Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2954
Peer-review started: September 22, 2023
First decision: October 17, 2023
Revised: October 30, 2023
Accepted: December 6, 2023
Article in press: December 6, 2023
Published online: December 27, 2023
Processing time: 96 Days and 8.1 Hours
In recent years, minimally invasive liver resection has become a standard of care for liver tumors. Considering the need to treat increasingly fragile patients, general anesthesia is sometimes avoided due to respiratory complications. Therefore, surgical treatment with curative intent is abandoned in favor of a less invasive and less radical approach. Epidural anesthesia has been shown to reduce respiratory complications, especially in elderly patients with pre-existing lung disease.
A 77-year-old man with hepatitis-C-virus-related chronic liver disease underwent robotic liver resection for hepatocellular carcinoma. The patient was suffering from hypertension, diabetes and chronic obstructive pulmonary disease. The National Surgical Quality Improvement Program score for developing pneumonia was 9.2%. We planned a combined spinal–epidural anesthesia with conscious sedation to avoid general anesthesia. No modification of the standard surgical technique was necessary. Hemodynamics were stable and bleeding was minimal. The postoperative course was uneventful.
Robotic surgery in locoregional anesthesia with conscious sedation could be considered a safe and suitable approach in specialized centers and in selected patients.
Core Tip: Liver resection represents a gold standard for the treatment of liver malignancies. Minimally invasive approach guarantees less invasiveness and faster postoperative recovery. Frail and older patients undergoing liver resection under general anesthesia have a high risk of respiratory and cardiac complications. Locoregional anesthesia preserves better intestinal, cardiac and pulmonary function compared to general anesthesia. The combination of robotic surgery and locoregional anesthesia guarantees minimal surgical and anesthetic invasiveness and could be considered a safe approach in selected patients and specialized centers.
- Citation: Delvecchio A, Pavone G, Conticchio M, Piacente C, Varvara M, Ferraro V, Stasi M, Casella A, Filippo R, Tedeschi M, Pullano C, Inchingolo R, Delmonte V, Memeo R. Awake robotic liver surgery: A case report. World J Gastrointest Surg 2023; 15(12): 2954-2961
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2954.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2954
Liver resection is a gold standard for the treatment of liver malignancies. In recent years, first laparoscopic and then robotic approaches have emerged as first-line options for liver resection[1,2]. The minimally invasive approach guarantees less invasiveness and faster postoperative recovery. This kind of surgery is usually performed under general or combined general and epidural anesthesia.
In most cases, liver resection was performed on frail patients with many comorbidities and liver diseases[3]. The surgical approach is not the only one to ensure a good perioperative course but also the type of anesthetic approach. In frail and older patients with many comorbidities, general anesthesia is associated with a high risk of respiratory and cardiac complications.
Sometimes the high perioperative risk excludes the possibility to perform surgery with curative intent. In this type of patient, compared with general anesthesia, locoregional anesthesia reduces cardiac and pulmonary complications and the use of opioids, while preserving better intestinal, cardiac and pulmonary function[4,5]. Locoregional anesthesia is integrated with conscious sedation to avoid patient movements under the robotic platform, as reported for robotic partial nephrectomy[6].
The combination of robotic surgery and locoregional anesthesia guarantees both surgical and anesthetic minimal invasiveness. We report a case of robotic liver resection under epidural and spinal anesthesia in a frail patient with many comorbidities.
A 77-year-old man with hepatocellular carcinoma (HCC) and hepatitis-C-virus-related chronic liver disease was enrolled for robotic wedge resection of segment II.
During the 6-monthly follow-up for chronic liver disease, investigations revealed a liver lesion in segment II.
The patient had previously undergone subtotal gastrectomy for peptic ulcer disease, lumbar discectomy for disc herniation, and bilateral carotid endarterectomy with stenting. The patient had: Hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease (COPD). The home medications were: Olmesartan, clopidogrel, insulin, doxazosin, and pravastatin. No COPD therapy had ever been started.
The patient denied any family history of malignant tumors.
Body mass index was 28 kg/m2. Chest auscultation revealed mild rhonchi on expiration in both lung bases. No pathological sounds were heard on cardiac auscultation. The abdomen was treatable over the entire area.
Levels of serum tumor markers were: Carcinoembryonic antigen, 5.3 ng/mL; carbohydrate antigen 19-9, 5.0 U/mL; and -fetoprotein, 50 ng/mL. No abnormality was found in routine blood analyses.
Computed tomography and magnetic resonance imaging detected a 3-cm lesion in liver segment II characterized by wash in in the arterial phase and wash out in the portal phase.
Preoperative diagnosis was based on the noninvasive criteria established by the European Association for the Study of the Liver[7].
After combining the patient’s medical history, and laboratory and imaging examinations, the final diagnosis was HCC.
Before the procedures, we performed a thromboelastography (TEG) platelet mapping test (TEG 6s), which showed normal values for ADP and arachidonic acid (AA) test; ADP test: 64.4 mm (normal values: 45–69 mm) and AA test: 66.4 mm (normal values: 51–71 mm).
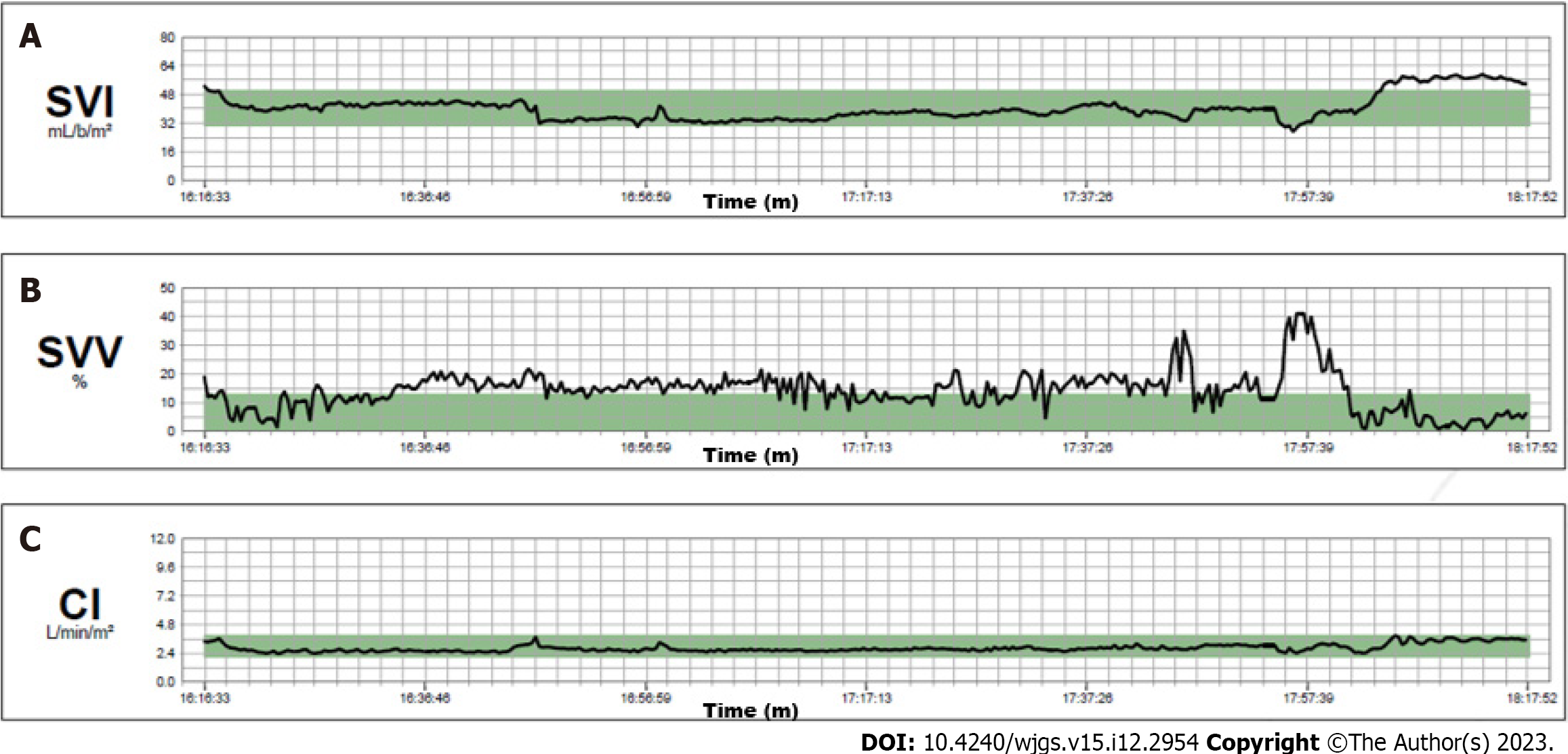
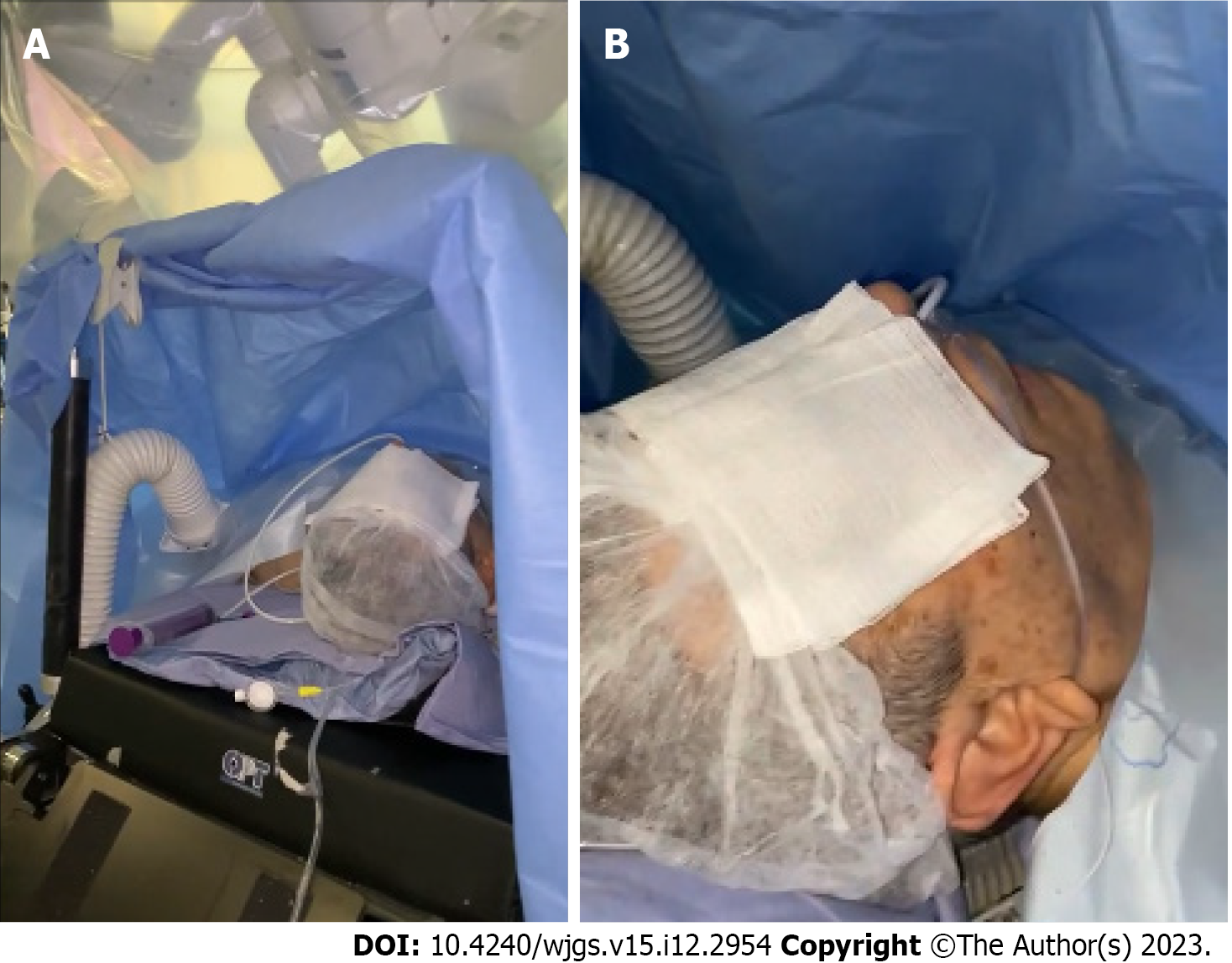
Noninvasive monitoring with multiparameter monitoring (Figure 1) was initiated: Electrocardiography, noninvasive blood pressure and pulse oximetry. The mechanical ventilation monitor was turned off, as shown in Figure 2. A combined spinal–epidural anesthetic was planned.
An epidural catheter was inserted at T6–T7, followed by subarachnoid anesthesia at T12–L1 with ropivacaine 10 mg + dexmedetomidine 10 g (total volume 5 mL, diluted with distilled water). After patient positioning, an epidural bolus of ropivacaine 0.2% + midazolam 5 mg (total volume 10 mL) was administered. Ten minutes after the epidural bolus, the pinprick was negative at C4, with Bromage IV. We added intravenous sedation with dexmedetomidine 100 g + ketamine 50 mg + remifentanil 1 mg in 50 mL saline at 3 mL/h and with a Richmond Agitation–Sedation Scale 2/3 target around remifentanil at 0.01 g/kg/min, dexmedetomidine 0.07 g/kg/h, ketamine 0.03 mg/kg/h. Supplemental oxygen was administered via nasal cannula at 2 L/min. We did not routinely monitor central venous pressure for liver resection[8], but intra-arterial cannulation of the left radial artery was placed without local anesthesia and patient discomfort. We use the FloTrac® sensor for intraoperative monitoring[9].
Given the unreliability of stroke volume variation in spontaneous respiration in the resection phase, we aimed to have a 10% reduction in stroke volume until the end of the resection, with restrictive fluid therapy and using diuretics. Anesthesia, in spontaneous respiration, was maintained with epidural injection of ropivacaine 0.2%, 10 mL every 1 h.
The hemodynamics remained stable throughout the entire operation. Continuous infusion of crystalloid solution 1 mg/kg/h was given until the end of the liver resection. The cardiac index was in the normal range and the stroke volume index decreased from the initial 50 mL/m2 to 30–35 mL/m2 at the end of the resection (Figure 3). We aimed for a mean arterial pressure of 65 mmHg and four boluses of 5 mg ephedrine were required (Figure 4).
Robotic surgery was performed using the Da Vinci Xi robotic platform. The patient was awake and placed in a supine position with 30° reverse Trendelenburg position (Figure 5). A pneumoperitoneum was created using an open technique through a supraumbilical incision. Insufflation of pneumoperitoneum at 12 mmHg was uneventful. The other three robotic trocars and two laparoscopic 12-mm trocar assistants were inserted under direct vision. The trocar position was standardized as shown in Figure 6. The surgeon was sitting at the robotic console and the assistant surgeon was between the patient’s legs. The assistant performed retraction, suction, cutting and stapling.
The procedure began with exploration of the abdominal cavity and intraoperative ultrasound to check the liver tumor. Transection of the hepatoduodenal and falciform ligaments was performed for preparation of the hepatic hilum for the Pringle maneuver. We placed the Foley catheter around the hepatic hilum for intermittent intracorporeal clamping. Liver parenchymal transection was performed with double crash clamping and vessel sealing for the main pedicle. Indocyanine green was administered the day before the procedure and used in positive staining in association with intraoperative ultrasound to better highlight the parenchymal transection margin. Hem-o-lock clips were used for resection of vascular and biliary structures. Specimens were placed in a laparoscopic extraction bag and removed through enlargement of the trocar. No modification of the usually adopted surgical technique was necessary and no Pringle maneuver was used to minimize blood loss. The patient had an uneventful postoperative course and was discharged on postoperative day 4.
At 1 year postoperatively, the patient was still alive and free of disease.
In the last few decades, minimally invasive surgery has emerged as the gold standard for treatment of liver tumor. During the Consensus Conferences of Louisville in 2008[10] and Morioka in 2014[11], and the Southampton Guidelines Meeting in 2017[1], the minimally invasive techniques were standardized also for complex procedures. The minimally invasive approach guarantees less invasiveness, reduction of postoperative complications and faster postoperative recovery.
Despite technological advances, laparoscopic liver resection is still limited by rigid instruments and 2D vision, resulting in steep learning curves and difficulties in performing major hepatectomy and resection of lesions located in posterosuperior segments[12].
The robotic platform overcomes some limits of laparoscopic surgery thanks to the high definition of 3D vision, magnified field of vision, tremor filter, EndoWrist articulated instruments, and improved surgeon’s ergonomy. This allows surgeons to perform some complex procedures, such as hilar dissection or biliary-enteric anastomosis and resection of posterosuperior segments[13]. These technical properties guarantee a lower estimated blood loss, less narcotic use, shorter length of hospital stay, and improved short-term quality of life for complex liver resection[13].
However, the benefits of the robotic approach in liver surgery have not yet been clearly defined and some recently published studies comparing robotic and laparoscopic techniques have not provided conclusive results in favor of either approach[14,15]. Use of the robotic platform has increased exponentially in the last 10 years and, in 2018, the first international consensus statement on robotic liver surgery was published[2].
In most cases, liver resection has been performed on frail patients with many comorbidities and liver diseases[3]. The surgical approach is not the only way to ensure a good perioperative course, and the anesthetic approach is also important. Liver resection is usually performed under general or combined general and epidural anesthesia. In more frail and older patients with many comorbidities, general anesthesia is associated with a high risk of respiratory and cardiac complications. Sometimes, the high perioperative risk excludes the possibility of surgery with curative intent. General anesthesia for high-risk surgical patients with significant lung disease may trigger some adverse effects, including: Pneumonia, heart failure, biotrauma and barotrauma, and subsequently, intra- and postoperative hypoxemia[4,16].
Epidural anesthesia reduces the decrease in tissue oxygen tension caused by surgical stress and adrenergic vasoconstriction during major abdominal surgery, which provides sufficient oxygenation of organs with improved cardiac, respiratory and gastrointestinal function[17]. Locoregional anesthesia reduces the invasiveness of general anesthesia and the use of opioids, preserving better intestinal and pulmonary function, and could be used in frail patients. The reduction in postoperative pulmonary complications with epidural anesthesia has been confirmed by two systemic reviews[5,18].
Awake surgery is a specialized surgical technique performed on patients who remains awake and conscious during the procedure. The patient receives sedative drugs to help them feel relaxed and less anxious during the operation. The decision to perform awake abdominal surgery depends on several factors, including the type of surgery, the patient’s medical condition and the surgeon’s expertise. This approach may be preferred in certain situations, such as for gynecological procedures, brain surgery, hernia repair or appendectomy. In 1994, a case of colectomy under awake epidural anesthesia was reported in a patient with high perioperative risk. The procedure was safe and effective[19]. Since then, other awake procedures under epidural anesthesia have been reported including awake laparoscopic cholecystectomy in COPD patients[20].
During the COVID-19 pandemic, the surgical community focused on the potential risks of minimally invasive surgery and general anesthesia because they are both aerosol-generating procedures that could cause the spread of contamination in operating theaters[21]. For these reasons, increased use of regional anesthesia was supported and encouraged during the pandemic. Locoregional anesthesia was reported also for emergency abdominal surgery in high-risk patients with decreased morbidity and mortality[22,23]. The combination of robotic surgery and locoregional anesthesia guarantees minimal surgical and anesthetic invasiveness, excellent quality of surgery and enhanced recovery[17].
There are cases in the literature of awake robotic cardiac surgery[24], awake robotic partial nephrectomy[6] and awake video-assisted thoracic surgery[25]. However, to our knowledge, there are no cases in the literature of awake robotic liver resection, but just a case series of awake open hepatectomy[26]. Our patient was frail with liver cirrhosis, and cardiac, vascular and lung diseases. In other hospitals, the patient was not a candidate for radical surgery because of the high perioperative risk. Our patient received the least invasive treatment possible, thanks to the close collaboration between surgeons and anesthesiologists. The patient had an uneventful postoperative course and was discharged on postoperative day 4, which was average for our robotic liver resection procedures.
We believe that spinal epidural anesthesia and analgesia are suitable techniques, especially for selected frail patients. These techniques, associated with robotic surgery, offer a better short-term outcome, especially in comorbid patients with serve lung disease. The patients benefit from a quicker return home and hospital costs are also reduced. Robotic surgery associated with locoregional anesthesia and conscious sedation could be considered a safe and suitable approach but only in specialized centers and in selected patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alzerwi NAN, Saudi Arabia; Navarrete Arellano M, Mexico S-Editor: Fan JR L-Editor: Kerr C P-Editor: Zhao S
| 1. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 496] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 2. | Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, He J, Boggi U, Troisi RI, Efanov M, Azoulay D, Panaro F, Pessaux P, Wang XY, Zhu JY, Zhang SG, Sun CD, Wu Z, Tao KS, Yang KH, Fan J, Chen XP. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 2019;25:1432-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 3. | Story DA. Postoperative complications in elderly patients and their significance for long-term prognosis. Curr Opin Anaesthesiol. 2008;21:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Jin F, Chung F. Minimizing perioperative adverse events in the elderly. Br J Anaesth. 2001;87:608-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Jayr C, Thomas H, Rey A, Farhat F, Lasser P, Bourgain JL. Postoperative pulmonary complications. Epidural analgesia using bupivacaine and opioids versus parenteral opioids. Anesthesiology. 1993;78:666-76; discussion 22A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Gontero P, Oderda M, Calleris G, Allasia M, Balagna R, Gobbi F. Awake Da Vinci robotic partial nephrectomy: First case report ever in a situation of need. Urol Case Rep. 2022;42:102008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6063] [Article Influence: 866.1] [Reference Citation Analysis (3)] |
| 8. | Dunki-Jacobs EM, Philips P, Scoggins CR, McMasters KM, Martin RC 2nd. Stroke volume variation in hepatic resection: a replacement for standard central venous pressure monitoring. Ann Surg Oncol. 2014;21:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Ratti F, Cipriani F, Reineke R, Catena M, Paganelli M, Comotti L, Beretta L, Aldrighetti L. Intraoperative monitoring of stroke volume variation versus central venous pressure in laparoscopic liver surgery: a randomized prospective comparative trial. HPB (Oxford). 2016;18:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS; World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 11. | Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 403] [Reference Citation Analysis (0)] |
| 12. | Lee SY, Goh BKP, Sepideh G, Allen JC, Merkow RP, Teo JY, Chandra D, Koh YX, Tan EK, Kam JH, Cheow PC, Chow PKH, Ooi LLPJ, Chung AYF, D'Angelica MI, Jarnagin WR, Peter Kingham T, Chan CY. Laparoscopic Liver Resection Difficulty Score-a Validation Study. J Gastrointest Surg. 2019;23:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Lafaro KJ, Stewart C, Fong A, Fong Y. Robotic Liver Resection. Surg Clin North Am. 2020;100:265-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Montalti R, Scuderi V, Patriti A, Vivarelli M, Troisi RI. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc. 2016;30:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Troisi RI, Patriti A, Montalti R, Casciola L. Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int J Med Robot. 2013;9:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Strøm C, Rasmussen LS, Sieber FE. Should general anaesthesia be avoided in the elderly? Anaesthesia. 2014;69 Suppl 1:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Abd Elrazek E, Thornton M, Lannigan A. Effective awake thoracic epidural anesthetic for major abdominal surgery in two high-risk patients with severe pulmonary disease--a case report. Middle East J Anaesthesiol. 2010;20:891-895. [PubMed] |
| 18. | Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T, MacMahon S. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1266] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 19. | Koltun WA, McKenna KJ, Rung G. Awake epidural anesthesia is effective and safe in the high-risk colectomy patient. Dis Colon Rectum. 1994;37:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Pursnani KG, Bazza Y, Calleja M, Mughal MM. Laparoscopic cholecystectomy under epidural anesthesia in patients with chronic respiratory disease. Surg Endosc. 1998;12:1082-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Romanzi A, Boleso N, Di Palma G, La Regina D, Mongelli F, Milanesi M, Putortì A, Rossi F, Scolaro R, Zanardo M, Vannelli A. Awake Major Abdominal Surgeries in the COVID-19 Era. Pain Res Manag. 2021;2021:8763429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Le Roux JJ, Wakabayashi K, Jooma Z. Emergency Awake Abdominal Surgery Under Thoracic Epidural Anaesthesia in a High-Risk Patient Within a Resource-Limited Setting. Cureus. 2023;15:e34856. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Niraj G, Basar SHM, Warusawitharana C, Sebastian S, Camacho E. Continuous Spinal Anaesthesia (CSA) for Emergency Laparotomy in High-Risk Elderly patients: Technique and Outcomes of a Prospective Service Evaluation. J Anesth Surg. 2017;4:130-133. [DOI] [Full Text] |
| 24. | Ishikawa N, Watanabe G. Ultra-minimally invasive cardiac surgery: robotic surgery and awake CABG. Surg Today. 2015;45:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zheng H, Hu XF, Jiang GN, Ding JA, Zhu YM. Nonintubated-Awake Anesthesia for Uniportal Video-Assisted Thoracic Surgery Procedures. Thorac Surg Clin. 2017;27:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Yamamoto K, Fukumori D, Yamamoto F, Yamamoto M, Igimi H, Yamashita Y. First report of hepatectomy without endotracheal general anesthesia. J Am Coll Surg. 2013;216:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |