Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2222
Peer-review started: July 6, 2023
First decision: July 27, 2023
Revised: August 2, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: October 27, 2023
Processing time: 113 Days and 6.9 Hours
Patients with gastrointestinal tumors often suffer from poor nutritional status during treatment. Surgery is the main treatment for these patients, but the long postoperative recovery period is often accompanied by digestive and absorption dysfunction, leading to further deterioration of the nutritional status. Early enteral nutrition support is hypothesized to be helpful in improving this situation, but the exact effects have yet to be studied in depth.
To observe the effect of early enteral nutritional support on postoperative recovery in patients with surgically treated gastrointestinal tract tumors, with the expectation that by improving the nutritional status of patients, the recovery process would be accelerated and the incidence of complications would be reduced, thus improving the quality of life.
A retrospective analysis of 121 patients with gastrointestinal tract tumors treated in our hospital from January 2020 to January 2023 was performed. Fifty-three of these patients received complete parenteral nutrition support as the control group for this study. The other 68 patients received early enteral nutritional support as the observation group of this study. The clinical indicators comparing the two groups included time to fever, time to recovery of postoperative bowel function, time to postoperative exhaustion, and length of hospital stay. The changes in immune function and nutritional indexes in the two groups were compared. Furthermore, we utilized the SF-36 scale to compare the changes in the quality of life between the two groups of patients. Finally, the occurrence of postoperative complications between the two patient groups was also compared.
The postoperative fever time, postoperative bowel function recovery time, postoperative exhaustion time, and hospitalization time were all higher in the control group than in the observation group (P < 0.05). The levels of CD3+, CD4+, immunoglobulin (Ig) A, IgM, and IgG in the observation group were significantly higher than those in the control group at 1 d and 7 d postoperatively, while CD8+ was lower than in the control group (P < 0.05). Total protein, albumin, prealbumin, and transferrin levels were significantly higher in the observation group than in the control group at 7 d postoperatively (P < 0.05). The SF-36 scores of patients in the observation group were significantly higher than those in the control group (P < 0.0001). The overall incidence of adverse reactions after the intervention was significantly lower in the control group than in the observation group (P = 0.021).
We found that patients with gastrointestinal tumors are nutritionally vulnerable, and early enteral nutrition support programs can improve the nutritional status of patients and speed up postoperative recovery. This program can not only improve the immune function of the patient and protect the intestinal function, but it can also help to improve the quality of life of the patient. However, this program will increase the incidence of complications in patients. Caution should be taken when adopting early enteral nutrition support measures for patients with gastric cancer. The patient's condition and physical condition should be comprehensively evaluated and closely monitored to prevent possible complications.
Core Tip: This study demonstrated the critical role of early enteral nutritional support in the postoperative recovery of patients undergoing surgery for gastrointestinal tract tumors. This strategy not only helped to improve patient nutritional status, accelerate postoperative recovery, and reduce the incidence of complications but also improved patient quality of life by enhancing immune function and protecting intestinal function. Early enteral nutritional support becomes an important component of postsurgical care for gastrointestinal tumors and helps to improve the overall outcome of patients.
- Citation: Chen Z, Hong B, He JJ, Ye QQ, Hu QY. Examining the impact of early enteral nutritional support on postoperative recovery in patients undergoing surgical treatment for gastrointestinal neoplasms. World J Gastrointest Surg 2023; 15(10): 2222-2233
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2222.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2222
The continuous development of medical technology has significantly improved the effectiveness of prevention and treatment of many diseases[1]. Nonetheless, the comprehensive fight against cancer remains an unfinished task that seriously threatens the lives and health of patients[2]. According to the Global Cancer Statistics 2020 report[3], colorectal and gastric cancers ranked third and fifth in global cancer incidence and second and fourth in mortality, respectively. Gastrointestinal tract tumors, especially gastric and colorectal cancers, are commonly seen in people over 50 years of age and are among the common diseases in the malignancy category[4]. These two types of cancers often have no obvious specific clinical manifestations in their early stages, resulting in most patients not receiving timely and effective interventions[5]. As the cancer progresses, the demand for nutrients by the cancerous cells increases and the location occupied by the tumor affects the normal absorption function of the intestine, leading to depletion of the body’s nutrient reserves and destruction of immune function[6]. For elderly patients with gastric and colorectal cancer, their nutritional status and immune function are often substantially reduced by the effects of cancer, while the stress response of the body triggered by surgery may further exacerbate this situation[7]. Therefore, it is particularly important to implement postoperative clinical nutritional support measures for this specific population.
Parenteral nutrition is widely used in clinical practice by a wide range of physicians to provide effective nutritional supplementation for patients with impaired postoperative gastrointestinal function[8]. It has demonstrated remarkable efficacy in energy supplementation and maintenance of water-electrolyte balance in patients in the perioperative period[9]. However, as its application has expanded, medical professionals have identified progressive shortcomings after in-depth studies and comparisons[10]. Long-term reliance on parenteral nutrition after gastrointestinal surgery may go against the normal physiology of the gut, resulting in reduced basic activity of the gastrointestinal mucosa, impaired absorption and secretion, and disturbance of the balance of the intestinal flora[11,12]. These effects disrupt the normal microecological environment of the gut, raising the risk of infectious complications, and further delaying the patient’s postoperative recovery.
Conventional wisdom tends to suggest that early feeding after gastrointestinal surgery may increase the patient’s risk of abdominal pain, bloating, nausea, and vomiting as well as complications such as anastomotic fistula and abdominal infection and that patients are usually allowed to eat after gastrointestinal function has been restored[13]. However, with the in-depth study of early enteral nutrition support modalities after gastrointestinal surgery, some studies have pointed out that providing enteral nutrition support early to patients after radical gastric cancer surgery can promote the absorption and secretion function of the gastrointestinal tract and shorten the length of hospital stay of patients[14]. Moreover, compared with parenteral nutrition support, enteral nutrition support early after gastric cancer surgery does not increase the incidence of postoperative complications[15]. Early postoperative feeding is more in line with the normal physiological needs of the body and has a stimulating effect on the digestive and secretory functions of the gastrointestinal tract, which is conducive to the recovery of the gastrointestinal mucosa, promotes the absorption of nutrients, and reduces postoperative adverse reactions. However, there are insufficient studies on whether early enteral nutritional support has an impact on the postoperative recovery of patients with gastrointestinal tumors.
In the present study, we analyzed the effect of early enteral nutritional support on postoperative recovery in patients with surgically treated gastrointestinal tract tumors and provided reference for clinical gastrointestinal tract tumor nutritional supplementation protocols.
This study was conducted with the approval of Xiangshan First People’s Hospital Medical and Health Group Medical Ethics Committee, ethical approval number: 2023-(k)-41.
We conducted a retrospective analysis of 121 patients with gastrointestinal tract tumors treated at our hospital from January 2020 to January 2023. Fifty-three of these patients received complete parenteral nutrition support as the control group for this study. The other 68 patients received early enteral nutritional support as the observation group of this study.
Inclusion criteria: Patients with diagnosed gastric and colorectal cancer; patients having undergone radical tumor resection or partial gastrointestinal resection; patients over 60 years of age; patients with a clear state of consciousness and normal communication skills; and patients having complete clinical information.
Exclusion criteria: presence of severe psychological or psychiatric illness; presence of other types of malignancy; insufficiency of vital organs such as the heart, liver, and kidneys; and comorbidities at other sites.
Control group: Complete parenteral nutrition support was used in the control group. After central venous cannulation, prepared nutritional solutions such as sodium chloride injection and compounded amino acids were administered via intravenous drip starting 20 h after surgery. The indwelling gastric tube was removed after the patient’s gastrointestinal function was restored.
Observation group: The observation group received early enteral nutritional support. A nasojejunal tube and a gastrointestinal decompression tube were left in place during the operation. The jejunal tube was placed under direct vision along a guide wire into the jejunal output collaterals, while the end of the gastric tube was placed into the stomach. Twenty hours after surgery, warm saline was dripped through the enteral tube, and a slow drip of enteral nutrition mix was administered. The temperature of the nutrient solution should be kept at 37 to 40 C. After the anus has begun to pass, remove the gastric tube and start a small amount of liquid food by mouth, while reducing the amount of nutrient solution injected through the jejunal tube. Once the patient was able to eat normal liquid food, the nutrition tube was removed. The nursing staff used positive and optimistic words to encourage the patient during daily rounds, identified any negative emotions in time, and provided targeted psychological counselling to help the patient build up confidence in overcoming the disease.
Immune function: Venous blood was collected on an empty stomach before surgery and 1 d and 7 d after surgery, and peripheral blood T cell subset (CD3+, CD4+, and CD8+) activity was measured by flow cytometry. Immunoglobulins (Igs) [IgA, IgM, and IgG] were measured by enzyme-linked immunoassay.
Nutritional parameters: Venous blood was collected before surgery and 1 d and 7 d after surgery on an empty stomach. Total protein (TP) and albumin (ALB) were measured by the bicuculline method, prealbumin (PAB) was measured by the immunoturbidimetric method, and transferrin (Tf) was measured by the immunoscattering turbidimetric method.
Main observation indexes: The clinical indexes of the two groups including time to fever, time to recover intestinal function after surgery, time to postoperative exhaustion, and length of hospital stay were compared. The changes in immune function and nutritional indicators between the two groups were also compared.
Secondary observation indexes: The clinical data and postoperative complications of the two groups of patients were compared. Using the SF-36 scale[16], the changes in quality of life of patients in the two groups was compared.
The data collected in this study were statistically analyzed using the SPSS 26.0 software package, and GraphPad Prism 9 was used to plot the pictures of this data. The count data were expressed in % using the χ2 test as well as the Fisher test. The measurement data were expressed using the mean ± SD. The preoperative and postoperative comparisons between the same groups were made using the paired t test, and independent samples t test was used for comparison between two groups. Statistical differences were indicated when P < 0.05.
A comparison of the clinical data between the two groups revealed that there was no statistical difference in age, sex, body mass index, tumor location, degree of tumor differentiation, history of diabetes mellitus, hypertension, smoking, and alcohol abuse between the control group and the observation group (P > 0.05; Table 1).
| Characteristic | Observation group, n = 53 | Control group, n = 68 | P value |
| Age in yr | |||
| ≥ 65 | 30 | 44 | 0.369 |
| < 65 | 23 | 24 | |
| Sex | |||
| Male | 25 | 37 | 0.444 |
| Female | 28 | 31 | |
| BMI in kg/m2 | |||
| ≥ 25 | 13 | 14 | 0.511 |
| < 25 | 40 | 54 | |
| Tumor location | |||
| Stomach | 24 | 37 | 0.275 |
| Colorectal | 29 | 31 | |
| Tumor differentiation | |||
| Low | 20 | 31 | 0.438 |
| Medium, high | 33 | 37 | |
| Diabetes mellitus | |||
| Yes | 6 | 12 | 0.363 |
| No | 47 | 56 | |
| Hypertension | |||
| Yes | 10 | 17 | 0.432 |
| No | 43 | 51 | |
| Smoking | |||
| Yes | 27 | 37 | 0.584 |
| No | 27 | 31 | |
| Alcohol | |||
| Yes | 4 | 3 | 0.500 |
| No | 49 | 65 |
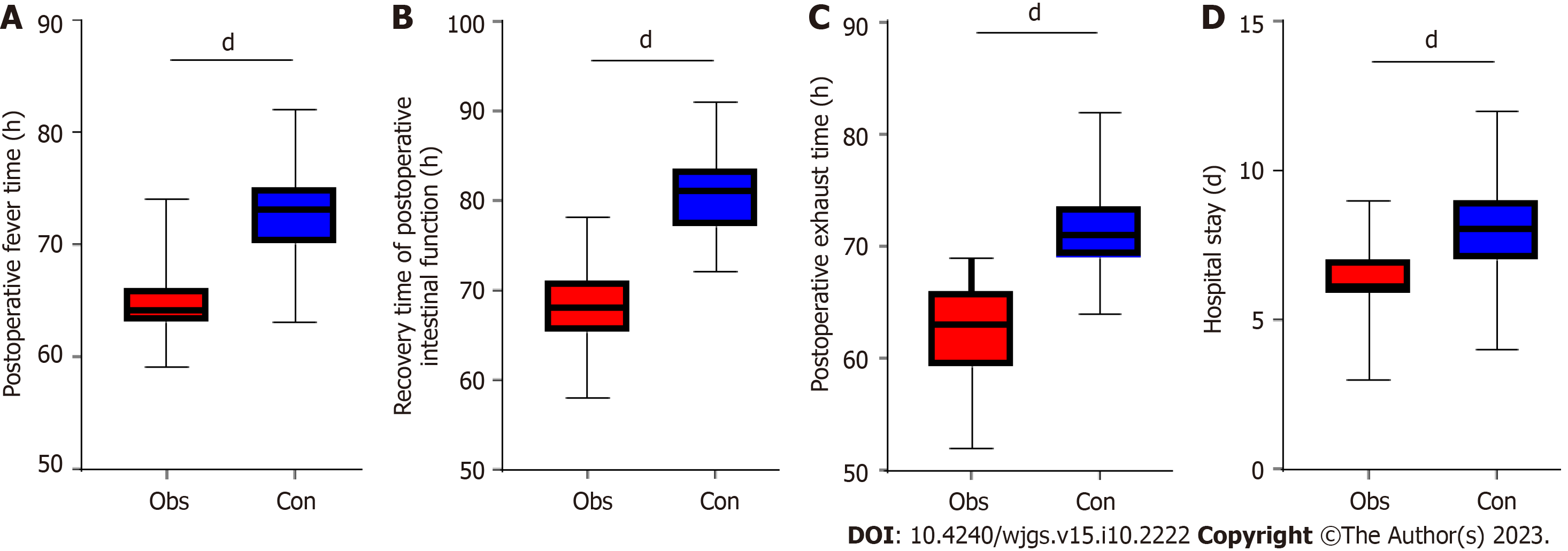
In this study we also compared the clinical indicators of the two groups of patients after treatment. Our results found that the time to postoperative fever, time to recovery of postoperative bowel function, time to postoperative evacuation, and time to hospitalization were all higher in the control group than in the observation group (P < 0.001; Figure 1).
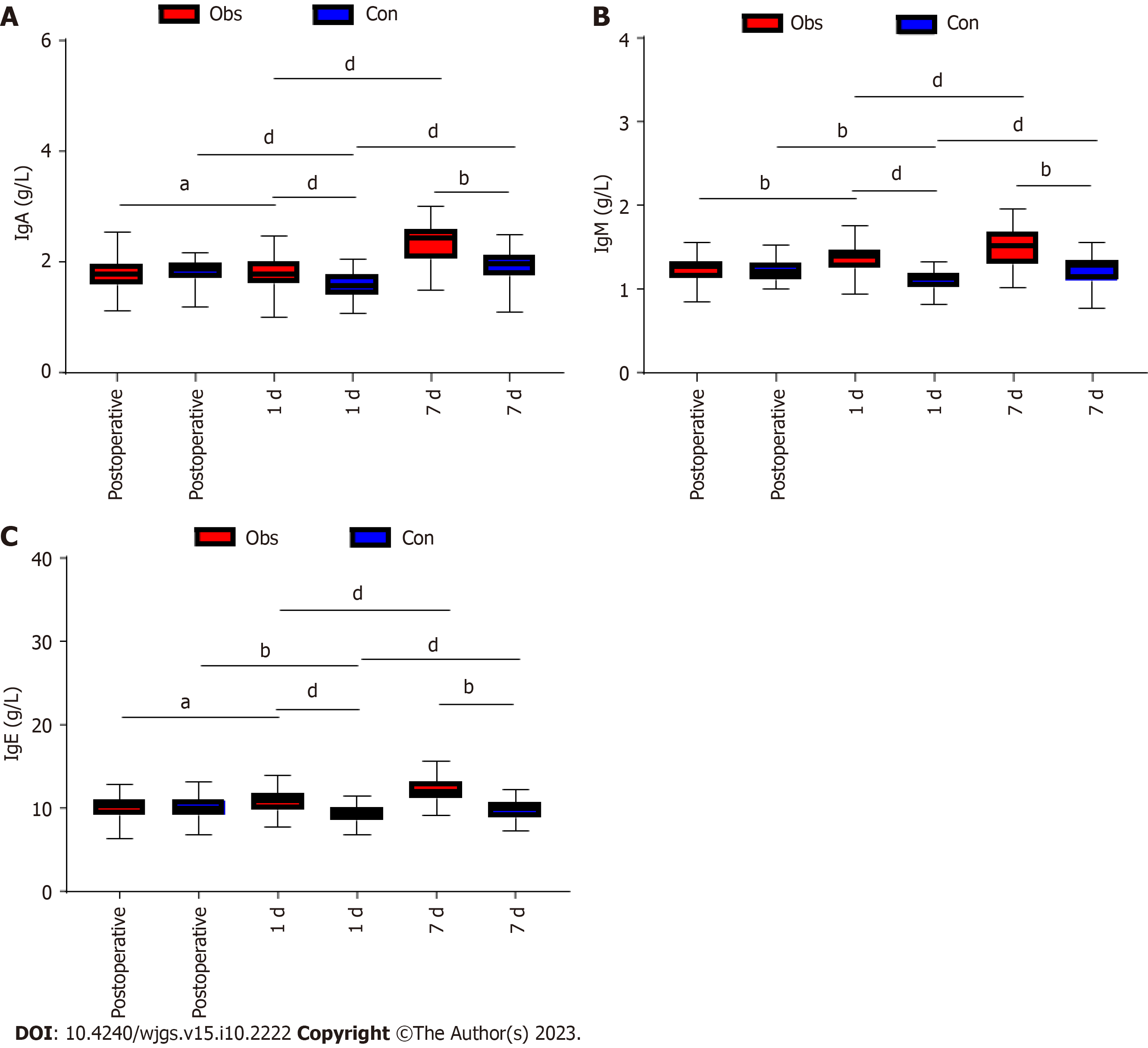
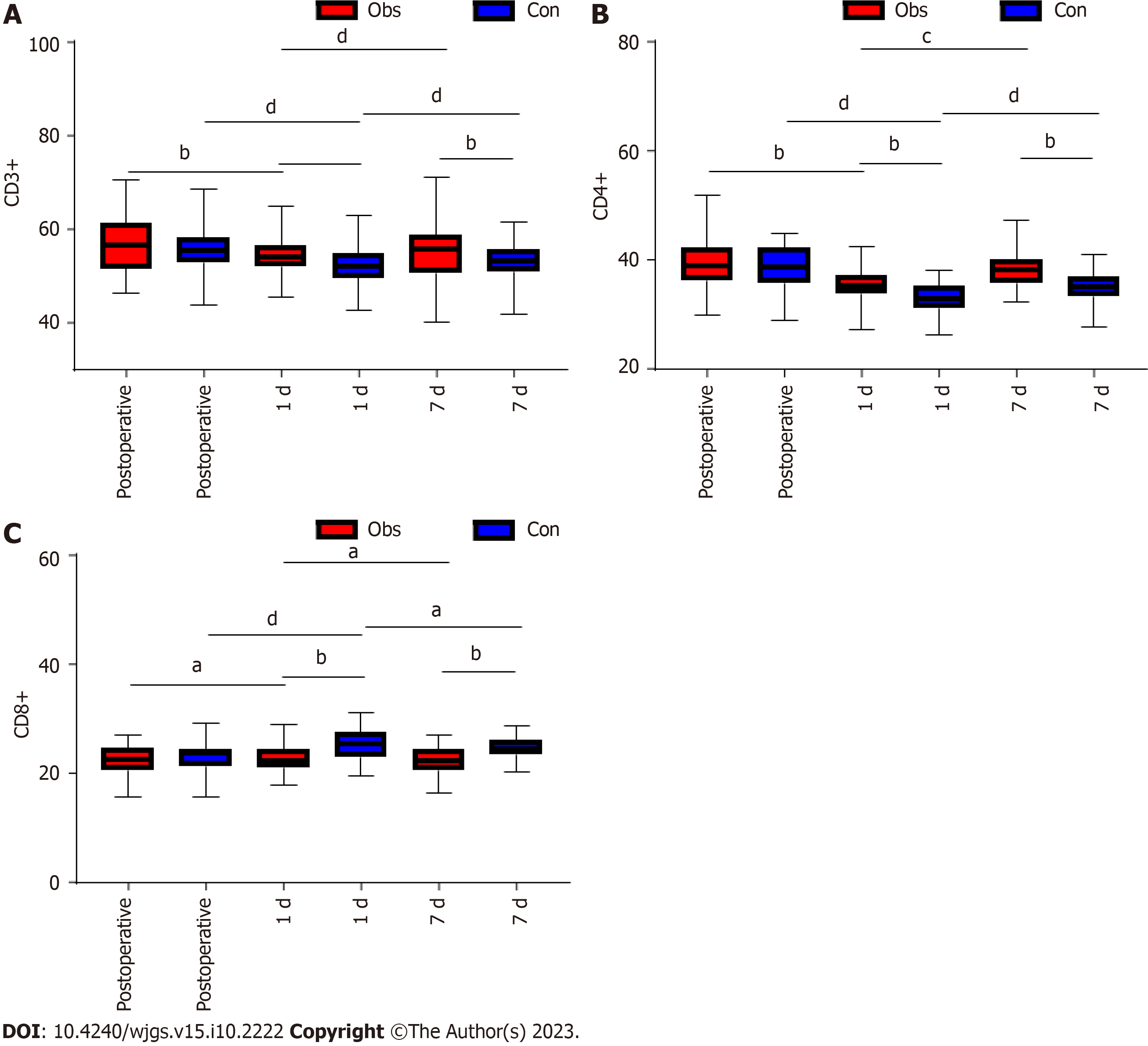
In this study, we compared the immune function of the two groups of patients. We found that there was no difference in CD3+, CD4+, CD8+, IgA, IgM, and IgG between the two groups of patients before surgery (P > 0.05). In both groups, there was a decrease in CD3+, CD4+, IgA, IgM, and IgG and an increase in CD8+ at 1 d postoperatively compared to the preoperative period (P < 0.05; Figures 2 and 3). However, further comparison revealed that CD3+, CD4+, IgA, IgM, and IgG levels were significantly higher in the observation group than in the control group at 1 d postoperatively, while CD8+ was lower than in the control group (P < 0.05; Figures 2 and 3). At the 7th postoperative day, CD3+, CD4+, IgA, IgM, and IgG levels increased in both groups, while CD8+ decreased in both groups (P < 0.05; Figures 2 and 3). In addition, the levels of CD3+, CD4+, IgA, IgM, and IgG in the observation group were significantly higher than those in the control group at 7 d postoperatively compared with those at 1 d, while CD8+ was lower than that in the control group (P < 0.05; Figures 2 and 3). Further comparison revealed that the CD3+, CD4+, IgA, IgM, and IgG levels in the observation group were significantly higher than those in the control group at 7 d postoperatively, while CD8+ was lower than that in the control group (P < 0.05; Figures 2 and 3).
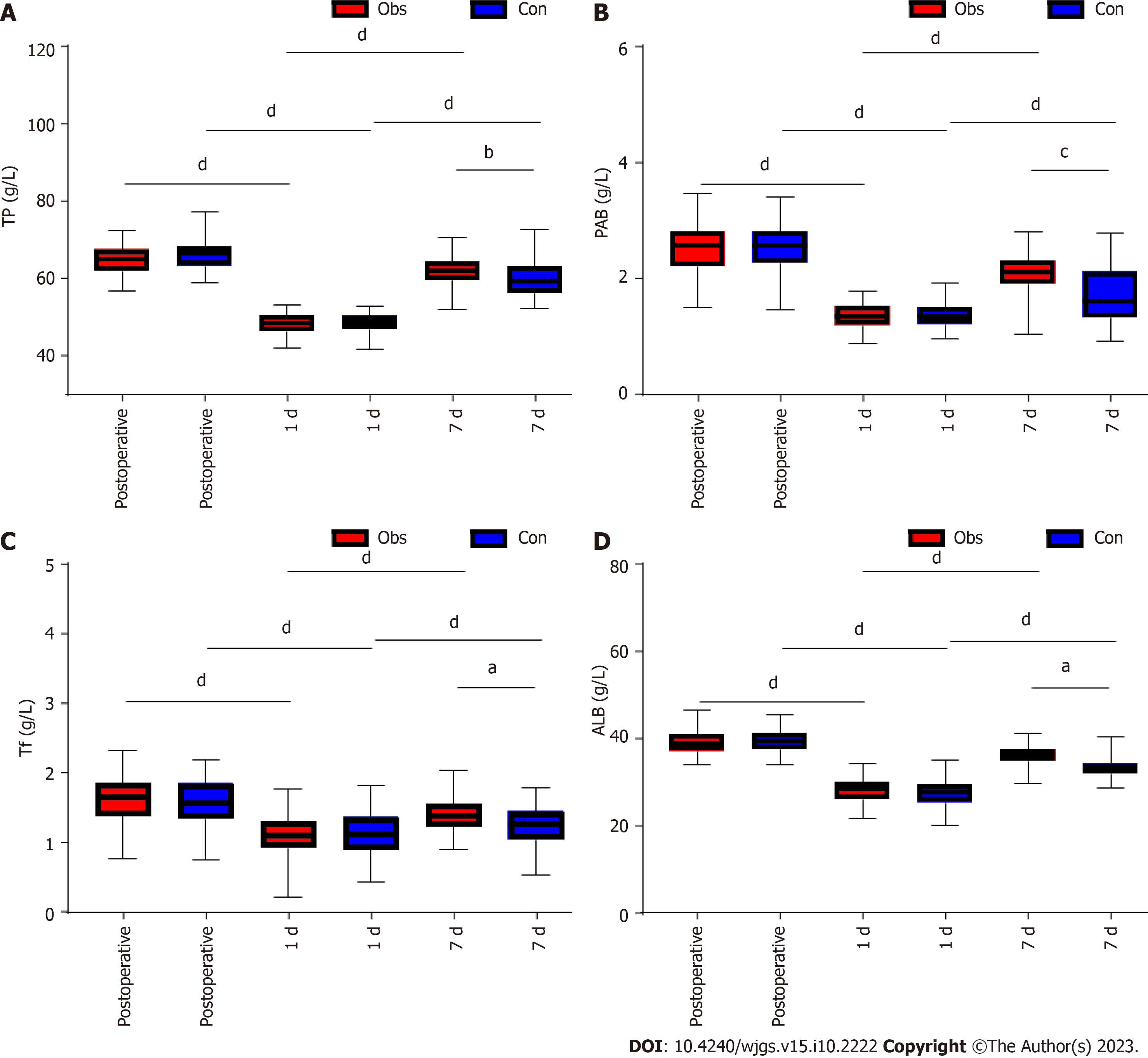
In this study we compared the nutritional function of the two groups of patients. Our comparison revealed no difference in preoperative TP, ALB, PAB, and Tf between the two groups (P > 0.05). TP, ALB, PAB, and Tf decreased in both groups at 1 d postoperatively compared to preoperatively (P < 0.05; Figure 4). However, further comparison revealed that TP, ALB, PAB, and Tf levels were significantly higher in the observation group than in the control group at 1 d postoperatively (P < 0.05; Figure 4). At the 7th postoperative day, TP, ALB, PAB, and Tf were all increased in both groups (P < 0.05; Figure 4). The TP, ALB, PAB, and Tf levels in the observation group were significantly higher than those in the control group at day 7 compared to day 1, while CD8+ was lower than that in the control group (P < 0.05; Figure 4). Further comparison revealed that TP, ALB, PAB,, and Tf levels in the observation group were significantly higher than those in the control group at 7 d postoperatively (P < 0.05; Figure 4).
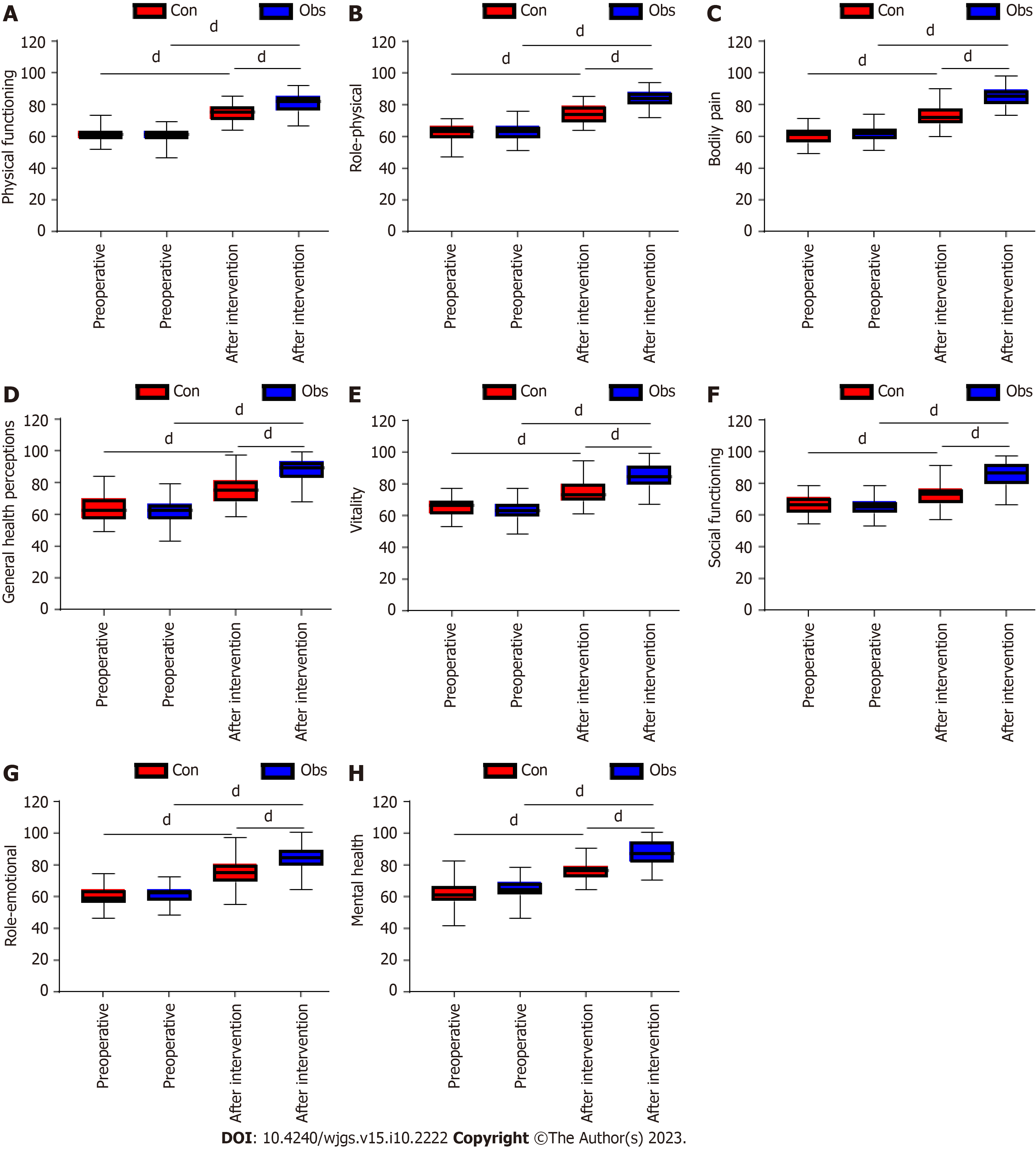
The quality of life was assessed between the two groups of patients before surgery and before discharge. The results showed that there was no difference in the preoperative SF-36 scores between the two groups (P > 0.05), and the SF-36 scores of patients in both groups increased significantly after the intervention compared with those before the intervention (P < 0.0001). The SF-36 score was significantly higher in the observation group than in the control group before discharge (P < 0.0001; Figure 5).
Statistics on adverse reactions after treatment in both groups showed that the overall incidence of adverse reactions after intervention was significantly lower in the control group than in the observation group (P = 0.021; Table 2).
| Groups | Gastrointestinal symptoms | Incisional infection | Anastomotic leak | Incidence |
| Observation group, n = 53 | 4 | 3 | 2 | 9 |
| Control group, n = 68 | 1 | 1 | 1 | 3 |
| χ2 | 5.267 | |||
| P value | 0.021 |
The nutritional management of patients with gastrointestinal tract tumors remains a difficult issue globally. Studies have shown that these patients are at very high nutritional risk and that patients suffer from concomitant malnutrition[17]. The stomach and intestines are important components of the digestive system. They play an important role in the digestion and absorption of nutrients, and tumor invasion can severely interfere with these normal digestive and absorption functions[18]. Surgery is the main treatment modality, but it is also associated with significant surgical trauma, long recovery times, and the absence of parts of the gastrointestinal tract[19]. These problems, together with the metabolic disorders caused by the tumor, can affect the nutritional status of the patient.
Patient digestive and metabolic capacity is reduced as a result of the disease, and they eat less, which in turn leads to a decrease in immune function[20]. This immunosuppression is more likely to occur in postsurgical patients due to their inability to eat normally[21]. Therefore, it is crucial to provide professional nutritional support after surgery for patients with gastrointestinal tumors, not only to improve the safety of surgery but also to speed up the recovery of the disease. In this study, we found that patients in the observation group who received the early enteral nutrition support protocol had significantly lower postoperative fever time, postoperative bowel function recovery time, postoperative exhaustion time, and hospital stay than the control group who did not receive the protocol. But, the overall incidence of postoperative complications was significantly lower in the control group than in the observation group.
These findings suggest that early enteral nutrition support programs can accelerate disease recovery. This finding is in line with the meta-analysis by Li et al[22], which found that patients with gastric cancer combined with diabetes were more effective in maintaining glycemic stability after early enteral nutrition intervention compared to those receiving total parenteral nutrition intervention, resulting in better outcomes. Another study conducted by Yan et al[23] also pointed out that early enteral nutritional support after surgery for patients with gastrointestinal tract tumors significantly reduced the incidence of postoperative complications and shortened the length of hospital stay. However, our study found that the early enteral nutrition program increased the incidence of postoperative complications in patients with gastric cancer. The reason for this may be related to the risk of early enteral nutrition and the patient's condition. Specifically, early enteral nutrition may cause excessive enteral nutrition and increase gastrointestinal symptoms such as diarrhea; at the same time, early enteral nutrition also carries risk of complications such as infection, bleeding and intestinal obstruction. In addition, considering the large surgical wound produced by gastric cancer treatment, early enteral nutrition may increase the probability of serious complications such as anastomotic leakage. As for the patients themselves, all were of advanced age, a factor that increases the risk of various postoperative complications. Therefore, the results of this study suggest that early enteral nutritional support measures for gastric cancer patients should be applied with caution, and each patient’s condition and physical status should be comprehensively assessed, while close monitoring should be performed to prevent complications or promptly address any that may arise.
We believe that early enteral nutrition support protocol is effective because it helps the synthesis of visceral proteins, thus shortening the recovery time of postoperative bowel function and postoperative fever. In addition, early enteral nutrition can reduce the damage to the intestinal mucosa and is more in line with the physiological state of the body, thus reducing the impact on the circulatory system and decreasing the time of postoperative exhaustion. This effect not only helps to reduce the length of the patient’s hospital stay but also reduces the cost of treatment to a certain extent.
The immune system plays a vital role in the body. However, in serious diseases such as gastrointestinal tumors, the function of the immune system may be compromised[24]. For example, treatment modalities such as surgery, chemotherapy, or radiotherapy may lead to a reduction in immune cells, which may affect immune function[25]. In such cases, enteral nutritional support can play an important role. In the current study, we found that patients in the observation group had significantly higher levels of CD3+, CD4+, IgA, IgM, and IgG and lower levels of CD8+ than the control group at postoperative day 1 and day 7, suggesting that early enteral nutritional support can maintain the stability of immune function in patients.
Previously, in a study by Wang et al[26], early enteral nutritional support was found to improve immune function in patients after radical chemotherapy gastric cancer to reduce the occurrence of postoperative complications. This is largely consistent with our study. We hypothesize this is due to the immune system requiring high amounts of energy and nutrients, including protein, fat, carbohydrates, vitamins, and minerals, to function properly. Early enteral nutritional support ensures that patients receive these essential nutrients to maintain and enhance the function of the immune system. Early enteral nutritional support can protect the function of the intestinal tract from damage caused by tumor treatment (e.g., surgery, chemotherapy) and maintain the immune function of the intestinal tract. Early enteral nutritional support can speed up postoperative recovery and reduce surgical complications, such as infection and delayed wound healing.
At the end of the study we analyzed the nutritional function and quality of life of patients in both groups. In our results, TP, ALB, PAB, and Tf levels in the observation group were not different from those in the control group before and 1 d after the intervention, but at 7 d after the intervention TP, ALB, PAB and Tf were higher than those in the control group. These results suggested that early enteral nutrition support can improve nutritional function and enhance quality of life. This is because early enteral nutrition support, in line with the natural physiological characteristics of human diet, is beneficial to the growth of intestinal mucosal cells and maintains the integrity of the intestinal mucosal barrier, thus ensuring the normal intake of nutrients[27-29]. The application of this nutritional support strategy can rapidly provide patients with the necessary nutrients to effectively improve their nutritional status, enhance their body protein content, and improve their negative nitrogen balance. Effective psychological care by nursing staff also plays an important role, which helps to improve the emotional state of patients, their confidence in treatment, and their resistance to the disease. This holistic approach to care, which includes nutritional and psychological support, provides strong support for the patient’s full recovery.
In this study, we determined the value of early enteral nutritional support in patients with gastrointestinal tumors, but there are still limitations to this study. First, we did not obtain long-term prognostic data in this study, and more experimental data are needed to verify whether early enteral nutrition support has an effect on patient prognosis. Second, this study is a single-center retrospective study, and the sample size was small. Therefore, more data are needed to support whether it is representative. Finally, we anticipate that more clinical studies will be conducted in subsequent studies to refine our findings.
We found that patients with gastrointestinal tumors are nutritionally vulnerable, and early enteral nutrition support programs can improve the nutritional status of patients and speed up postoperative recovery. This program can not only improve the immune function of the patient and protect the intestinal function but also help to improve the quality of life of the patient. However, this program will increase the incidence of complications in patients. Caution should be taken when adopting early enteral nutrition support measures for patients with gastric cancer. Each patient's condition and physical status should be comprehensively evaluated and closely monitored with the aim of preventing the possible complications.
Patients with gastrointestinal tumors often suffer from malnutrition, and surgical treatment may further affect nutrient absorption and metabolism. In this context, nutritional interventions to improve patients’ postoperative recovery and quality of life become critical. Early enteral nutrition support as a form of nutritional management can theoretically help to improve the nutritional status of patients and accelerate recovery, but its actual effectiveness needs to be supported by clinical evidence.
The postoperative nutritional management of patients with gastrointestinal tumors remains a global challenge that has a significant impact on patient recovery and overall prognosis. Our motivation was to investigate the impact of early enteral nutritional support on postoperative recovery in patients with gastrointestinal tract tumors.
The main objective of this study was to evaluate the use of early enteral nutrition support in patients undergoing surgery for gastrointestinal tract tumors and to determine how it improves postoperative complications, enhances quality of life, promotes immune function, and improves nutritional status.
In a retrospective study, we compared patients who received early enteral nutrition support with those who did not, examining the incidence of postoperative complications, time to recovery, nutritional parameters, and quality of life.
The results showed that early enteral nutrition support significantly improved recovery time and nutritional status, increased the incidence of postoperative complications, and improved quality of life.
We concluded that early enteral nutrition support plays a key role in the postoperative recovery of patients with surgically treated gastrointestinal tract tumors, suggesting that the importance of early enteral nutrition support in the postoperative management of these patients should not be overlooked.
Further research is needed to examine the impact of early enteral nutrition support on the long-term prognosis of patients with gastrointestinal tumors and its potential application in a broader clinical context. We look forward to future clinical studies that will provide more data and insight into this area.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Praktiknjo M, Germany; Ren-Fielding CJ, United States S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Miani C, Wandschneider L, Batram-Zantvoort S, Covi B, Elden H, Nedberg IH, Drglin Z, Pumpure E, Costa R, Rozée V, Otelea MR, Drandić D, Radetic J, Abderhalden-Zellweger A, Ćerimagić A, Arendt M, Mariani I, Linden K, Ponikvar BM, Jakovicka D, Dias H, Ruzicic J, de Labrusse C, Valente EP, Zaigham M, Bohinec A, Rezeberga D, Barata C, Pfund A, Sacks E, Lazzerini M; IMAgiNE EURO study group. Individual and country-level variables associated with the medicalization of birth: Multilevel analyses of IMAgiNE EURO data from 15 countries in the WHO European region. Int J Gynaecol Obstet. 2022;159 Suppl 1:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Roy PS, Saikia BJ. Cancer and cure: A critical analysis. Indian J Cancer. 2016;53:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 122] [Reference Citation Analysis (0)] |
| 3. | Dyba T, Randi G, Bray F, Martos C, Giusti F, Nicholson N, Gavin A, Flego M, Neamtiu L, Dimitrova N, Negrão Carvalho R, Ferlay J, Bettio M. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 370] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 4. | Mantese G. Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol. 2019;35:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 5. | Zhao Y, Li J, Li D, Wang Z, Zhao J, Wu X, Sun Q, Lin PP, Plum P, Damanakis A, Gebauer F, Zhou M, Zhang Z, Schlösser H, Jauch KW, Nelson PJ, Bruns CJ. Tumor biology and multidisciplinary strategies of oligometastasis in gastrointestinal cancers. Semin Cancer Biol. 2020;60:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16:282-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 400] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 7. | Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018;153:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 8. | Groh-Wargo S, Barr SM. Parenteral Nutrition. Clin Perinatol. 2022;49:355-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Simmer K, Rakshasbhuvankar A, Deshpande G. Standardised parenteral nutrition. Nutrients. 2013;5:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients. 2013;5:608-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Fuentes Padilla P, Martínez G, Vernooij RW, Urrútia G, Roqué I Figuls M, Bonfill Cosp X. Early enteral nutrition (within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Bielawska B, Allard JP. Parenteral Nutrition and Intestinal Failure. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Tian F, Heighes PT, Allingstrup MJ, Doig GS. Early Enteral Nutrition Provided Within 24 Hours of ICU Admission: A Meta-Analysis of Randomized Controlled Trials. Crit Care Med. 2018;46:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Jordan EA, Moore SC. Enteral nutrition in critically ill adults: Literature review of protocols. Nurs Crit Care. 2020;25:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Srinivasan V, Hasbani NR, Mehta NM, Irving SY, Kandil SB, Allen HC, Typpo KV, Cvijanovich NZ, Faustino EVS, Wypij D, Agus MSD, Nadkarni VM; Heart and Lung Failure-Pediatric Insulin Titration (HALF-PINT) Study Investigators. Early Enteral Nutrition Is Associated With Improved Clinical Outcomes in Critically Ill Children: A Secondary Analysis of Nutrition Support in the Heart and Lung Failure-Pediatric Insulin Titration Trial. Pediatr Crit Care Med. 2020;21:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 631] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 17. | Ding P, Guo H, Sun C, Yang P, Tian Y, Liu Y, Zhang Z, Wang D, Zhao X, Tan B, Li Y, Zhao Q. Relationship Between Nutritional Status and Clinical Outcome in Patients With Gastrointestinal Stromal Tumor After Surgical Resection. Front Nutr. 2022;9:818246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, Shi YY, Hu W, Ba Y, Li SY, Li ZN, Wang KH, Wu J, He Y, Yang JJ, Xie CH, Song XX, Chen GY, Ma WJ, Luo SX, Chen ZH, Cong MH, Ma H, Zhou CL, Wang W, Luo Q, Shi YM, Qi YM, Jiang HP, Guan WX, Chen JQ, Chen JX, Fang Y, Zhou L, Feng YD, Tan RS, Li T, Ou JW, Zhao QC, Wu JX, Deng L, Lin X, Yang LQ, Yang M, Wang C, Song CH, Xu HX, Shi HP; Investigation on the Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer. 2020;28:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | Deftereos I, Kiss N, Isenring E, Carter VM, Yeung JM. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur J Surg Oncol. 2020;46:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Cao J, Xu H, Li W, Guo Z, Lin Y, Shi Y, Hu W, Ba Y, Li S, Li Z, Wang K, Wu J, He Y, Yang J, Xie C, Zhou F, Song X, Chen G, Ma W, Luo S, Chen Z, Cong M, Ma H, Zhou C, Wang W, Qi Luo, Qi Y, Jiang H, Guan W, Chen J, Fang Y, Zhou L, Feng Y, Tan R, Ou J, Zhao Q, Xin Lin, Yang L, Fu Z, Wang C, Deng L, Li T, Song C, Shi H; Investigation on Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group, Chinese Society of Nutritional Oncology. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr Probl Cancer. 2021;45:100638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Carrillo Lozano E, Osés Zárate V, Campos Del Portillo R. Nutritional management of gastric cancer. Endocrinol Diabetes Nutr (Engl Ed). 2021;68:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Li K, Wang D, Zhang X, Yang J, Chen X. Efficacy of early enteral nutrition versus total parenteral nutrition for patients with gastric cancer complicated with diabetes mellitus: A systematic review and meta-analysis. Nutr Diet. 2022;79:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Yan X, Zhou FX, Lan T, Xu H, Yang XX, Xie CH, Dai J, Fu ZM, Gao Y, Chen LL. Optimal postoperative nutrition support for patients with gastrointestinal malignancy: A systematic review and meta-analysis. Clin Nutr. 2017;36:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Wu GH. [Application of immune nutrition in patients with gastrointestinal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:1021-1024. [PubMed] |
| 25. | Cheng Y, Zhang J, Zhang L, Wu J, Zhan Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Wang J, Wang L, Zhao M, Zuo X, Zhu W, Cui K, Yan X, Liu X. Effect of Early Enteral Nutrition Support Combined with Chemotherapy on Related Complications and Immune Function of Patients after Radical Gastrectomy. J Healthc Eng. 2022;2022:1531738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Shen Y, Zhou Y, He T, Zhuang X. Effect of Preoperative Nutritional Risk Screening and Enteral Nutrition Support in Accelerated Recovery after Resection for Esophageal Cancer. Nutr Cancer. 2021;73:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Jankowski M, Las-Jankowska M, Sousak M, Zegarski W. Contemporary enteral and parenteral nutrition before surgery for gastrointestinal cancers: a literature review. World J Surg Oncol. 2018;16:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Zheng R, Devin CL, Pucci MJ, Berger AC, Rosato EL, Palazzo F. Optimal timing and route of nutritional support after esophagectomy: A review of the literature. World J Gastroenterol. 2019;25:4427-4436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |