Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.374
Peer-review started: December 21, 2022
First decision: January 3, 2023
Revised: January 11, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 27, 2023
Processing time: 96 Days and 6.6 Hours
Pain after transcatheter arterial chemoembolisation (TACE) can seriously affect the prognosis of patients and the insertion of additional medical resources.
To develop an early warning model for predicting pain after TACE to enable the implementation of preventive analgesic measures.
We retrospectively collected the clinical data of 857 patients (from January 2016 to January 2020) and prospectively enrolled 368 patients (from February 2020 to October 2022; as verification cohort) with hepatocellular carcinoma (HCC) who received TACE in the Hepatic Surgery Center of Tongji Hospital. Five predictive models were established using machine learning algorithms, namely, random forest model (RFM), support vector machine model, artificial neural network model, naive Bayes model and decision tree model. The efficacy of these models in predicting postoperative pain was evaluated through receiver operating characteristic curve analysis, decision curve analysis and clinical impact curve analysis.
A total of 24 candidate variables were included in the predictive models using the iterative algorithms. Age, preoperative pain, number of embolised tumours, distance from the liver capsule, dosage of iodised oil and preoperative prothrombin activity were closely associated with postoperative pain. The accuracy of the predictive model was compared between the training [area under the curve (AUC) = 0.798; 95% confidence interval (CI): 0.745-0.851] and verification (AUC = 0.871; 95%CI: 0.818-0.924) cohorts, with RFM having the best predictive efficiency (training cohort: AUC = 0.869, 95%CI: 0.816-0.922; internal verification cohort: AUC = 0.871; 95%CI: 0.818-0.924).
The five predictive models based on advanced machine learning algorithms, especially RFM, can accurately predict the risk of pain after TACE in patients with HCC. RFM can be used to assess the risk of pain for facilitating preventive treatment and improving the prognosis.
Core Tip: Machine learning-based pre-warning models can be used to predict post-transcatheter arterial chemoembolisation (TACE) pain for hierarchical management of patients at high risk of moderate and severe pain after TACE. In particular, random forest model (RFM) combined with preoperative predictors (i.e., age, preoperative pain, distance from liver capsule ≤ 2 cm, prothrombin activity, iodine oil dose and increased number of emboli) has optimal discriminating power and high predictive accuracy. Therefore, RFM can be used for early prediction of the risk of pain, which can facilitate prompt pain management after TACE and improve the prognosis of patients.
- Citation: Guan Y, Tian Y, Fan YW. Pain management in patients with hepatocellular carcinoma after transcatheter arterial chemoembolisation: A retrospective study. World J Gastrointest Surg 2023; 15(3): 374-386
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/374.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.374
As a first-line treatment for patients with mid-stage hepatocellular carcinoma (HCC), transcatheter arterial chemoembolisation (TACE) is especially suitable for patients with multifocal HCC who are not eligible for radical treatment[1,2]. Compared with the classic supportive treatment, TACE can significantly improve the quality of life and prolong the survival time of patients[3]. However, because TACE can block the blood supply of main blood vessels and lead to local liver tissue swelling and tumour necrosis, most patients experience pain of varying intensity after receiving TACE[4]. Previous studies have shown that the incidence of pain in patients with HCC after TACE is 60%-80%, and approximately 20%-40% of patients have severe pain, prolonged bed rest time and increased likelihood of postoperative complications, resulting in increased medical costs[5-7]. Therefore, prompt and effective pain management and nursing care are of great significance for improving the prognosis and quality of life of patients receiving TACE.
Early pain management can not only significantly reduce the incidence of pain but also improve the quality of life of patients receiving TACE. Therefore, identifying predictive factors related to postoperative pain may help to assess the risk of pain after TACE to implement pain relief interventions in advance[8]. Previous studies have shown that age, portal vein tumour thrombosis and tumour diameter are associated with an increased risk of pain after TACE[9]. In addition, preoperative anxiety, depression and other psychological factors can promote postoperative pain[10,11]. However, no effective scoring strategy is available for evaluating the risk of pain in patients with HCC after TACE. Developing such strategies may facilitate hierarchical management of patients with HCC with different degrees of pain.
In recent years, scholars have constructed nomographs for quantitative scoring by integrating multi-dimensional pain-related variables. Nomographs are based on traditional logic algorithms, which can help to indicate the risk of pain in patients with HCC receiving TACE[9]. However, with the continuous innovation and improvement of machine learning algorithms, several advanced algorithms have been gradually applied to the medical field for improving the accuracy and robustness of risk stratification[12-14]. To the best of our knowledge, an early warning model integrating machine learning algorithms and clinical indicators of post-TACE pain has not yet been developed for the management of pain in patients with HCC. Therefore, in this study, we constructed a machine learning-based model to predict post-TACE pain for early identification and prompt treatment of high-risk patients in clinical settings.
We retrospectively included 857 patients with HCC who received TACE in the Hepatic Surgery Center of Tongji Hospital from January 2016 to January 2020 through the electronic record system of the hospital. Additionally, we prospectively included 368 patients with HCC who underwent TACE in the hospital from February 2020 to October 2022 as the external verification cohort. The inclusion criteria were as follows: (1) Patients aged > 18 years; (2) Patients diagnosed with HCC via histopathological examination; (3) Patients receiving traditional TACE; and (4) The patients received corticosteorids as part of the protocol and the pain-management protocols has not changed between 2016 and 2022. The exclusion criteria were as follows: (1) Patients with incomplete medical records; and (2) Patients who underwent other surgeries and those with long-term use of painkillers before surgery. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, and Huazhong University of Science and Technology and complies with the Declaration of Helsinki (2013 version). All patients with HCC who participated in this study signed an informed consent form. Figure 1 demonstrates the process of patient selection and construction of predictive models.
We retrospectively collected the perioperative clinical data of patients: (1) Demographic data, including age, sex and body mass index; (2) History of TACE and hepatobiliary surgery; (3) Relevant preoperative imaging data, including maximum tumour diameter, number of embolised tumours, location of embolised tumours, portal vein tumour thrombosis and distance from the liver capsule; (4) Surgery-related data, including preoperative pain (PrP) perception, Child-Pugh classification, surgical duration, use of embolic supplement (except for iodised oil, gelatin sponge, blank microspheres, polyvinyl alcohol and other granular embolic agents) and iodised oil dosage; and (5) Preoperative biochemical data, including the levels of albumin, total bilirubin, alanine aminotransferase and aspartate aminotransferase; prothrombin time; prothrombin activity and platelet count. The quality control standards for retrospective data were as follows: Variables with missing data in ≤ 5% of the total number of cases were considered for inclusion in the analysis (the missing value is filled in using median interpolation); however, those with missing data in > 5% of cases were excluded to avoid bias caused by filling the missing value.
The intensity of postoperative pain was evaluated by trained professionals using the numeric rating scale. The subjective feelings of patients were considered the main observation index. Patients were evaluated every 2 h after receiving TACE and scored as follows: 0 points, no pain; 1-3 points, mild pain; 4-6, moderate pain; 7-10, severe pain. Patients with scores of ≥ 4 points are identified as having moderate and severe pain, and opioids should be considered for analgesic treatment. We considered moderate and severe pain within 24 h after surgery as the outcome variables. Patients with pain scores of ≥ 4 points within 24 h of surgery were included in the pain group, whereas those with < 4 points were included in the non-pain group.
Five common machine learning algorithms were used to develop predictive models: Random forest model (RFM), support vector machine model (SVMM), artificial neural network model (ANNM), decision tree model (DTM) and naive Bayes model (NBM). The efficacy of these models in predicting postoperative pain was evaluated through receiver operating characteristic analysis and decision curve analysis (DCA). In addition, continuous correction curves were plotted to evaluate the robustness of predictive models, and clinical impact curves (CICs) were plotted to evaluate the differentiation efficiency of the optimal predictive model (RFM). All predictive models were tested in the internal training, internal verification and external verification cohorts.
Statistical analysis was performed using the R software (https://www.r-project.org/). For descriptive analysis, the median (interquartile range) and frequency (%) of continuous variables and categorical variables were evaluated, respectively. Pearson correlation coefficients were evaluated to measure the degree of correlation between variables, and least absolute shrinkage and selection operator (LASSO) regression was performed for selecting significant variables for models. For variable screening and inter-group comparison (pain vs non-pain group), P-values of < 0.05 were considered significant.
Patients were divided into pain and non-pain groups based on whether they had moderate or severe pain within 24 h of TACE. Among 1225 patients, 205 (16.73%) had pain after TACE. The cumulative incidence of moderate and severe post-TACE pain at < 6 h, 6-12 h and 12-24 h after TACE was 15.12%, 17.26% and 13.15%, respectively, in the training cohort, and 14.86%, 18.26% and 15.11%, respectively, in the external verification cohort.
On comparing the baseline data of patients with HCC between the pain and non-pain groups, significant differences (P < 0.05) were observed in age, PrP, maximum tumour diameter, number of embolised tumours (NOETs), distance from the liver capsule (DFLS), use of embolic supplements and dosage of iodised oil. On comparison of biochemical indicators, post-TACE pain was found to be significantly associated with prothrombin activity and platelet count (P < 0.05). The detailed baseline data of the two groups are summarised in Table 1.
| Variables | Training set | P value | Testing set | ||
| Overall (n = 857) | Pain (n = 139) | No-pain (n = 718) | Overall (n = 368) | ||
| Age (%), yr | |||||
| ≤ 50 | 199 (23.2) | 119 (85.6) | 80 (11.1) | < 0.001 | 86 (23.4) |
| > 50 | 658 (76.8) | 20 (14.4) | 638 (88.9) | 282 (76.6) | |
| Gender (%) | |||||
| Male | 445 (51.9) | 74 (53.2) | 371 (51.7) | 0.806 | 151 (41.0) |
| Female | 412 (48.1) | 65 (46.8) | 347 (48.3) | 217 (59.0) | |
| BMI [median (IQR)], kg/m2 | 24.00 (21.10, 27.10) | 23.70 (21.10, 27.45) | 24.00 (21.02, 27.08) | 0.658 | 24.00 (21.20, 26.70) |
| Pathogeny (%) | |||||
| Hepatitis B | 218 (25.4) | 31 (22.3) | 187 (26.0) | 0.089 | 104 (28.3) |
| HCV | 226 (26.4) | 28 (20.1) | 198 (27.6) | 88 (23.9) | |
| Alcoholic liver | 214 (25.0) | 39 (28.1) | 175 (24.4) | 95 (25.8) | |
| Others | 199 (23.2) | 41 (29.5) | 158 (22.0) | 81 (22.0) | |
| ECOG (%) | |||||
| 0 | 417 (48.7) | 63 (45.3) | 354 (49.3) | 0.443 | 187 (50.8) |
| 1 | 440 (51.3) | 76 (54.7) | 364 (50.7) | 181 (49.2) | |
| TACE (%) | |||||
| Yes | 445 (51.9) | 70 (50.4) | 375 (52.2) | 0.756 | 169 (45.9) |
| No | 412 (48.1) | 69 (49.6) | 343 (47.8) | 199 (54.1) | |
| HHS (%) | |||||
| Yes | 414 (48.3) | 73 (52.5) | 341 (47.5) | 0.321 | 195 (53.0) |
| No | 443 (51.7) | 66 (47.5) | 377 (52.5) | 173 (47.0) | |
| PrP (%) | |||||
| Yes | 204 (23.8) | 130 (93.5) | 74 (10.3) | < 0.001 | 91 (24.7) |
| No | 653 (76.2) | 9 (6.5) | 644 (89.7) | 277 (75.3) | |
| MDT (%), cm | |||||
| ≤ 10 | 416 (48.5) | 76 (54.7) | 340 (47.4) | 0.137 | 193 (52.4) |
| > 10 | 441 (51.5) | 63 (45.3) | 378 (52.6) | 175 (47.6) | |
| LOET (%) | |||||
| Left | 437 (51.0) | 80 (57.6) | 357 (49.7) | 0.11 | 189 (51.4) |
| Right | 420 (49.0) | 59 (42.4) | 361 (50.3) | 179 (48.6) | |
| NOET (%) | |||||
| Single | 683 (79.7) | 21 (15.1) | 662 (92.2) | < 0.001 | 280 (76.1) |
| Multiple | 174 (20.3) | 118 (84.9) | 56 (7.8) | 88 (23.9) | |
| PVTT (%) | |||||
| Yes | 438 (51.1) | 72 (51.8) | 366 (51.0) | 0.932 | 185 (50.3) |
| No | 419 (48.9) | 67 (48.2) | 352 (49.0) | 183 (49.7) | |
| DFLS (%), cm | |||||
| > 2 | 646 (75.4) | 16 (11.5) | 630 (87.7) | < 0.001 | 268 (72.8) |
| ≤ 2 | 211 (24.6) | 123 (88.5) | 88 (12.3) | 100 (27.2) | |
| CTPG (%) | |||||
| Grade A | 442 (51.6) | 75 (54.0) | 367 (51.1) | 0.602 | 176 (47.8) |
| Grade B | 415 (48.4) | 64 (46.0) | 351 (48.9) | 192 (52.2) | |
| OpD (%), h | |||||
| ≤ 1 | 452 (52.7) | 83 (59.7) | 369 (51.4) | 0.088 | 184 (50.0) |
| > 1 | 405 (47.3) | 56 (40.3) | 349 (48.6) | 184 (50.0) | |
| ES (%) | |||||
| Yes | 437 (51.0) | 72 (51.8) | 365 (50.8) | 0.908 | 187 (50.8) |
| No | 420 (49.0) | 67 (48.2) | 353 (49.2) | 181 (49.2) | |
| LOD (%), mL | |||||
| ≤ 10 | 630 (73.5) | 21 (15.1) | 609 (84.8) | < 0.001 | 260 (70.7) |
| > 10 | 227 (26.5) | 118 (84.9) | 109 (15.2) | 108 (29.3) | |
| Albumin [median (IQR)], g/L | 36.12 (33.45, 38.63) | 36.11 (33.50, 38.81) | 36.12 (33.43, 38.57) | 0.688 | 36.03 (33.42, 38.73) |
| PT [median (IQR)], s | 12.70 (12.30, 13.20) | 12.60 (12.30, 13.10) | 12.70 (12.30, 13.20) | 0.559 | 12.70 (12.30, 13.10) |
| PTA [median (IQR)], % | 82.25 (77.12, 86.60) | 90.20 (87.26, 93.18) | 80.33 (76.45, 84.36) | < 0.001 | 82.29 (77.81, 86.83) |
| TBIL [median (IQR)], g/L | 16.17 (12.95, 19.37) | 16.20 (13.34, 19.27) | 16.16 (12.88, 19.39) | 0.972 | 16.12 (13.25, 19.23) |
| ALT [median (IQR)], U/L | 33.00 (27.00, 40.00) | 34.00 (24.50, 40.00) | 33.00 (27.00, 40.75) | 0.446 | 34.00 (26.00, 41.00) |
| AST [median (IQR)], U/L | 42.00 (35.00, 48.00) | 44.00 (35.00, 49.00) | 41.00 (35.00, 48.00) | 0.091 | 42.00 (34.00, 48.25) |
| PLT [median (IQR)], 109 | 136.00 (104.00, 163.00) | 138.00 (104.00, 160.00) | 135.50 (104.00, 164.00) | 0.749 | 130.50 (100.00, 160.25) |
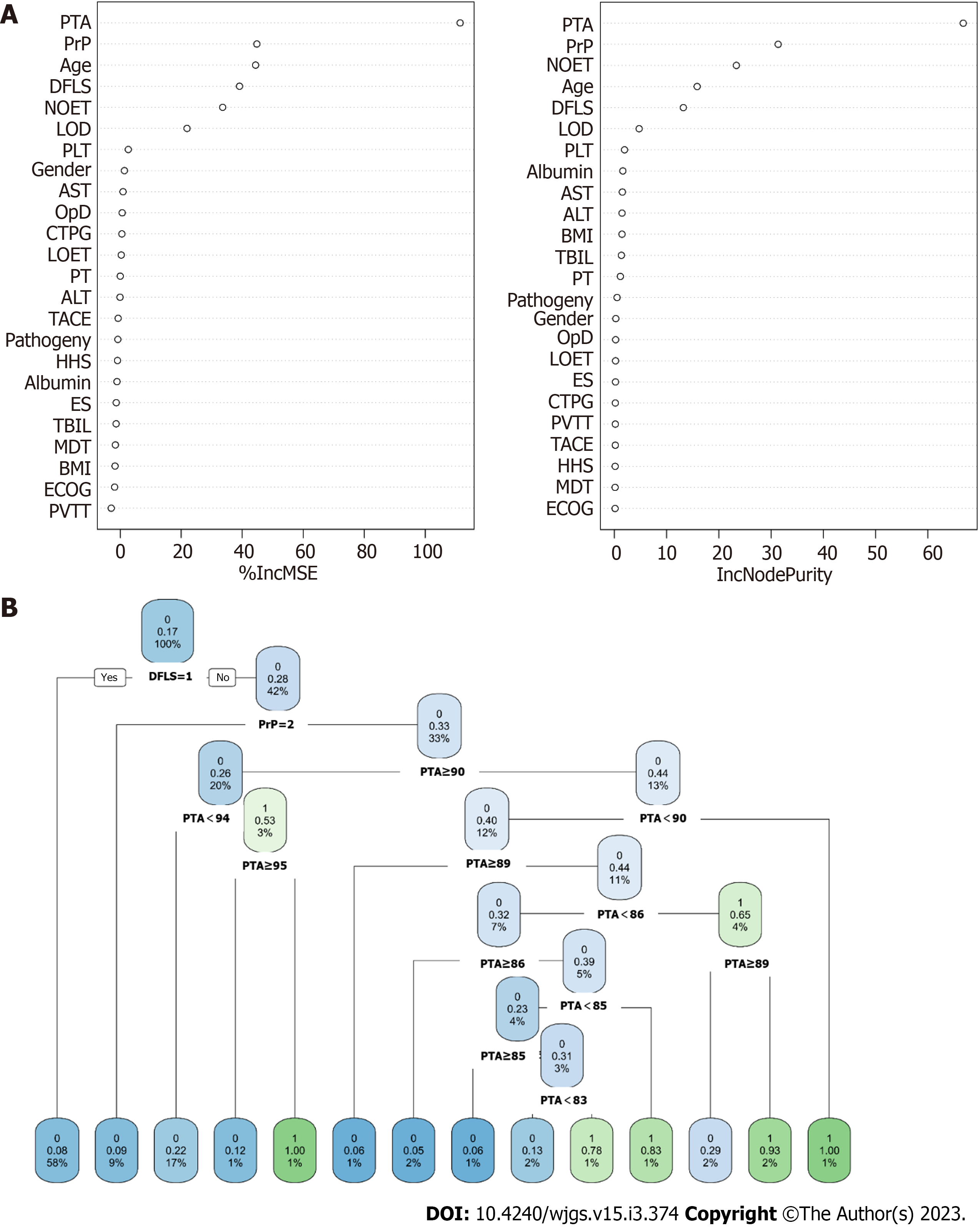
According to the distribution of Pearson correlation coefficients, postoperative pain was considered an ‘outcome variable’, and its correlation with 24 candidate variables was examined (Figure 2A). Postoperative pain was significantly correlated with age, PrP, NOET, DFLS, dosage of iodised oil (LOD) and preoperative prothrombin activity (PTA). Similarly, LASSO regression was used to determine the optimal penalty coefficient (Figures 2B and 2C) to screen for candidate variables for the predictive models. For four models, age, PrP, NOET, DFLS, LOD and PTA were identified as significant predictive factors, which was consistent with the results obtained by generalized linear modelthrough univariate and multivariate logistic regression analyses (Figure 2D). Altogether, these results indicate that age, PrP, NOET, DFLS, LOD and PTA can efficiently predict postoperative pain in patients with HCC.
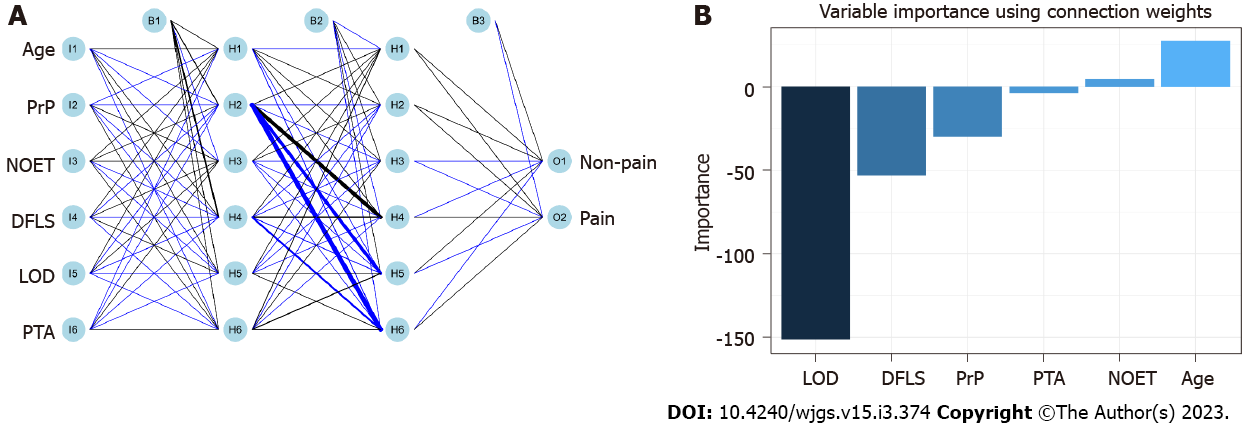
RFM and DTM were constructed based on the principle of ‘branching’ to discriminate and classify each included variable (Figure 3 and Supplementary Table 1). Age, PrP, NOET, DFLS, LOD and PTA were the major variables included in RFM, whereas the ‘branch’ variable in the decision tree included only PTA and DFLS. In addition, ANNM was constructed based on the algorithms of the ‘input layer’, ‘hidden layer’ and ‘output layer’ (Figure 4). After iteration of the input and hidden layers, age, tumour size and pathological type could accurately stratify the pain risk. Consistent with the candidate variables included in ANNM, SVMM was based on a class of generalised linear classifiers that categorise data in a binary way according to supervised learning, which can convert the problem into a convex quadratic programming problem. Furthermore, age, NOET, DLFS, LOD and PTA were the major variables included in NBM. These results suggest that predictive models of postoperative pain can be developed using the abovementioned variables, and the contribution of the intersecting variables among these models cannot be ignored.
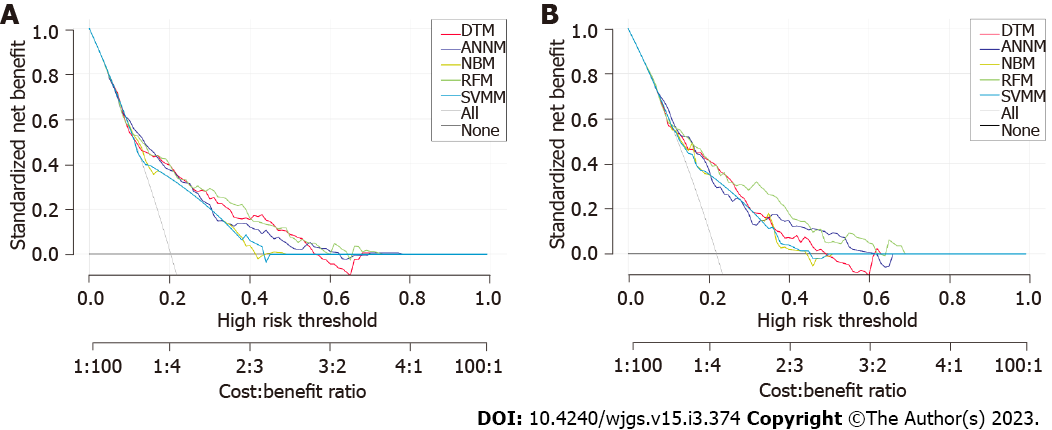
DCA was performed to evaluate the differentiation efficiency and robustness of the five predictive models. The predictive efficiency of RFM was most optimal, followed by DTM. The predictive efficiency of ANNM and SVMM was better than that of NBM (Figure 5). The area under the curve (AUC) values of RFM were 0.869 [95% confidence interval (CI): 0.816-0.922) and 0.871 (95%CI: 0.818-0.924) in the training and internal verification sets, respectively. Additionally, the prediction accuracy of DTM, ANNM, SVMM, and NBM in the training cohort was between (AUC = 0.798; 95%CI: 0.745-0.851) and (AUC = 0.871; 95%CI: 0.818-0.924) (Table 2). These results indicate that although the candidate variables used in the five predictive models were similar, the predictive efficiency of the models was significantly different, with RFM having the best predictive efficiency.
| Model | Training set | Testing set | ||
| AUC mean | AUC 95%CI | AUC mean | AUC 95%CI | |
| RFM | 0.869 | 0.816-0.922 | 0.871 | 0.818-0.924 |
| DTM | 0.861 | 0.808-0.914 | 0.864 | 0.811-0.917 |
| ANNM | 0.826 | 0.773-0.879 | 0.827 | 0.774-0.880 |
| SVMM | 0.803 | 0.750-0.856 | 0.808 | 0.755-0.861 |
| NBM | 0.798 | 0.745-0.851 | 0.803 | 0.750-0.856 |
CIC curves were plotted to verify the predictive efficiency of RFM. As shown in SupplementaryFigure
At present, TACE is considered the first-line non-surgical treatment for HCC. TACE can effectively control the growth of HCC cells, significantly prolong the survival of patients and benefit patients with HCC; therefore, it is the first therapeutic option and the most effective treatment for patients with advanced HCC who are not eligible for surgical resection[15,16]. Although the trauma of TACE is minor, several adverse reactions may occur postoperatively[17]. Pain is one of the common postoperative complications; however, its pathophysiological mechanism remains unclear[18]. It may be caused by acute liver parenchyma ischaemia, liver capsule tension caused by transient liver swelling and chemical damage of hepatic arteries[19]. Previous studies have shown that pain can prolong the length of hospital stay, reduce the quality of life of patients and harm the physiology and psychology of patients[19,20]. Therefore, early treatment of postoperative pain is necessary for improving the prognosis of patients with HCC. To the best of our knowledge, this study is the first to use machine learning algorithms to build a multi-course model for predicting post-TACE pain. The model can help to assess the risk of post-TACE pain objectively in order to improve clinical diagnosis and treatment.
Previous studies have shown that the incidence of pain is high among patients with HCC after 6-12 h of TACE[19,21,22]. However, this study showed that the incidence of moderate and severe pain in patients with HCC was 16.73% within 24 h of TACE. Based on the analysis of pain occurrence at various time points, the incidence of pain was highest at 6 h after TACE. A possible reason is that postoperative pain is mostly caused by tumour tissue embolism and necrosis, the liver volume increases, and the right upper quadrant pain is caused by pulling the capsule. In this study, patients with HCC were uniformly administered preventive analgesic drugs before surgery, thus delaying the occurrence of pain. However, medical staff should strengthen the early inspection of patients with HCC, pay close attention to the symptoms and signs of patients and implement preventive measures, whenever necessary, to alleviate postoperative pain symptoms.
In this study, machine learning-based models were developed to predict post-TACE pain, and candidate predictors were identified based on the clinical baseline data of patients before surgery. The results were consistent with those of previous studies[20,23,24]. For example, this study showed that age of > 50 years was an independent risk factor for moderate-to-severe pain after TACE. The reason for the high incidence of postoperative pain in young patients is that the pain threshold of the human body increases with age, and the sensitivity of elderly patients to pain is lower than that of young patients, resulting in changes in pain tolerance[25]. Therefore, medical staff should closely observe the symptoms and signs of young patients after surgery, and if necessary, pre-emptive analgesia should be implemented to reduce the incidence of postoperative pain. Furthermore, PrP was also identified as an important predictor of moderate-to-severe pain after TACE, which is consistent with the results of previous studies[26,27]. A meta-analysis reported that PrP is an important predictor of postoperative pain[26]. A possible reason is that the influx of PrP signals can enhance the excitability of spinal dorsal horn neurons and their responsiveness to pain transmission, which can be further maintained through transcriptional changes[28]. For example, cyclooxygenase-2 is induced to produce prostaglandin E2, which leads to postoperative pain[21]. Therefore, selective cyclooxygenase-2 inhibitors should be administered to high-risk patients preoperatively to reduce the incidence of moderate and severe pain postoperatively.
Furthermore, the distance between the tumour and liver capsule (≤ 2 cm), presence of multiple embolic tumours and dosage of lipiodol greater than 10 mL were also identified as risk factors for moderate-to-severe pain after TACE, which is consistent with the results of previous studies[10,29]. If the tumour tissue is close to the liver capsule, it may become necrotic and oedematous after hepatic arterial chemoembolisation, leading to increased tension in the liver capsule. Consequently, the patient is more likely to feel pain and discomfort. Moreover, if more tumours are embolised, more iodised oil is required, and a larger embolised area may increase the pain caused by tumour necrosis. we speculated that embolization of nodules close to the gallbladder might also be an alternative cuase of pain, especially if cystic artery vessels provided bllod to the nodules and had to be embolized. Prothrombin activity is an important indicator of liver coagulation because high prothrombin activity often indicates that the body is in a hypercoagulable state[30,31]. High prothrombin activity may be a primary cause of postoperative pain. Alternatively, injured tissue cells release a large amount of thromboplastin during surgery, which can also lead to a temporary hypercoagulable state in the early postoperative period[32,33]. Consequently, the blood flow is slow, and the risk of postoperative micro-thrombosis and pain is increased. Therefore, medical staff should promptly evaluate the liver function of patients, detect changes in prothrombin time and control the abnormal indicators of coagulation function for effective pain management.
Although the candidate variables included in this study can be used to develop different machine learning-based predictive models, the predictive accuracy of the models may differ. In this study, RFM was found to have the best prediction efficiency, which is consistent with the results reported in previous studies[34,35]. RFM can realise multiple iterations of subsequent variables based on the ‘bagging’ algorithm, which signifies that the predictive efficiency of the included variables can be optimised after adding numerous ‘branches’ and ‘pruning’[35,36]. Although ANNM can be used for risk stratification of patients with post-TACE pain, its predictive efficiency is slightly inferior to that of RFM, which reflects the practicability of the input- and hidden-layer algorithms in this study. However, the algorithm requires to be constantly updated to reflect the robustness of its ‘output layer’[37]. The predictive performance of machine learning algorithms is undoubtedly better than that of logical regression algorithms because machine learning has incomparable advantages in terms of the number of iterations. Altogether, in this study, we developed an efficient predictive model based on candidate variables that can be adopted clinically. The model can be used for stratifying the risk of post-TACE pain to facilitate early management and improve the prognosis of patients.
However, this study has some limitations. First, this study had a retrospective design and only focused on patients undergoing traditional lipiodol-based embolisation, while the latter focused on patients using drug-eluting microspheres for embolisation. Therefore, the results may have been affected by selection bias. Second, although some clinical variables were included in this study, the psychological status and psychosocial factors of patients were not included, and no suggestion was made to further improve the predictive model by adding these factors. Third, this study relied on only single-centre internal verification; therefore, external spatial verification is required to accurately evaluate the predictive efficiency of the predictive models. Fourth, this study included only patients receiving traditional TACE; therefore, patients undergoing different types of TACE should be included in future studies to improve the universality of the predictive model.
Machine learning-based pre-warning models can be developed to predict post-TACE pain for hierarchical management of patients at high risk of moderate and severe pain after TACE. In particular, RFM combined with preoperative predictors (i.e., age, PrP, DFLS ≤ 2cm, prothrombin activity, iodine oil dose and presence of multiple emboli) has optimal discriminating power and high predictive accuracy. Therefore, RFM can be used for early prediction of the risk of postoperative pain, which can facilitate prompt pain management after TACE and improve the prognosis of patients.
Pain after transcatheter arterial chemoembolisation (TACE) can seriously affect the prognosis of patients and the insertion of additional medical resources.
To develop a practical model for predicting pain after TACE.
This study aimed to predict pain after TACE to enable the implementation of preventive analgesic measures.
Of 857 patients (from January 2016 to January 2020) and prospectively enrolled 368 patients (from February 2020 to October 2022; as verification cohort) with hepatocellular carcinoma (HCC) who received TACE were collected from the Hepatic Surgery Center of Tongji Hospital. Five predictive models were established using machine learning algorithms were used to predicting postoperative pain and receiver operating characteristic curve analysis, decision curve analysis and clinical impact curve analysis were used to evaluate the effectiveness of the model.
Of 24 candidate variables were to build prediction model, among them, the age, preoperative pain, number of embolised tumours, distance from the liver capsule, dosage of iodised oil and preoperative prothrombin activity were closely associated with postoperative pain. The random forest model (RFM) had the best predictive efficiency [training cohort: Area under the curve (AUC) = 0.869, 95% confidence interval (CI): 0.816-0.922; internal verification cohort: AUC = 0.871; 95%CI: 0.818-0.924].
The five prediction models based on advanced machine learning algorithms are extremely suitable for the pain management of liver cancer patients after TACE, especially the RFM can accurately classify the pain risk of patients.
Machine learning-based pre-warning models can be developed to predict post-TACE pain for hierarchical management of patients at high risk of moderate and severe pain after TACE. Alarmingly, the RFM can be used for early prediction of the risk of postoperative pain, which can facilitate prompt pain management after TACE and improve the prognosis of patients.
The researchers thanked all the participants in this study and offered their health data free of charge.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Brazil; Tovoli F, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Liu JB, Chu KJ, Ling CC, Wu TM, Wang HM, Shi Y, Li ZZ, Wang JH, Wu ZJ, Jiang XQ, Wang GR, Ma YS, Fu D. Prognosis for intrahepatic cholangiocarcinoma patients treated with postoperative adjuvant transcatheter hepatic artery chemoembolization. Curr Probl Cancer. 2020;44:100612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Cahill BA. Management of patients who have undergone hepatic artery chemoembolization. Clin J Oncol Nurs. 2005;9:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Li S, Lyu N, Han X, Li J, Lai J, He M, Deng H, Shi M, Wang H, Zhao M. Hepatic Artery Infusion Chemotherapy Using Fluorouracil, Leucovorin, and Oxaliplatin versus Transarterial Chemoembolization as Initial Treatment for Locally Advanced Hepatocellular Carcinoma: A Propensity Score-Matching Analysis. J Vasc Interv Radiol. 2021;32:1267-1276.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Kokudo N, Makuuchi M. Current role of portal vein embolization/hepatic artery chemoembolization. Surg Clin North Am. 2004;84:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 6. | Wang ZX, Liu SL, Sun CH, Wang Q. Psychological intervention reduces postembolization pain during hepatic arterial chemoembolization therapy: a complementary approach to drug analgesia. World J Gastroenterol. 2008;14:931-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Romano M, Giojelli A, Tamburrini O, Salvatore M. Chemoembolization for hepatocellular carcinoma: effect of intraarterial lidocaine in peri- and post-procedural pain and hospitalization. Radiol Med. 2003;105:350-355. [PubMed] |
| 8. | Hyun D, Cho SK, Shin SW, Park KB, Lee SY, Park HS, Choo SW, Do YS. Combined transarterial chemoembolization of the right inferior phrenic artery and radiofrequency ablation for small hepatocellular carcinoma near the diaphragm: its efficacy and safety. Abdom Radiol (NY). 2018;43:2851-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Bian LF, Zhao XH, Gao BL, Zhang S, Ge GM, Zhan DD, Ye TT, Zheng Y. Predictive model for acute abdominal pain after transarterial chemoembolization for liver cancer. World J Gastroenterol. 2020;26:4442-4452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 10. | Benzakoun J, Ronot M, Lagadec M, Allaham W, Garcia Alba C, Sibert A, Vilgrain V. Risks factors for severe pain after selective liver transarterial chemoembolization. Liver Int. 2017;37:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Chen SC, Wu SF, Wang TJ, Rosenberg J, Lu YY, Liang SY. Factors influencing the coping strategies of liver cancer patients undergoing transarterial chemoembolization. Int J Nurs Pract. 2022;28:e13033. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1943] [Article Influence: 215.9] [Reference Citation Analysis (6)] |
| 13. | Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284:603-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 492] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 14. | Arslan E, Schulz J, Rai K. Machine Learning in Epigenomics: Insights into Cancer Biology and Medicine. Biochim Biophys Acta Rev Cancer. 2021;1876:188588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Takaki H, Sato Y, Yamakado K. [Transcatheter arterial chemoembolization for hepatocellular carcinoma:current topics]. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Paul SB, Guglani B, Gulati MS, Batra Y, Mukhopadhyay S. Transcatheter arterial chemoembolization in hepatocellular carcinoma: technique, effects and present status. Trop Gastroenterol. 2003;24:176-184. [PubMed] |
| 17. | Hassanin TM, Fouad Y, Hassnine A, Eisawy M, Farag N, Abdel Ghany W. Quality of Life after Transcatheter Arterial Chemoembolization Combined with Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Compared with Transcatheter Arterial Chemoembolization alone. Asian Pac J Cancer Prev. 2021;22:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Sueyoshi E, Hayashida T, Sakamoto I, Uetani M. Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2010;195:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Chang KT, Liu CJ, Tsai HT, Hsu TP, Chen PT, Hu SH. Effects and safety of body positioning on back pain after transcatheter arterial chemoembolization in people with hepatocellular carcinoma: A randomized controlled study. Int J Nurs Stud. 2020;109:103641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | He JJ, Yin XX, Wang T, Chen MY, Li XL, Yang XJ, Shao HY. Factors influencing postembolization syndrome in patients with hepatocellular carcinoma undergoing first transcatheter arterial chemoembolization. J Cancer Res Ther. 2021;17:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Lv N, Kong Y, Mu L, Pan T, Xie Q, Zhao M. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol. 2016;26:3492-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Gu J, Liang Y. Clinical Nursing Paths Benefit Patient Outcomes Undergoing Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. Evid Based Complement Alternat Med. 2022;2022:4655293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Wang TC, Zhang ZS, Xiao YD. Determination of Risk Factors for Pain After Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma. J Pain Res. 2020;13:649-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Pachev A, Raynaud L, Paulatto L, Dioguardi Burgio M, Roche V, Garcia Alba C, Sibert A, Lagadec M, Kavafyan-Lasserre J, Paugam-Burtz C, Vilgrain V, Ronot M. Predictive factors of severe abdominal pain during and after transarterial chemoembolization for hepatocellular carcinoma. Eur Radiol. 2021;31:3267-3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | El Tumi H, Johnson MI, Dantas PBF, Maynard MJ, Tashani OA. Age-related changes in pain sensitivity in healthy humans: A systematic review with meta-analysis. Eur J Pain. 2017;21:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 26. | Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 741] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, Riva-Cambrin J. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9:e025091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 28. | Kehlet H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain. 2018;159 Suppl 1:S11-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Cohen MJ, Bloom AI, Barak O, Klimov A, Nesher T, Shouval D, Levi I, Shibolet O. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:2521-2528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Hoffman M. Coagulation in Liver Disease. Semin Thromb Hemost. 2015;41:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Chow JH, Lee K, Abuelkasem E, Udekwu OR, Tanaka KA. Coagulation Management During Liver Transplantation: Use of Fibrinogen Concentrate, Recombinant Activated Factor VII, Prothrombin Complex Concentrate, and Antifibrinolytics. Semin Cardiothorac Vasc Anesth. 2018;22:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Moon YJ, Kim SH, Kim JW, Lee YK, Jun IG, Hwang GS. Comparison of postoperative coagulation profiles and outcome for sugammadex versus pyridostigmine in 992 living donors after living-donor hepatectomy. Medicine (Baltimore). 2018;97:e0129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Oo J, Allen M, Loveday BPT, Lee N, Knowles B, Riedel B, Burbury K, Thomson B. Coagulation in liver surgery: an observational haemostatic profile and thromboelastography study. ANZ J Surg. 2020;90:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Wang H, Zhou L. Random survival forest with space extensions for censored data. Artif Intell Med. 2017;79:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Rigatti SJ. Random Forest. J Insur Med. 2017;47:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 36. | Buri M, Hothorn T. Model-based random forests for ordinal regression. Int J Biostat. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Zhou L, Wang C. Innovation of Platform Economy Business Model Driven by BP Neural Network and Artificial Intelligence Technology. Comput Intell Neurosci. 2022;2022:3467773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |