Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2926
Peer-review started: August 17, 2023
First decision: October 17, 2023
Revised: October 24, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 27, 2023
Processing time: 132 Days and 0.1 Hours
Marked arterioportal shunt (APS) can be a contraindication for transarterial radioembolization (TARE) because of the risk of radiation-induced liver toxicity or pneumonitis. To date, the best method to close marked APS to reduce intrahepatic shunt (IHS) and hepatopulmonary shunt (HPS) before TARE has not been elucidated.
This case report describes a novel strategy of embolization of the portal venous outlet to reduce IHS and HPS caused by marked APS before TARE in a patient with advanced hepatocellular carcinoma (HCC). The patient had a significant intratumoral shunt from the tumor artery to the portal vein and had already been suspected based on pre-interventional magnetic resonance angiography, and digital subtraction angiography (DSA) confirmed the shunt. Selective right portal vein embolization (PVE) was performed to close the APS outlet and DSA con
Closure of APS with PVE during mapping angiography of advanced-stage HCC to enable reduction of HPS and subsequent TARE is feasible.
Core Tip: Marked arterioportal shunt (APS) can be a contraindication for transarterial radioembolization (TARE) because of the risk of radiation-induced liver toxicity or pneumonitis. In this case report, portal vein embolization was performed, for the first time, to close the APS outlet in a patient with advanced hepatocellular carcinoma. Single photon emission computed tomography revealed a low intrahepatic shunt and hepatopulmonary shunt, and TARE was performed successfully.
- Citation: Wang XD, Ge NJ, Yang YF. Portal vein embolization for closure of marked arterioportal shunt of hepatocellular carcinoma to enable radioembolization: A case report. World J Gastrointest Surg 2023; 15(12): 2926-2931
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2926.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2926
Surgical resection and liver transplantation are the main curative treatment options for hepatocellular carcinoma (HCC), which ranks as the 5th most common malignancy and the 4th leading cause of cancer-related mortality worldwide[1]. However, most patients do not meet these treatment selection criteria at the time of diagnosis[2]. Transarterial radioembolization (TARE) with yttrium-90 is a mature method for unresectable HCC, because it can deliver high radiation energy selectively targeting the tumor while sparing the surrounding normal parenchyma[3].
An arterioportal shunt (APS) between a hepatic artery and portal vein is frequently observed in patients with HCC[4], resulting in potentially life-threatening complications, such as esophageal varicose rupture, refractory ascites, and hepatic encephalopathy[5,6]. It was reported that marked APS of the left, right or main portal vein occurred in 30% of HCC patients[7]. Marked APS can be a contraindication for TARE because the radioactive microspheres can easily pass through the shunts, potentially resulting in radiation-induced liver toxicity or pneumonitis[8,9].
To date, closure of a marked APS has been carried out with various approaches, including systemic treatment[9], transcatheter arterial occlusion[10,11] and portal vein occlusion balloons[12], before patients undergo transarterial chemoembolization or TARE. However, the optimal therapy has not yet been elucidated, and other new techniques may be necessary to successfully alleviate the intrahepatic shunt (IHS) and hepatopulmonary shunt (HPS) caused by APS. Portal vein embolization (PVE) is a widely used technique for liver regeneration. PVE can completely embolize the outlet of the APS, indicating its potential to reduce IHS and HPS. This report describes PVE for closure of marked APS in a patient with HCC to enable TARE.
A 58-year-old man presented himself with fatigue, poor appetite and weight loss.
The symptoms started 2 mo previously. Ultrasonography detected multifocal hepatic lesions in the left outer lobe and right lobe.
The patient had no medical history previously.
Nothing in particular.
There are no obvious abnormalities in the physical examination, and the vital signs are within the normal range. No jaundice was observed.
Hepatitis B virus DNA was 2 × 109 IU/mL (< 40); liver function tests were normal; tumor markers were as follows: α-fetoprotein 4.1 ng/mL (< 20.0), PIVKA-II 3454 mAU/mL (< 40.0).
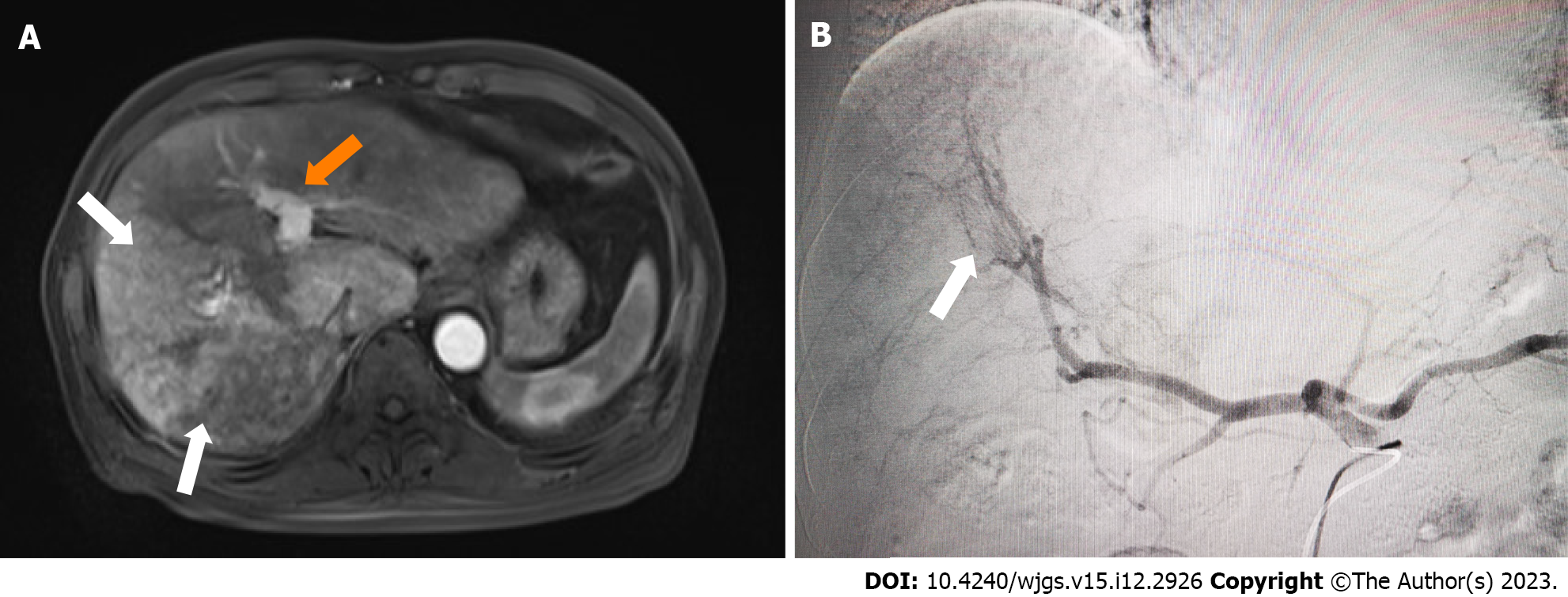
At admission, abdominal enhanced computed tomography (CT) and magnetic resonance imaging (MRI) showed that most lesions were in the right liver lobe; carcinoma thrombus formation in the main and right portal vein branches; and collateral circulation with spongy degeneration. A marked APS was revealed, and hence, early contrast enhancement of the left portal vein was seen on MRI (Figure 1A).
The final diagnosis of the presented case was BCLC-C stage HCC.
The MDT made the treatment plan of combining Y-90 TARE with the anti-PD-1 antibody and the anti-VEGF beva
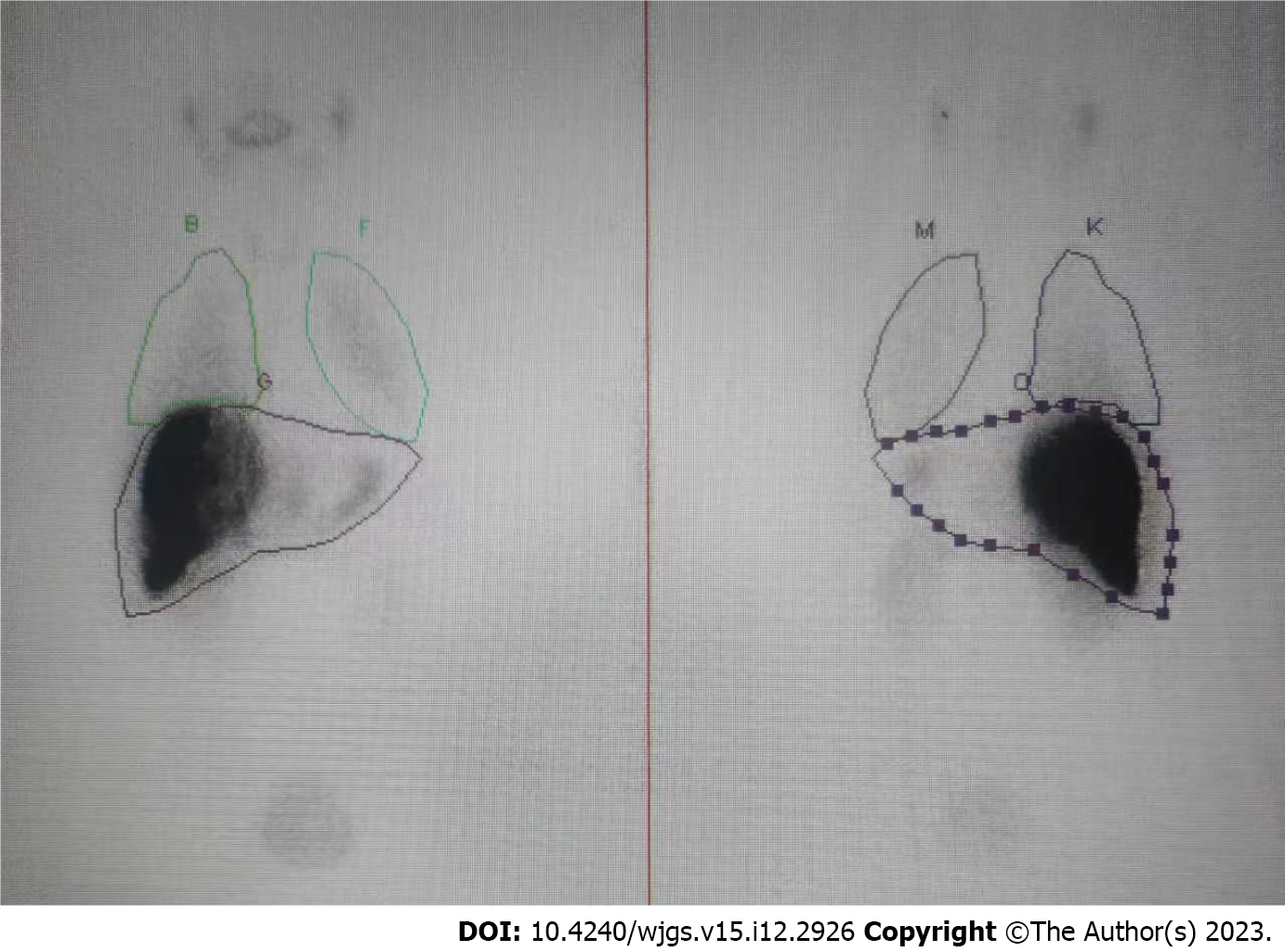
SPECT confirmed the absence of a relevant HPS with 8.4% (Figure 3). TARE was carried out successfully in the next week. Referring to the recommendations of the Cardiovascular and Interventional Radiological Society of Europe[13], the patient had no serious adverse event (grades 3-6) in the perioperative period.
There has been increased use of TARE in patients with intermediate- to advanced-stage HCC in recent years. Not all patients are candidates for this treatment due to a number of technical and clinical factors that need to be taken into consideration. One is the presence of APS bypass in the tumor capillary bed because TARE can cause liver failure due to extensive radioembolization in nontumorous liver parenchyma, or lung injury due to elevated HPS. Therefore, timely and complete closure of shunts is necessary before TARE for advanced HCC with marked APS.
Systemic antiangiogenic therapy has been described for reduction of IHS and HPS[9,14] caused by APS or arteriovenous shunt. Theysohn et al[14] reported on seven patients with elevated HPS who treated with oral sorafenib for an average of 138 d (ranging from 72-297 d). Four of the patients had significant reduction in their HPS and had successful TARE. The remaining three patients had disease progression and did not survive to undergo TARE. For patients to be treated with systemic antiangiogenic drug to relieve high HPS, it is a main challenge to balance the time between treatment and conducting TARE[15]. To reduce the HPS, sufficient time must be given for observing the efficacy of the systemic antiangiogenic therapy regimen. In patients with rapidly growing tumor types, the window of opportunity for TARE treatment may be lost. Standard techniques of transarterial bland embolization or chemoembolization can be used to shut down large arteriovenous and APS[16,17]. Ward et al[17] reported a 29%-69% decrease in HPS in five patients receiving embolization procedures. However, excessive transarterial embolization may theoretically lead to unsatisfactory treatment response following TARE due to uneven microsphere distribution. Therefore, the best treatment strategy to reduce IHS and HPS for HCC complicated with APS remains to be determined.
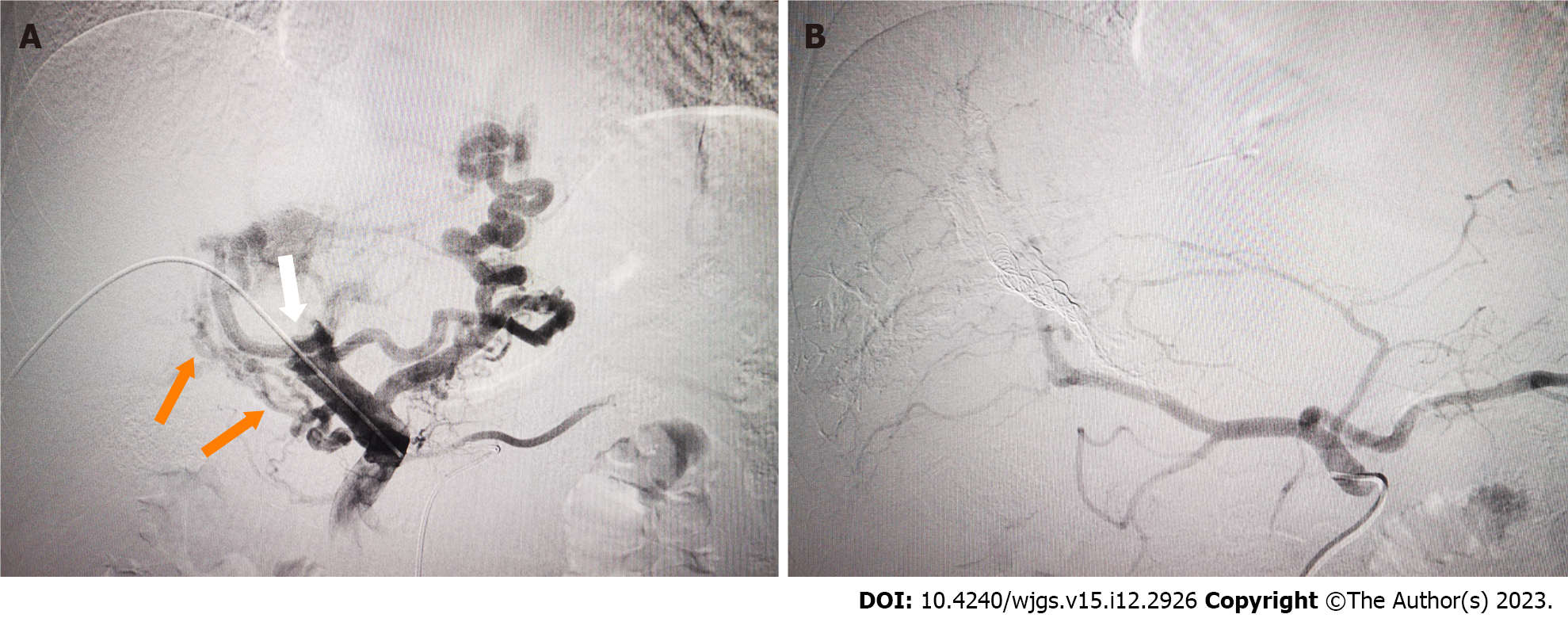
PVE has been widely used to expand the indications for hepatectomy for HCC in patients with insufficient future liver remnant. In the present case, PVE was applied to reduce IHS and HPS in advanced HCC with marked APS by complete embolization of the corresponding portal veins to prevent Tc-99m MAA or radio-microspheres moving through the APS. In our case, marked APS was shown by initial DSA. It has been shown that TARE for large IHS and HPS is unsafe, and it is necessary to reduce the therapeutic dose, but the efficacy may be compromised[17]. After we performed PVE using interlock microcoils and NBCA, the APS disappeared, and SPECT after injection of TC99m-MAA showed low uptake in normal liver and lung tissue, which made treatment with TARE viable.
Timely and completely embolization of shunts using PVE to reduce extensive portal vein radioembolization and HPS before TARE may represent a suitable treatment approach for HCC with marked APS. However, this technique remains technically difficult in cases with multiple APSs. In addition, PVE may further exacerbate portal hypertension and lead to gastrointestinal bleeding. Future prospective studies should investigate the safety and efficiency of PVE in pretreatment angiography with Tc-99m MAA mapping.
Our clinical observations suggest the feasibility of closure of APS with PVE during mapping angiography for patients with advanced-stage HCC to enable reduction of HPS and subsequent TARE.
The authors would like to express their deep gratitude to all staff who participated in the endovascular procedures for the study patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Baiocchi L, Italy; Giacomelli L, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wu RR
| 1. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 558] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 2. | Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Ponziani FR, Santopaolo F, Posa A, Pompili M, Tanzilli A, Maestri M, Pallozzi M, Ibba F, Manfredi R, Gasbarrini A, Iezzi R. SIRT in 2025. Cardiovasc Intervent Radiol. 2022;45:1622-1633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Wu H, Zhao W, Zhang J, Han J, Liu S. Clinical characteristics of hepatic Arterioportal shunts associated with hepatocellular carcinoma. BMC Gastroenterol. 2018;18:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ratti F, Cipriani F, Paganelli M, Ferla G, Aldrighetti LA. Surgical approach to multifocal hepatocellular carcinoma with portal vein thrombosis and arterioportal shunt leading to portal hypertension and bleeding: a case report. World J Surg Oncol. 2012;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 7. | Okuda K, Musha H, Yamasaki T, Jinnouchi S, Nagasaki Y, Kubo Y, Shimokawa Y, Nakayama T, Kojiro M, Sakamoto K, Nakashima T. Angiographic demonstration of intrahepatic arterio-portal anastomoses in hepatocellular carcinoma. Radiology. 1977;122:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Park J, Oh D, Paeng JC, Lee M, Chung JW, Kim HC. Radioembolization for Hepatocellular Carcinoma: The Effects of Arterioportal Shunts on Nontargeted Liver Hypertrophy. J Vasc Interv Radiol. 2022;33:787-796.e4. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Balli HT, Aikimbaev K, Burak IG, Pehlivan UA, Piskin FC, Sozutok S. Reduction of Hepatopulmonary and Intrahepatic Shunts after Treatment with Sorafenib in Hepatocellular Carcinoma Patients. Cardiovasc Intervent Radiol. 2022;45:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Hoshiai S, Mori K, Ishiguro T, Konishi T, Uchikawa Y, Fukuda K, Minami M. Balloon-Assisted Chemoembolization Using a Micro-Balloon Catheter Alongside a Microcatheter for a Hepatocellular Carcinoma with a Prominent Arterioportal Shunt: A Case Report. Cardiovasc Intervent Radiol. 2017;40:625-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Duan F, Bai Y, Cui L, Li X, Yan J, Zhu H. Transarterial embolization with N-butyl 2-cyanoacrylate for the treatment of arterioportal shunts in patients with hepatocellular carcinoma. J Cancer Res Ther. 2017;13:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Murata S, Tajima H, Nakazawa K, Onozawa S, Kumita S, Nomura K. Initial experience of transcatheter arterial chemoembolization during portal vein occlusion for unresectable hepatocellular carcinoma with marked arterioportal shunts. Eur Radiol. 2009;19:2016-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199-S202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1340] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 14. | Theysohn JM, Schlaak JF, Müller S, Ertle J, Schlosser TW, Bockisch A, Lauenstein TC. Selective internal radiation therapy of hepatocellular carcinoma: potential hepatopulmonary shunt reduction after sorafenib administration. J Vasc Interv Radiol. 2012;23:949-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Schiro BJ, Amour ES, Harnain C, Gandhi RT. Management of High Hepatopulmonary Shunts in the Setting of Y90 Radioembolization. Tech Vasc Interv Radiol. 2019;22:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | McGregor H, Hill M, Kuo P, Woodhead G, Patel M. Same-Day Repeat Hepatopulmonary Shunt Measurement during Planning Angiography for Hepatic Radioembolization. J Vasc Interv Radiol. 2020;31:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Ward TJ, Tamrazi A, Lam MG, Louie JD, Kao PN, Shah RP, Kadoch MA, Sze DY. Management of High Hepatopulmonary Shunting in Patients Undergoing Hepatic Radioembolization. J Vasc Interv Radiol. 2015;26:1751-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |