local remnant lesions or recurrences were observed during the follow-up period in both groups.
NETs of the rectum are a heterogeneous group of tumors. The pathological types mainly include NET, neuroendocrine carcinoma, mixed gland neuroendocrine carcinoma, and site-specific and functional NETs[18,19]. Less than 2% and 0.7% of rectal NETs < 10 mm in diameter are related to lymph nodes and distant metastases, respectively[10]. According to the current European Neuroendocrine Tumor Society guidelines, ER is considered curative for tumors smaller than 10 mm and well differentiated[20]. EMR has the advantages of simple and rapid operation and low complication rate[21-23], while its high recurrence rate of residual lesions is a limiting factor for its application[10]. On the other hand, ESD is an effective method with a higher complete resection rate, while its technical requirements and the rate of complications are relatively high[12,24,25]. The consensus about the optimal endoscopic treatment modality for rectal NETs has not yet been established.
Previous studies have proved that EMR using a band-ligation device (EMR-B) (EMR-L) is sufficient for tumors ≤ 10 mm in diameter[12,13,15]. However, the histopathological examination shows a positive margin for some lesions that have invaded the submucosa or deeper layers of the rectal wall. Therefore, we presented the EMR-dB technique, a new approach derived from EMR-B, containing an extra band below the first one. With EMR-dB, the second band could lengthen the distance from the lesion to the vertical resection margin, especially for some flat lesions and tumors that invaded the submucosa or deeper layers of the rectal wall. Therefore, this approach might better improve the complete resection rate and reduce the risk of residual tumors. However, there have been no more research reports about the EMR-dB technique.
The present study was the first randomized controlled trial to compare the safety and efficacy of EMR-dB with ESD for treating small rectal NETs. To remove the tumor completely, we carried out a series of optimization and improvement on operation steps. Firstly, we marked the head-end of the tumor to avoid deflecting the tumor during the ligation, making it easier to be suctioned it into the ligating device completely. Secondly, a submucosal injection was given to completely lift the submucosal layer of the tumor and set the basal layer of the tumor apart from the muscularis propria. This procedure could achieve a better complete resection and prevent the muscularis propria from being suctioned into the cap leading to perforation. Thirdly, given intestinal inflation when the NETs and part of the muscularis propria layer were ligated by band ligation, the muscularis propria layer will fall out of the band ligation over time due to the ductility of muscularis propria layer, leaving only the mucosal layer and submucosa, which may reduce the risk of perforation during resection.
EMR-dB showed an en bloc resection of all lesions with a tumor-free margin in both the lateral and vertical margins. Moreover, no complications occurred, and there were free of local remnant lesions or recurrence during the follow-up period, indicating similar efficacy with ESD. However, the procedure time of the EMR-dB group was significantly shorter compared with the ESD group (6.28 ± 0.75 min vs 14.30 ± 1.51 min) and the devices cost was significantly lower in the EMR-dB group than in the ESD group ($ 494.04 ± $ 85.47 vs $ 808.98 ± $ 143.67). When compared with EMR-B, the use of the Multiple Band Ligators for continuous ligations at one time in EMR-dB procedure may resulted in a little increase in technical difficulty, cost, and procedure time, and the size of the resection specimen might enlarge. However, it could better reduce residual tumor infiltration within vertical and lateral margins and potentially reduce recurrence rates. Recently, a case of rectal NET removal using the EMR-dB technique has been reported[26], and the pathological examination reveals a G1 NET with a negative margin and without complications, indicating that EMR-dB could work more significantly, which is consistent with our result.
In addition, the EMR-dB technique has several other advantages. Firstly, the tightening of the elastic band in EMR-dB could shrink the wound size. Therefore, the required use of clips is less. Secondly, as demonstrated in our study, the cost of the devices in the EMR-dB group was much lower compared with the ESD group. It is mainly attributed to the fact that the cost of the ligation device of EMR-dB is lower than that of ESD group using a dual knife. Moreover, EMR-dB requires fewer clips, which leads to the reduction of the operation and hospitalization cost. Moreover, there was no complication in the EMR-dB group. In contrast, two cases in the ESD group had delayed bleeding and needed further treatment, which also increased the hospitalization cost and days, bringing more physical and mental pain to patients.
The present study has some limitations. First, this study was a single-center study with limited sample size. In addition, considering that rectal NET is a slow-growing tumor, further prospective studies with a long-term follow-up period are needed to verify our findings.
In conclusion, our study demonstrated that the EMR-dB technique took less time than ESD, and it displayed a similar curative effect to ESD. If no lymph nodes and distant metastases are revealed by either EUS or CT, EMR-dB is a feasible and safe option for the treatment of small rectal NETs.
In conclusion, our study demonstrated that the EMR-dB technique took less time than ESD, and it displayed a similar curative effect to ESD. If no lymph nodes and distant metastases are revealed by either EUS or CT, EMR-dB is a feasible and safe option for the treatment of small rectal NETs.
Endoscopic resection remains an effective method for the treatment of small rectal neuroendocrine tumors (NETs) ( ≤ 10 mm). However, the consensus about the optimal endoscopic treatment modality for rectal NETs has not been established yet.
To overcome the shortcomings of endoscopic mucosal resection(EMR) with band ligation (EMR-B)(EMR-L), we presented a new EMR technique. EMR with double band ligation (EMR-dB), a simplified modification of EMR-B, could achieve a deeper vertical resection margin compared with EMR-B. However, the safety and efficacy of EMR-dB technique in treating small rectal NETs has not been determined.
In the present study, we compared the safety and efficacy of EMR-dB and endoscopic submucosal dissection (ESD) in the treatment of rectal NETs. We aimed to evaluate the feasibility of EMR-dB for the treatment of small rectal NETs ( ≤ 10 mm) in comparison to ESD.
A randomized controlled trial comparing EMR-dB and ESD was conducted. The primary outcome was the histological complete resection rate; secondary outcomes included en bloc resection rate, procedure time, complications and so on. Follow-up was also performed.
A total of 50 patients were analyzed and were 25 patients in each group. The demographic and baseline characteristics of the participants were similar between the two groups, including age, gender, lesion location (average distance from anus), lesion sizes, and resected lesion sizes. histological complete resection and en bloc resection were achieved in all 50 patients. No significant difference in the complication rate between the two groups [delayed bleeding occurred in 0 patients in the EMR-dB group and two patients in the ESD group (8.0%) (P = 0.47)], indicating that EMR-dB is non-inferior to ESD with a similar complete resection rate and complication rate. However, the procedure time was significantly shorter in the EMR-dB group (6.28 ± 0.75 min) compared with the ESD group (14.30 ± 1.51 min) (P < 0.001) and the devices cost was significantly lower in the EMR-dB group than in the ESD group ($ 494.04 ± $ 85.47 vs $ 808.98 ± $ 143.67, P < 0.05), which demonstrated that EMR-dB had shorter procedure duration time and lower operation costs. No local remnant lesions or recurrences were observed during the follow-up period in both groups, further prospective studies with a long-term follow-up period are needed to verify our findings.
EMR-dB, a new EMR technique presented in our study, took less time than ESD, and displayed a similar curative effect to ESD. If no lymph nodes and distant metastases are revealed by either endoscopic ultrasound or computerized tomography, EMR-dB is a feasible and safe option for the treatment of small rectal NETs.
First, this study was a single-center study with limited sample size. In addition, considering that rectal NET is a slow-growing tumor, further prospective studies with a long-term follow-up period are needed to verify our findings. Moreover, statistical analysis between EMR-B and EMR-dB can be further investigate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Matsui S, Japan; Osawa S, Japan S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: the investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther. 2016;44:332-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 3. | Koura AN, Giacco GG, Curley SA, Skibber JM, Feig BW, Ellis LM. Carcinoid tumors of the rectum: effect of size, histopathology, and surgical treatment on metastasis free survival. Cancer. 1997;79:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Schindl M, Niederle B, Häfner M, Teleky B, Längle F, Kaserer K, Schöfl R. Stage-dependent therapy of rectal carcinoid tumors. World J Surg. 1998;22:628-33; discussion 634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Zhou PH, Yao LQ, Qin XY, Xu MD, Zhong YS, Chen WF, Ma LL, Zhang YQ, Qin WZ, Cai MY, Ji Y. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc. 2010;24:2607-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 8. | Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, Cassese G, Pagano G, Tropeano FP, Luglio G, De Palma GD. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Gotoda T, Kaltenbach T, Soetikno R. Is en bloc resection essential for endoscopic resection of GI neoplasia? Gastrointest Endosc. 2008;67:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Yang DH, Park Y, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2016;83:1015-22; quiz 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Choi CW, Kang DH, Kim HW, Park SB, Jo WS, Song GA, Cho M. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol. 2013;47:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Lim HK, Lee SJ, Baek DH, Park DY, Lee BE, Park EY, Park JW, Kim GH, Song GA. Resectability of Rectal Neuroendocrine Tumors Using Endoscopic Mucosal Resection with a Ligation Band Device and Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2019;2019:8425157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Huang J, Lu ZS, Yang YS, Yuan J, Wang XD, Meng JY, Du H, Wang HB. Endoscopic mucosal resection with circumferential incision for treatment of rectal carcinoid tumours. World J Surg Oncol. 2014;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Lee J, Park YE, Choi JH, Heo NY, Park J, Park SH, Moon YS, Nam KH, Kim TO. Comparison between cap-assisted and ligation-assisted endoscopic mucosal resection for rectal neuroendocrine tumors. Ann Gastroenterol. 2020;33:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Zhang D, Lin Q, Shi R, Wang L, Yao J, Tian Y. Ligation-assisted endoscopic submucosal resection with apical mucosal incision to treat gastric subepithelial tumors originating from the muscularis propria. Endoscopy. 2018;50:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2409] [Article Influence: 481.8] [Reference Citation Analysis (3)] |
| 18. | Lim JY, Pommier RF. Clinical Features, Management, and Molecular Characteristics of Familial Small Bowel Neuroendocrine Tumors. Front Endocrinol (Lausanne). 2021;12:622693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Tran CG, Sherman SK, Howe JR. Small Bowel Neuroendocrine Tumors. Curr Probl Surg. 2020;57:100823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 21. | Kim GU, Kim KJ, Hong SM, Yu ES, Yang DH, Jung KW, Ye BD, Byeon JS, Myung SJ, Yang SK, Kim JH. Clinical outcomes of rectal neuroendocrine tumors ≤ 10 mm following endoscopic resection. Endoscopy. 2013;45:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Puli SR, Kakugawa Y, Gotoda T, Antillon D, Saito Y, Antillon MR. Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J Gastroenterol. 2009;15:4273-4277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Zheng JC, Zheng K, Zhao S, Wang ZN, Xu HM, Jiang CG. Efficacy and safety of modified endoscopic mucosal resection for rectal neuroendocrine tumors: a meta-analysis. Z Gastroenterol. 2020;58:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Yong JN, Lim XC, Nistala KRY, Lim LKE, Lim GEH, Quek J, Tham HY, Wong NW, Tan KK, Chong CS. Endoscopic submucosal dissection versus endoscopic mucosal resection for rectal carcinoid tumor. A meta-analysis and meta-regression with single-arm analysis. J Dig Dis. 2021;22:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Wang XY, Chai NL, Linghu EQ, Qiu ST, Li LS, Zou JL, Xiang JY, Li XX. The outcomes of modified endoscopic mucosal resection and endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors and the value of endoscopic morphology classification in endoscopic resection. BMC Gastroenterol. 2020;20:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | He L, Wen W, Ye L, Liao K, Hu B. Endoscopic Mucosal Resection With Double Band Ligation for Small Rectal Neuroendocrine Tumors. Am J Gastroenterol. 2021;116:1827-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |