Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2879
Peer-review started: July 26, 2023
First decision: October 9, 2023
Revised: October 23, 2023
Accepted: November 27, 2023
Article in press: November 27, 2023
Published online: December 27, 2023
Processing time: 154 Days and 11.5 Hours
Surgical site infections (SSIs) increase mortality, hospital stays, additional medical treatment, and medical costs. Subcutaneous drains prevent SSIs in gynecological and breast surgeries; however, their clinical impact in abdominal surgery remains unclear.
To investigate whether subcutaneous drains were beneficial in abdominal surgery using a systematic review and meta-analysis.
The database search used PubMed, MEDLINE, and the Cochrane Library. The following inclusion criteria were set for the systematic review: (1) Randomized controlled trial studies comparing SSIs after abdominal surgery with or without subcutaneous drains; and (2) Studies that described clinical outcomes, such as SSIs, seroma formation, the length of hospital stays, and mortality.
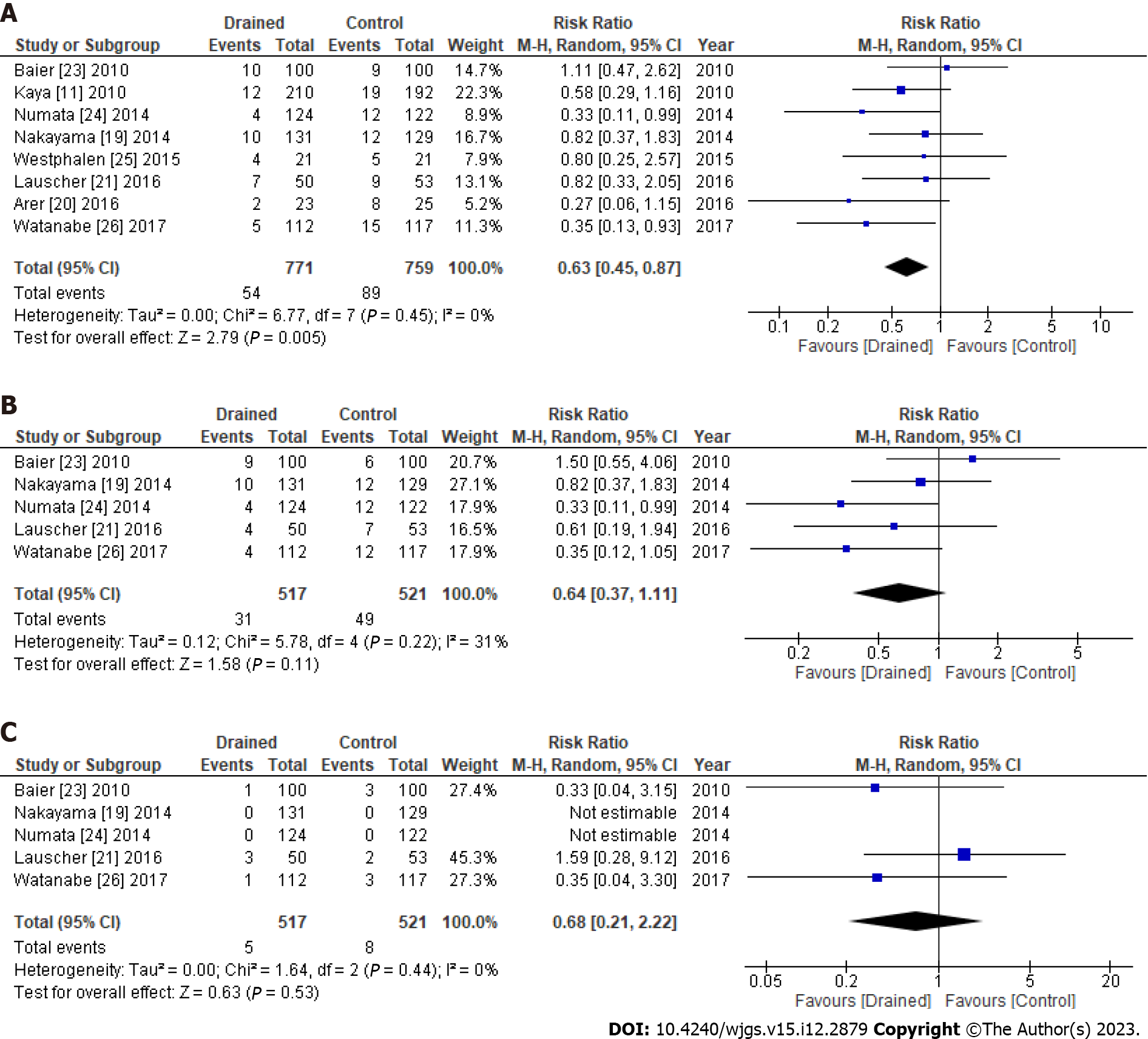
Eight studies were included in this meta-analysis. The rate of total SSIs was significantly lower in the drained group (54/771, 7.0%) than in the control group (89/759, 11.7%), particularly in gastrointestinal surgery. Furthermore, the rate of superficial SSIs was slightly lower in the drained group (31/517, 6.0%) than in the control group (49/521, 9.4%). No significant differences were observed in seroma formation between the groups. Hospital stays were shorter in the drained group than in the control group.
Subcutaneous drains after abdominal surgery prevented SSIs and reduced hospital stays but did not significantly affect seroma formation. The timing of drain removal needs to be reconsidered in future studies.
Core Tip: This review supports the beneficial effects of subcutaneous drains after abdominal surgery. Subcutaneous drains may not prevent seroma formation. Most studies removed drains within 3 d regardless of the amount of fluid discharge. The timing of drain removal may affect the clinical outcome. Nevertheless, subcutaneous drains can prevent surgical site infections and shorten the length of hospital stays after abdominal surgery.
- Citation: Ishinuki T, Shinkawa H, Kouzu K, Shinji S, Goda E, Ohyanagi T, Kobayashi M, Kobayashi M, Suzuki K, Kitagawa Y, Yamashita C, Mohri Y, Shimizu J, Uchino M, Haji S, Yoshida M, Ohge H, Mayumi T, Mizuguchi T. Recent evidence for subcutaneous drains to prevent surgical site infections after abdominal surgery: A systematic review and meta-analysis. World J Gastrointest Surg 2023; 15(12): 2879-2889
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2879.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2879
Surgical site infections (SSIs) are a common complication after gastrointestinal surgery and increase patient mortality[1,2]. They were previously reported to occur in approximately 20% of patients after colorectal and hepatobiliary surgeries[3-5]. SSIs prolong hospital stays and increase additional medical management with high medical costs[6]. Therefore, their prevention during the perioperative period after surgery is essential.
Fluid collection in subcutaneous tissue is a risk factor for the development of SSIs through its provision of an environment for bacterial growth[7]. The Centers for Disease Control and Prevention guidelines recommend the use of preoperative antimicrobial agents, the removal of serous fluid with drains, including negative pressure wound therapy, and antibacterial-coated absorbent sutures for the prevention of SSIs[8]. The World Health Organization global guidelines also recommend the use of negative pressure wound therapy and antibacterial, absorbent sutures in any surgical procedure[9]. Subcutaneous drains are commonly used after gynecological and breast surgeries[10]; however, conflicting findings have been reported on their clinical impact on SSIs after abdominal surgery[11-15].
The majority of previous studies that examined the clinical benefits of subcutaneous drains were retrospective analyses without cause responsiveness. Therefore, further evidence is needed from randomized controlled trials (RCTs). This systematic review and meta-analysis were designed to compare clinical outcomes among subcutaneous drained and control groups after abdominal surgery.
The review followed the statement of Preferred Reporting Items for Systematic Reviews and Meta-analyses[16]. The protocol was registered in INPLASY (INPLASY No. 202350115). Ethical approval was not required for this review because of the observational meta-analysis. The protocol was augmented and evaluated with the peer review of electronic search strategies guidelines before performing the search[17]. A comprehensive literature search was conducted using PubMed, MEDLINE, and the Cochrane Library for articles published between January 1, 1999 and April 1, 2023, and the search strategy is described in Supplementary Table 1.
Five independent authors (Ishinuki T, Kouzu K, Kobayashi Mo, Suzuki K, and Yamashita C) reviewed the title and abstract of each article based on the following criteria: (1) RCT studies published in 1999 or thereafter; (2) Patients underwent abdominal surgery, defined as gastrointestinal, hepatobiliary, and hernia repair surgery; (3) Comparisons between subcutaneous drains and no subcutaneous drains; and (4) Incidence of SSIs was presented. Exclusion criteria were duplicate studies and studies in the same database or population. In the case of a conflict of agreement among authors, a group consensus was used to reach acceptance or rejection. Six authors (Ishinuki T, Goda E, Kitagawa Y, Mohri Y, Uchino M, and Haji S) reviewed the full texts of studies that passed the first screening. In addition, references were checked to ensure that all studies were included in eligible studies.
Data selection was performed by four independent authors (Ishinuki T, Shinkawa H, Suzuki K, and Yoshida M). A data entry form was prepared in advance. The following information was selected: study details (title, first author, journal name, and year of publication), study design (purpose, study period, eligibility and exclusion criteria, age, and total number of participants), interventions delivered (procedure, surgical site, type of drain, timing of drain removal, length of hospital stays, and type of antimicrobial agents), and study results [number of SSIs, type of SSI (superficial or deep/organ), number of seroma formed, sample size, and P value]. The authors independently assessed the risk of bias for studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and Cochrane’s effective practice and organization of care guidance, such as random sequence generation, allocation concealment, blinding of participants, blinding of personnel, blinding of outcome assessments, incomplete outcome data, selective reporting, and other bias[18]. Conflicts of opinion among authors were discussed with all authors, and a consensus was reached.
The primary outcome was the rate of SSIs, including individual superficial or deep/organ SSIs. Secondary outcomes were the rate of seroma formation, the length of hospital stays, and mortality. Dichotomous data were analyzed for risk ratios using a random effects model and the Mantel-Haenszel method. Continuous data, such as the length of hospital stays, were analyzed using non-parametric tests[19-21]. Data were presented as means with standard deviations using a random effects model. Median and interquartile range data were calculated as means and standard deviations[22]. In addition, a subgroup analysis of SSIs was performed according to each type of drain or surgical site. Data synthesis and statistical analyses were performed with Review Manager (version 5.4; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
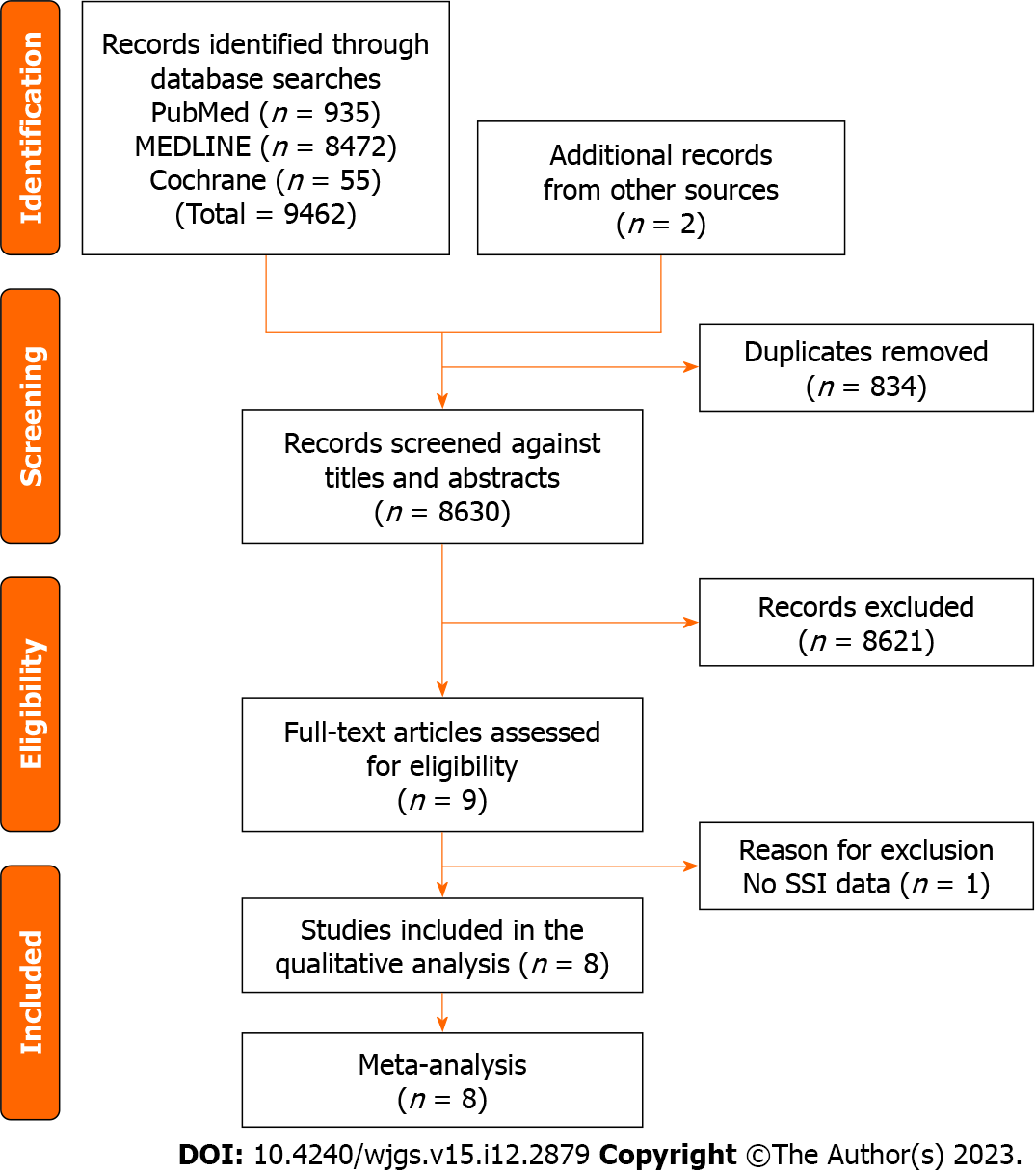
The Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram for this review is shown in Figure 1. Database search results showed that 9462 studies were eligible, with two additional studies being added to the references. We excluded 834 studies because of duplication, and 8621 were excluded after the screening of titles and abstracts. As a result, nine studies were reviewed as full texts[11,19-21,23-27]. Eight studies were ultimately included in the meta-analysis[11,19-21,23-26] after the exclusion of one study without SSI data[27]. The bias summary is shown in Supplementary Figure 1.
A summary of data in each study is shown in Table 1. Five studies disclosed the outcomes of superficial or deep/organ SSIs[19,23,24,26]. Three studies reported the number of seromas formed[21,24,25], and three described the length of hospital stays[19-21]. Although three studies discussed mortality[19,25,26], only one mortality occurred in the drained group; therefore, a further analysis of mortality was impossible[25,26].
| Ref. | Year | Drained, n | Control, n | Total SSI, n | Superficial SSI, n | Deep/organ SSI, n | Seroma, n | Hospital stay, mean ± SD | Mortality, n |
| Baier et al[23] | 2010 | 100 | 100 | 10:9 | 9:6 | 1:3 | ND | ND | ND |
| Kaya et al[11] | 2010 | 210 | 192 | 12:19 | ND | ND | ND | ND | ND |
| Nakayama et al[19] | 2014 | 131 | 129 | 10:12 | 10:12 | 0:0 | ND | 26.3 ± 39.7 : 30.3 ± 49.5 | 1:0 |
| Numata et al[24] | 2014 | 124 | 122 | 4:12 | 4:12 | 0:0 | 0:0 | ND | ND |
| Westphalen et al[25] | 2015 | 21 | 21 | 4:5 | ND | ND | 11:9 | ND | 0:0 |
| Arer et al[20] | 2016 | 23 | 25 | 2:8 | ND | ND | ND | 5.9 ± 2.4 : 7.9 ± 3.8 | ND |
| Lauscher et al[21] | 2016 | 50 | 53 | 7:9 | 4:7 | 3:2 | 1:1 | 8.3 ± 2.3 : 9.3 ± 3.8 | ND |
| Watanabe et al[26] | 2017 | 112 | 117 | 5:15 | 4:12 | 1:3 | ND | ND | 0:0 |
The characteristics of each study are shown in Supplementary Table 2. There were three studies on lower gastrointestinal surgery[21,24,26], one on hepatobiliary surgery[19], three on mixed surgery[11,20,23], and one on hernia repair[25]. The types of drains were the Redon drain[23], Hemovac drain[11], Blake drain[19,26], Penrose drain[24], and Tubular drain[25]. Two studies did not specify the type of drain used[20,21]. The timing of drain removal ranged between 2-5 postoperative days or when the drainage volume was less than 40 mL/day. Cefuroxime and metronidazole were used in one study[23], cefazoline in three[11,19,25], cefmetazole in one[24], ampicillin/sulbactam in one[21], and flomoxef in one[26]. One study did not describe the type of antibiotic prophylaxis[20]. An intent-to-treat analysis was performed in three out of seven studies[19,23,25].
The rate of total SSIs was significantly lower in the drained group (54/771, 7.0%) than in the control group (89/759, 11.7%) without heterogeneity (I2 = 0%) [risk ratio (RR): 0.63, 95% confidence interval (95%CI): 0.45-0.87, P = 0.005] (Figure 2A). Furthermore, the rate of superficial SSIs was slightly lower in the drained group (31/517, 6.0%) than in the control group (49/521, 9.4%) (RR: 0.64, 95%CI: 0.37-1.11, P = 0.11) (Figure 2B). In addition, no significant differences were observed in deep/organ SSIs between the groups (Figure 2C). Publication bias of the SSIs was assessed by the funnel plot (Supplementary Figure 2).
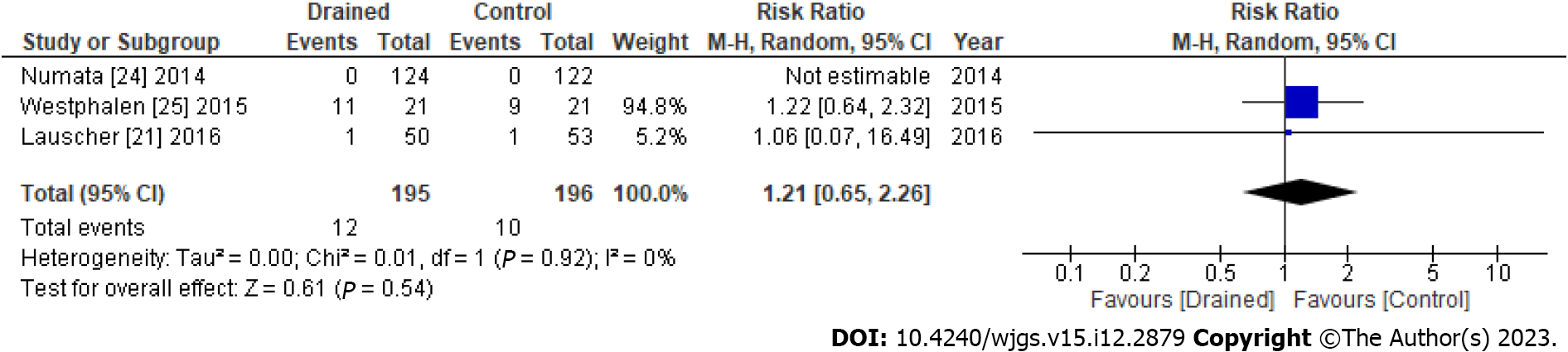
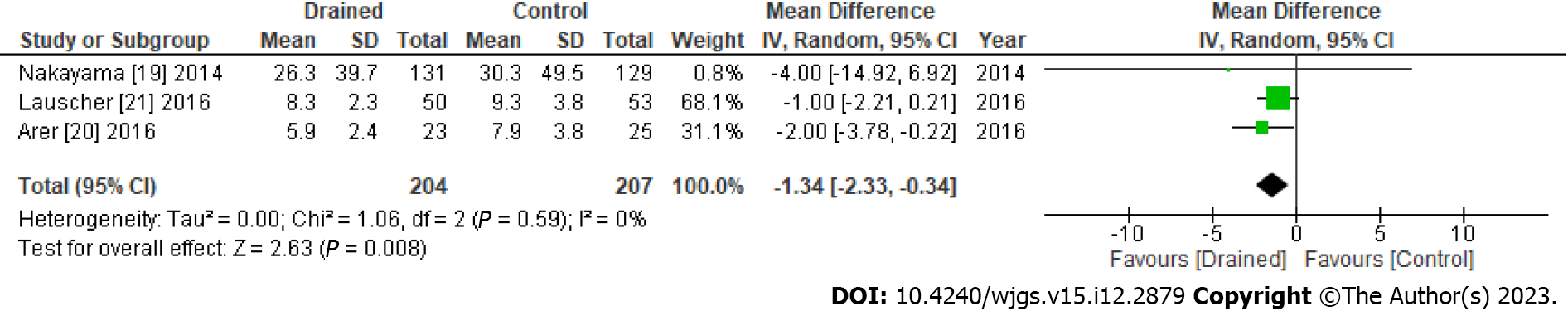
No significant differences were observed in seroma formation between the drained and control groups (RR: 1.21, 95%CI: 0.65-2.26, P = 0.54) (Figure 3). However, the length of hospital stays significantly differed (mean difference: -1.34, 95%CI: -2.33 to -0.34, P = 0.008) (Figure 4). Publication bias of the seroma formation and the hospital stays were assessed by the funnel plot (Supplementary Figures 3 and 4, respectively).
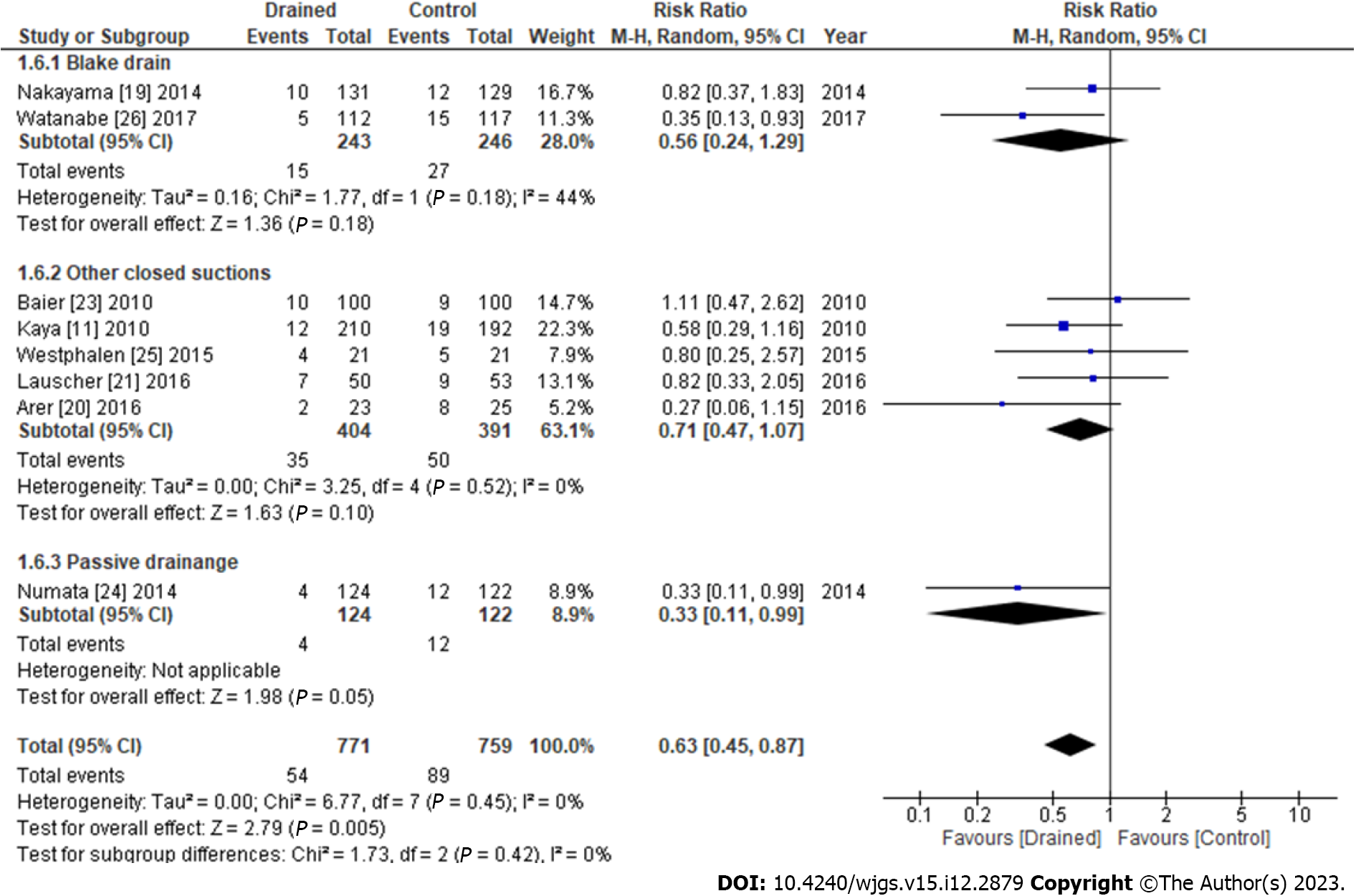
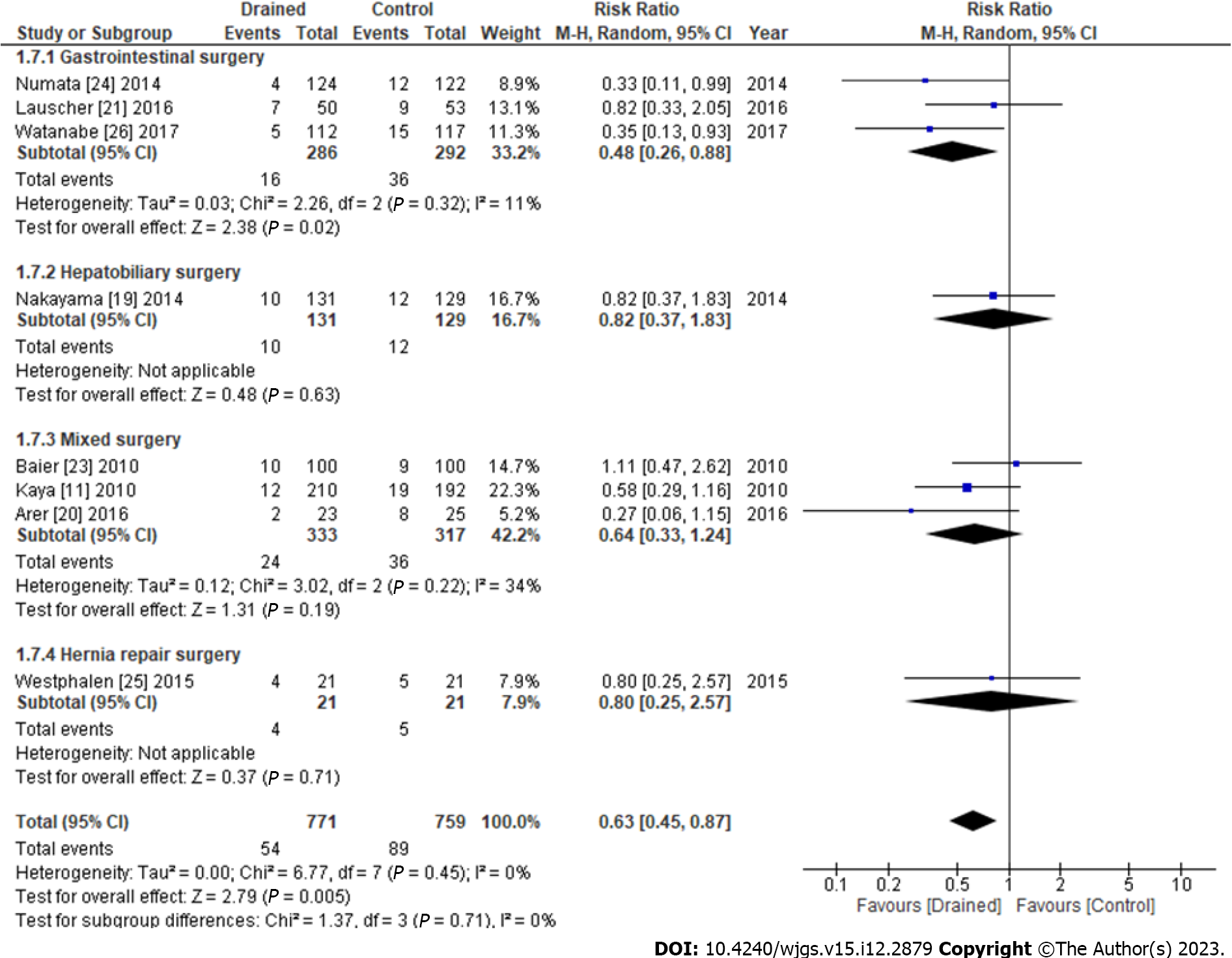
The results of subgroup analyses of the types of drains are shown in Figure 5. SSIs with the Blake drain (RR: 0.56, 95%CI: 0.24-1.29, P = 0.18) and other closed suction methods (RR: 0.71, 95%CI: 0.47-1.07, P = 0.52) did not significantly differ from those with the control, whereas SSIs using passive drainage significantly decreased more than with the control (RR: 0.33, 95%CI: 0.11-0.99, P = 0.05) (Figure 5). The results of subgroup analyses of surgical procedures are shown in Figure 6. Subcutaneous drains in gastrointestinal surgery decreased SSIs more than the control (RR: 0.48, 95%CI: 0.26-0.88, P = 0.02), whereas those in the hepatobiliary surgery (RR: 0.82, 95%CI: 0.37-1.83, P = 0.63), mixed surgery (RR: 0.64, 95%CI: 0.33-1.24, P = 0.22), and hernia repair surgery (RR: 0.80, 95%CI: 0.25-2.57, P = 0.71) did not (Figure 6).
We identified eight RCTs that compared SSIs after abdominal surgery with or without subcutaneous drains. Although the rate of total SSIs was significantly lower in the drained group than in the control group, no significant differences were observed in superficial or deep/organ SSIs because of statistical power. According to the surgical site, drains effectively prevented SSIs in gastrointestinal surgery. No significant differences were observed in SSIs among the drain types examined. We also showed that seroma formation did not significantly differ between the drained and control groups. On the other hand, subcutaneous drains effectively reduced hospital stays.
Subcutaneous drains remove interstitial fluid from a wound thereby reducing the risk of SSIs[28]. Watanabe et al[26] showed the benefits of subcutaneous drains in preventing SSIs after colorectal surgery, and putative reasons for reducing SSIs were proposed. The first reason is the specific shape of the drain itself. The classic shape of a subcutaneous drain is a tube with multiple small holes[11,21,23,25]. This drain type may damage the hypodermis because of high negative pressure from the holes[29]. The Blake drain is a closed suction drain made of a silicone elastomer with a solid core in the center and four slits along the sides. Since the Blake drain was developed to drain without causing tissue damage, it may be more beneficial than other drains[26]. Therefore, this review investigated whether the Blake drain significantly reduced SSIs; however, no significant differences were observed between the drain types examined. The small holes of a drain may become blocked with fat tissue during the flow of fluid, which eventually prevents drainage[30]. Therefore, drainage methods that are less likely to become blocked need to be developed.
The second proposed reason is the timing of drain removal. Long-term drain placement may increase the risk of contamination[26]. A previous study published in 2004 reported a high infection rate associated with drain placement[31]. On the other hand, studies published from the 2010s demonstrated that a sufficiently long drain time, such as longer than 72 h, prevented wound infection[12,30]. The majority of studies analyzed in the present study removed drains within 72 h, which may have been too short to prevent superficial infection[11,19,21,23,24]. Nakayama et al[19] also reported that the timing of drain removal was too early to detect clinical effects and showed that 41% of SSIs occurred within 72 h of drain removal. Watanabe et al[26] removed drains 120 h after surgery and found no incidence of SSIs. Therefore, the timing of drain removal needs to be investigated in further studies.
Although subcutaneous drains significantly decreased the rate of total SSIs, no significant differences were observed in the rate of superficial or deep/organ SSIs. Since two previous studies did not describe data on superficial or deep/organ SSIs, the statistical power in the subgroup analysis may have been lower than that in the total analysis[11,25]. The present study also examined clinical effects, which were slightly better in the drained group than in the control group. This result may be attributed to improvements in drain materials and management.
This review showed that the effectiveness of subcutaneous drains was dependent on the surgical site. The usefulness of subcutaneous drains for the prevention of SSIs has been demonstrated in cholecystectomy[32] and gastrointestinal surgery[11,23]. Baier et al[23] reported no benefit of subcutaneous drains in the abdominal field. On the other hand, Kaya et al[11] showed a significant reduction in SSIs after surgery for colorectal malignancies and lower abdominal incisions in their subgroup analysis. Previous studies also found that drains prevented SSIs in contaminated surgery, such as lower gastrointestinal surgery[33,34]. The present results provide support for the use of drains in lower gastrointestinal surgery.
The meta-analysis showed that subcutaneous drains did not significantly prevent seroma formation. Westphalen et al[25] noted that seroma formation mostly occurred after drain removal. The peak incidence of seroma formation was previously reported to be approximately 2 wk after surgery, at which time the majority of drains had already been removed[35-37]. Shima et al[12] demonstrated that the timing of drain removal in breast cancer surgery was important to avoid wound complications. They found that seroma formation may be prevented when subcutaneous drains were removed after 7 d or later without an increase in SSIs. Therefore, the timing of drain removal needs to be individually selected for specific surgical procedures.
The use of subcutaneous drains affects the length of hospital stays. The length of hospital stays is a socioeconomic factor that is increased by any complication[21]. Hospital stays were prolonged by SSIs, large seromas, hematomas, and other complications[21]. Therefore, the prevention of SSIs by appropriately placing a subcutaneous drain may shorten hospital stays.
To ensure the quality of evidence, this review performed a meta-analysis of RCTs only. However, the interpretation of the present results was limited. The small number of studies included and insufficient published data affected the statistical results obtained. Therefore, a subgroup analysis of factors, such as the material of the drain and the timing of drain removal, was not possible. In addition, surgical techniques may have improved over the study period. Furthermore, putative adverse effects, such as pain, cosmetic evaluations, and management costs, were not analyzed due to the lack of data. It was not possible to investigate a publication bias due to the lack of sufficiently large publications to estimate. A limitation of the present study was that some factors may not have been included in the analysis, such as the age of patients, preoperative conditions, the length of surgery, the surgical technique, and the surgeon’s experience.
In conclusion, the present study supports the use of subcutaneous drains to prevent SSIs and shorten the length of hospital stays after abdominal surgery. On the other hand, subcutaneous drains may not prevent seroma formation. Several specific clinical outcomes, such as the timing of drain removal, pain control, cosmetic evaluations, medical cost, and the quality of life of patients need to be clarified in future studies.
Surgical site infections (SSIs) are a common complication after gastrointestinal surgery and result in a worse clinical prognosis for the patients. Subcutaneous drains are commonly used after abdominal surgery to prevent SSIs. Nevertheless, further evidence is needed about subcutaneous drains. Therefore, this systematic review and meta-analysis were designed to compare clinical outcomes among subcutaneous drains and control groups after abdominal surgery.
There is a shortage of evidence as to whether subcutaneous draining is beneficial in preventing the development of SSIs, and the results are different in each of the previous studies.
The objective for this review was to determine whether subcutaneous drainage is beneficial in preventing the de
Independent authors reviewed the previous studies and data selection. The primary outcome was the rate of SSIs, including individual superficial or deep/organ SSIs. Secondary outcomes were the rate of seroma formation, the length of hospital stays, and mortality. Data were presented as means with standard deviations using a random effects model.
We identified eight RCTs that compared SSIs after abdominal surgery with or without subcutaneous drains. Although the rate of total SSIs was significantly lower in the drained group than in the control group, no significant differences were observed in superficial or deep/organ SSIs because of statistical power. According to the surgical site, drains effectively prevented SSIs in gastrointestinal surgery. No significant differences were observed in SSIs among the drain types examined. We also showed that seroma formation did not significantly differ between the drained and control groups. On the other hand, subcutaneous drains effectively reduced hospital stays.
This review supports the use of subcutaneous drains to prevent SSIs and shorten the length of hospital stays after abdominal surgery. On the other hand, subcutaneous drains may not prevent seroma formation.
Impact of subcutaneous drains for pain control, cosmetic evaluations, medical cost, and the quality of life of patients need to be clarified.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Jiang L, China; Manesis EK, Greece; Meng Y, China; Valek V, Czech Republic; Osman Nuri Dilek, Turkey S-Editor: Lin C L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132; quiz 133. [PubMed] |
| 2. | Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1178] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 3. | Romy S, Eisenring MC, Bettschart V, Petignat C, Francioli P, Troillet N. Laparoscope use and surgical site infections in digestive surgery. Ann Surg. 2008;247:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 5. | Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Kawai M, Hirono S, Tani M, Yamaue H. Risk factors for postoperative infectious complications after hepatectomy. J Hepatobiliary Pancreat Sci. 2011;18:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Haruki K, Shiba H, Fujiwara Y, Furukawa K, Wakiyama S, Ogawa M, Ishida Y, Misawa T, Yanaga K. Negative impact of surgical site infection on long-term outcomes after hepatic resection for colorectal liver metastases. Anticancer Res. 2013;33:1697-1703. [PubMed] |
| 7. | Panici PB, Zullo MA, Casalino B, Angioli R, Muzii L. Subcutaneous drainage vs no drainage after minilaparotomy in gynecologic benign conditions: a randomized study. Am J Obstet Gynecol. 2003;188:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1394] [Cited by in RCA: 2042] [Article Influence: 255.3] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, second edition. Dec 1, 2018. [cited 15 June 2023]. Available from: https://www.who.int/publications/i/item/9789241550475. |
| 10. | Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Kaya E, Paksoy E, Ozturk E, Sigirli D, Bilgel H. Subcutaneous closed-suction drainage does not affect surgical site infection rate following elective abdominal operations: a prospective randomized clinical trial. Acta Chir Belg. 2010;110:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Shima H, Kutomi G, Sato K, Kuga Y, Wada A, Satomi F, Uno S, Nisikawa N, Kameshima H, Ohmura T, Mizuguchi T, Takemasa I. An Optimal Timing for Removing a Drain After Breast Surgery: A Systematic Review and Meta-Analysis. J Surg Res. 2021;267:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Al-Inany H, Youssef G, Abd ElMaguid A, Abdel Hamid M, Naguib A. Value of subcutaneous drainage system in obese females undergoing cesarean section using pfannenstiel incision. Gynecol Obstet Invest. 2002;53:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Magann EF, Chauhan SP, Rodts-Palenik S, Bufkin L, Martin JN Jr, Morrison JC. Subcutaneous stitch closure vs subcutaneous drain to prevent wound disruption after cesarean delivery: a randomized clinical trial. Am J Obstet Gynecol. 2002;186:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ramsey PS, White AM, Guinn DA, Lu GC, Ramin SM, Davies JK, Neely CL, Newby C, Fonseca L, Case AS, Kaslow RA, Kirby RS, Rouse DJ, Hauth JC. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47178] [Article Influence: 2948.6] [Reference Citation Analysis (0)] |
| 17. | McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1398] [Cited by in RCA: 2988] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 18. | Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions. 8th ed. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor. Hoboken: Wiley, 2019. [DOI] [Full Text] |
| 19. | Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S. Subcutaneous drainage to prevent wound infection in liver resection: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2014;21:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Arer IM, Yabanoglu H, Aytac HO, Ezer A. The effect of subcutaneous suction drains on surgical site infection in open abdominal surgery A prospective randomized study. Ann Ital Chir. 2016;87:49-55. [PubMed] |
| 21. | Lauscher JC, Schneider V, Lee LD, Stroux A, Buhr HJ, Kreis ME, Ritz JP. Necessity of subcutaneous suction drains in ileostomy reversal (DRASTAR)-a randomized, controlled bi-centered trial. Langenbecks Arch Surg. 2016;401:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 7034] [Article Influence: 639.5] [Reference Citation Analysis (0)] |
| 23. | Baier PK, Glück NC, Baumgartner U, Adam U, Fischer A, Hopt UT. Subcutaneous Redon drains do not reduce the incidence of surgical site infections after laparotomy. A randomized controlled trial on 200 patients. Int J Colorectal Dis. 2010;25:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Numata M, Godai T, Shirai J, Watanabe K, Inagaki D, Hasegawa S, Sato T, Oshima T, Fujii S, Kunisaki C, Yukawa N, Rino Y, Taguri M, Morita S, Masuda M. A prospective randomized controlled trial of subcutaneous passive drainage for the prevention of superficial surgical site infections in open and laparoscopic colorectal surgery. Int J Colorectal Dis. 2014;29:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Westphalen AP, Araújo AC, Zacharias P, Rodrigues ES, Fracaro GB, Lopes Filho Gde J. Repair of large incisional hernias. To drain or not to drain. Randomized clinical trial. Acta Cir Bras. 2015;30:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Watanabe J, Ota M, Kawamoto M, Akikazu Y, Suwa Y, Suwa H, Momiyama M, Ishibe A, Watanabe K, Masui H, Nagahori K. A randomized controlled trial of subcutaneous closed-suction Blake drains for the prevention of incisional surgical site infection after colorectal surgery. Int J Colorectal Dis. 2017;32:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Peiper C, Conze J, Ponschek N, Schumpelick V. Value of subcutaneous drainage in repair of primary inguinal hernia. A prospective randomized study of 100 cases. Chirurg. 1997;68:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Colli A, Camara ML. First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg. 2011;6:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390-7; discussion 398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Tsujita E, Yamashita Y, Takeishi K, Matsuyama A, Tsutsui S, Matsuda H, Taketomi A, Shirabe K, Ishida T, Maehara Y. Subcuticular absorbable suture with subcutaneous drainage system prevents incisional SSI after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:1651-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, Poon RT, Wong J. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Chowdri NA, Qadri SA, Parray FQ, Gagloo MA. Role of subcutaneous drains in obese patients undergoing elective cholecystectomy: a cohort study. Int J Surg. 2007;5:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Kumar S, Chatterjee S, Gupta S, Satpathy A, Ray U. Role of subcutaneous closed vacuum drain in preventing surgical site infection in emergency surgery for perforative peritonitis: A randomized control study. Bangladesh Journal of Medical Science. 2017;16:85-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Pan HD, Wang L, Peng YF, Li M, Yao YF, Zhao J, Zhan TC, Du CZ, Gu J. Subcutaneous vacuum drains reduce surgical site infection after primary closure of defunctioning ileostomy. Int J Colorectal Dis. 2015;30:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Baroudi R, Ferreira CA. Seroma: how to avoid it and how to treat it. Aesthet Surg J. 1998;18:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Nahas FX, Ferreira LM, Ghelfond C. Does quilting suture prevent seroma in abdominoplasty? Plast Reconstr Surg. 2007;119:1060-4; discussion 1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Bercial ME, Sabino Neto M, Calil JA, Rossetto LA, Ferreira LM. Suction drains, quilting sutures, and fibrin sealant in the prevention of seroma formation in abdominoplasty: which is the best strategy? Aesthetic Plast Surg. 2012;36:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (1)] |