Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.809
Peer-review started: April 19, 2022
First decision: June 10, 2022
Revised: July 2, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 27, 2022
Processing time: 127 Days and 2.6 Hours
Mesenteric ischemia is significantly more common in end-stage kidney disease patients undergoing chronic dialysis than in the general population and is associated with high morbidity and mortality. However, reports on prognostic factors in this population are limited.
To elucidate the in-hospital outcomes of acute mesenteric ischemia in chronic dialysis patients and to analyze protective factors for survival.
The case data of 426 chronic dialysis patients who were hospitalized in a tertiary medical center for acute mesenteric ischemia over a 14-year period were retrospectively reviewed. Of these cases, 103 were surgically confirmed, and the patients were enrolled in this study. A Cox regression analysis was used to evaluate the protective factors for survival.
The in-hospital mortality rate among the 103 enrolled patients was 46.6%. Univariate analysis was performed to compare factors in survivors and nonsurvivors, with better in-hospital outcomes associated with a surgery delay (defined as the time from onset of signs and symptoms to operation) < 4.5 d, no shock, a higher potassium level on day 1 of hospitalization, no resection of the colon, and a total bowel resection length < 110 cm. After 1 wk of hospitalization, patients with lower white blood cell count and neutrophil counts, higher lymphocyte counts, and lower C-reactive protein levels had better in-hospital outcomes. Following multivariate adjustment, a higher potassium level on day 1 of hospitalization (HR 1.71, 95%CI 1.19 to 2.46; P = 0.004), a lower neutrophil count (HR 0.91, 95%CI 0.84 to 0.99; P = 0.037) at 1 wk after admission, resection not involving the colon (HR 2.70, 95%CI 1.05 to 7.14; P = 0.039), and a total bowel resection length < 110 cm (HR 4.55, 95%CI 1.43 to 14.29; P = 0.010) were significantly associated with survival.
A surgery delay < 4.5 d, no shock, no resection of the colon, and a total bowel resection length < 110 cm predicted better outcomes in chronic dialysis patients with acute mesenteric ischemia.
Core Tip: One hundred and three chronic dialysis patients with surgically confirmed acute mesenteric ischemia in a tertiary medical center over 14 years were retrospectively analyzed. Demographic data and clinical characteristics were compared between in-hospital survivors and nonsurvivors. Cox regression analysis was used to evaluate the protective factors for survival. Only 53.4% of the patients survived the index admission, and a surgery delay < 4.5 d, no shock, no resection of the colon, and a total bowel resection length < 110 cm predicted better outcomes in chronic dialysis patients with mesenteric ischemia.
- Citation: Liau SK, Kuo G, Chen CY, Lu YA, Lin YJ, Lee CC, Hung CC, Tian YC, Hsu HH. Identifying survival protective factors for chronic dialysis patients with surgically confirmed acute mesenteric ischemia. World J Gastrointest Surg 2022; 14(8): 809-820
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/809.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.809
Mesenteric ischemia is significantly more common in end-stage kidney disease (ESKD) patients undergoing chronic dialysis than in the general population and is associated with high morbidity and mortality. In chronic dialysis patients, mesenteric ischemia occurs in approximately 0.3%-1.9% of patients annually[1,2], whereas mesenteric ischemia is rare in the general population, with a frequency of 0.09%-2.0% per patient annually[3,4]. The nonocclusive type of mesenteric ischemia (NOMI) is a predominant feature in dialysis patients[5-8] and results from splanchnic hypoperfusion, vasoconstriction, and ischemia–reperfusion injury[9]. Previous investigations have reported mortality rates reaching 45% to 73%[2,5,6,10] in hemodialysis patients. However, reports on prognostic factors in this population are limited.
Acute mesenteric ischemia is usually surgically managed, and early surgical intervention is thought to favor NOMI survival in nondialysis patients. In an analysis of 54 nondialysis patients with mesenteric ischemia who underwent surgery, Duran et al[11] demonstrated a significantly worse prognosis in patients over 70 years of age and a higher mortality rate among those with delayed surgery, defined as the time from admission to surgery being > 24 h compared with ≤ 24 h. Aliosmanoglu et al[12] retrospectively analyzed 95 nondialysis patients who underwent emergent surgery for mesenteric ischemia and reported that advanced age, high leukocyte levels, a duration from the onset of symptoms to the operation of more than 24 h, and colon involvement had negative effects on the mortality rate. Similarly, among nondialysis patients, Acosta-Merida et al[13] found that age, time to surgery, shock, and acidosis significantly increased the risk of mortality due to acute mesenteric ischemia, whereas intestinal resection had a protective effect. A recent systematic review and meta-analysis analyzed 10425 patients with acute mesenteric ischemia and concluded that age, chronic renal disease, diabetes, patient dependency, arrhythmias, cardiac failure, hypotension, large bowel involvement, small and large bowel involvement, creatinine, lactate, delay to surgery, and inotropes were significantly associated with mortality, while anticoagulants, revascularization and bowel thickening on computerized tomography were associated with decreased mortality[14]. However, the in-hospital prognostic factors for survival among chronic dialysis patients with acute mesenteric ischemia are not well established. Moreover, the effect of bowel resection length, as the most important precipitating factor of short bowel syndrome, on the in-hospital survival of chronic dialysis patients with mesenteric ischemia has not been elucidated.
This retrospective study sought to identify the protective factors for mesenteric ischemia in chronic dialysis patients to promote earlier initiation of aggressive therapy in this targeted population and improve their poor prognosis.
The medical records of chronic dialysis patients who had been admitted to a tertiary medical center for mesenteric ischemia between January 2002 and December 2015 were retrospectively reviewed. The diagnosis of mesenteric ischemia was defined using the International Classification of Diseases, Ninth Revision, Clinical Modification codes 5570, 5571 and 5579 during the index admission. In total, 426 chronic dialysis patients with acute mesenteric ischemia were identified over a 14-year period. Of these patients, 103 received a surgically confirmed diagnosis and were therefore enrolled in this study. The study was approved by the Institutional Review Board of Chang Gung Medical Foundation (approval number: 202001647B0), which waived the requirement for written informed consent from each participant because personal information was anonymized for this study.
Baseline characteristics, including sex, age, body weight/height, ESKD-associated comorbidities (diabetes mellitus, hypertension, coronary artery disease, heart failure, atrial fibrillation, history of prior stroke, peripheral artery disease, cirrhosis, peptic ulcer disease, chronic obstructive pulmonary disease, malignancy, and immunosuppressive status), left ventricular ejection fraction (EF), and modality of renal replacement therapy were retrieved. For each patient, surgery delay, defined as the time from the onset of signs and symptoms to surgery, and complications during admission (shock, respiratory failure) were documented. The results of blood examinations upon admission and on day 7 of hospitalization were recorded. The etiology of mesenteric ischemia and the bowel resection sites and length were also documented. Each patient was followed for 3 years from the time of admission or until death.
This investigation was a retrospective cohort study. Demographic data and clinical information are presented as means ± SD and counts (%) for categorical data. The t test or chi-square test was used to compare continuous or categorical variables between survivors and nonsurvivors.
In the univariate and multivariate analyses, Cox regression analysis was used to identify the protective factors for in-hospital survival. Variables that were determined to be significant in the univariate analysis were calculated. Kaplan–Meier survival curves were plotted for groups with a surgery delay < 4.5 d or more, resection involving the colon or not, and total bowel resection length < 110 cm or more. We used the predictive model of classification and regression tree to define a cutoff value of 4.5 days for surgery delay and 110 cm for total bowel resection length.
R 3.0.2 statistical analysis software (Copyright the R Foundation for Statistical Computing, Vienna, Austria) was used. All reported P values were two-sided, and P < 0.05 was considered to indicate statistical significance.
Of the 426 chronic dialysis patients who were hospitalized with mesenteric ischemia, 103 patients whose diagnosis was surgically confirmed were enrolled in this study. The mean age was 68.3 ± 11.3 years, and the male-to-female ratio was 1:1.64 (Table 1). The distributions of age and sex did not differ between survivors and nonsurvivors. The number of patients who survived hospitalization was 55 (53.4%), and the number who did not survive hospitalization was 48 (46.6%). The average age of those who survived hospitalization was 68.5 ± 10.6 years, and that of those who did not survive hospitalization was 68.0 ± 12.3 years (P = 0.811). Among the chronic dialysis patients with acute mesenteric ischemia, 63.1% had hypertension, 54.4% had diabetes, 23.3% had peptic ulcer disease, 17.5% had coronary artery disease, 14.6% suffered a prior stroke, 12.6% had malignancy, 10.7% had heart failure, 9.7% had peripheral artery occlusive disease, 4.9% had atrial fibrillation, 2.9% had cirrhosis, 2.9% had chronic obstructive airway disease, and 1.9% had an immunosuppressed status. Hypertension and diabetes mellitus were the two most common comorbidities. No significant differences in baseline comorbidities existed between in-hospital survivors and nonsurvivors. Overall, 100 (97.1%) patients underwent hemodialysis, 8 (7.8%) underwent peritoneal dialysis, and 5 (4.9%) of 103 chronic dialysis patients underwent both hemodialysis and peritoneal dialysis. The frequencies of peritoneal dialysis as a renal replacement therapy modality differed significantly between in-hospital survivors (12.5%, n = 1) and nonsurvivors (87.5%, n = 7; P = 0.024), but the frequencies of hemodialysis did not.
| Variable | Total (n = 103) | Survival (n = 55) | Death (n = 48) | P value |
| Age (yr) (mean ± SD) | 68.3 ± 11.3 | 68.5 ± 10.6 | 68.0 ± 12.3 | 0.811 |
| BMI | 23.8 ± 3.7 | 23.5 ± 2.9 | 24.3 ± 4.6 | 0.323 |
| Sex, n (%) | 0.495 | |||
| Male | 39 (37.9) | 23 (59.0) | 16 (41.0) | |
| Female | 64 (62.1) | 32 (50.0) | 32 (50.0) | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 56 (54.4) | 32 (57.1) | 24 (42.9) | 0.527 |
| Hypertension | 65 (63.1) | 35 (53.8) | 30 (46.2) | 1.000 |
| Coronary artery disease | 18 (17.5) | 10 (55.6) | 8 (44.4) | 1.000 |
| Heart failure | 11 (10.7) | 6 (54.5) | 5 (45.5) | 1.000 |
| Atrial fibrillation | 5 (4.9) | 2 (40.0) | 3 (60.0) | 0.662 |
| Prior stroke | 15 (14.6) | 10 (66.7) | 5 (33.3) | 0.404 |
| Peripheral arterial occlusive disease | 10 (9.7) | 4 (40.0) | 6 (60.0) | 0.508 |
| Cirrhosis | 3 (2.9) | 1 (33.3) | 2 (66.7) | 0.597 |
| Peptic ulcer disease | 24 (23.3) | 11 (45.8) | 13 (54.2) | 0.539 |
| Chronic obstructive pulmonary disease | 3 (2.9) | 1 (33.3) | 2 (66.7) | 0.597 |
| Malignancy | 13 (12.6) | 8 (61.5) | 5 (38.5) | 0.740 |
| Immunosuppressive status | 2 (1.9) | 1 (50.0) | 1 (50.0) | 1.000 |
| RRT modality | ||||
| Hemodialysis | 100 (97.1) | 55 (55.0) | 45 (45.0) | 0.098 |
| Peritoneal dialysis | 8 (7.8) | 1 (12.5) | 7 (87.5) | 0.024a |
The average surgery delay, defined as the time from the onset of signs and symptoms to surgery, was 2.6 ± 3.1 d, without a significant difference between in-hospital survivors (2.3 ± 2.8 d) and nonsurvivors (2.9 ± 3.5 d; P = 0.296) (Table 2). The frequencies of shock defined as vasopressor or inotrope use during hospitalization, including norepinephrine, dopamine, and vasopressin (47.1% survivors vs 52.9% nonsurvivors; P < 0.007), significantly differed between the two groups. Patient hemogram and biochemical data on days 1 and 7 of hospitalization were recorded. On the first day of admission, the white blood cell (WBC) count was significantly lower (11.69 ± 5.49 k/μL vs 14.21 ± 6.74 k/μL, P = 0.041), and the serum potassium level was significantly higher (4.71 ± 1.08 g/dL vs 4.19 ± 0.89 g/dL; P < 0.008) in survivors than in nonsurvivors. On day 7 of hospitalization, a lower WBC count (10.05 ± 5.04 k/μL vs 13.96 ± 8.19 k/μL; P = 0.004) and a lower C-reactive protein (CRP) level (119.34 ± 81.27 mg/L vs 191.94 ± 82.54 mg/L; P = 0.000) were associated with higher in-hospital survival.
| Characteristics | Total (n = 103) | Survival (n = 55) | Death (n = 48) | P value |
| Surgery delay (d) (mean ± SD) | 2.6 ± 3.1 | 2.3 ± 2.8 | 2.9 ± 3.5 | 0.296 |
| Complications, n (%) | ||||
| Shock | 87 (84.5) | 41 (47.1) | 46 (52.9) | 0.007a |
| Laboratory data | ||||
| Hospital day 1 | ||||
| WBC (k/μL) | 12.86 ± 6.21 | 11.69 ± 5.49 | 14.21 ± 6.74 | 0.041a |
| Hemoglobin (g/dL) | 11.06 ± 2.40 | 11.22 ± 2.30 | 10.88 ± 2.53 | 0.476 |
| Platelet (k/μL) | 195.47 ± 76.10 | 189.76 ± 65.39 | 202.00 ± 87.03 | 0.418 |
| PMN (%) | 82.36 ± 10.74 | 80.87 ± 11.59 | 84.08 ± 9.50 | 0.126 |
| Lymphocytes (%) | 9.28 ± 6.10 | 10.12 ± 6.34 | 8.32 ± 5.72 | 0.132 |
| CRP (mg/L) | 180.12 ± 138.86 | 167.23 ± 136.09 | 193.60 ± 142.03 | 0.377 |
| Potassium (mEq/L) | 4.47 ± 1.02 | 4.71 ± 1.08 | 4.19 ± 0.89 | 0.008a |
| Albumin (g/dL) | 2.82 ± 0.59 | 2.91 ± 0.41 | 2.70 ± 0.74 | 0.080 |
| Total bilirubin (mg/dL) | 0.86 ± 0.61 | 0.78 ± 0.34 | 0.94 ± 0.79 | 0.230 |
| Hospital day 7 | ||||
| WBC count (k/μL) | 11.87 ± 6.94 | 10.05 ± 5.04 | 13.96 ± 8.19 | 0.004a |
| Hemoglobin (g/dL) | 9.56 ± 1.74 | 9.39 ± 1.76 | 9.75 ± 1.72 | 0.297 |
| Platelets (k/μL) | 159.35 ± 94.81 | 173.94 ± 72.26 | 142.94 ± 113.62 | 0.099 |
| PMN (%) | 79.71 ± 11.77 | 78.69 ± 8.83 | 80.90 ± 14.49 | 0.347 |
| Lymphocytes (%) | 10.16 ± 8.08 | 10.19 ± 5.60 | 10.13 ± 10.33 | 0.975 |
| CRP (mg/L) | 157.71 ± 89.16 | 119.34 ± 81.27 | 191.94 ± 82.54 | 0.000a |
| Potassium (mEq/L) | 4.08 ± 0.85 | 3.94 ± 0.68 | 4.24 ± 1.00 | 0.075 |
| Albumin (g/dL) | 2.50 ± 0.43 | 2.50 ± 0.47 | 2.50 ± 0.41 | 0.977 |
| Total bilirubin (mg/dL) | 1.62 ± 1.93 | 1.11 ± 1.75 | 2.05 ± 2.00 | 0.053 |
| Echocardiographyin hospital | ||||
| LVEF | 0.65 ± 0.13 | 0.66 ± 0.11 | 0.62 ± 0.16 | 0.199 |
| Etiology of mesenteric ischemia, n (%) | ||||
| Arterial embolism | 0 (0) | 0 (0.0) | 0 (0.0) | NA |
| Arterial thrombosis | 5 (4.9) | 2 (40.0) | 3 (60.0) | 0.664 |
| Venous thrombosis | 0 (0) | 0 (0.0) | 0 (0.0) | NA |
| Nonocclusive | 97 (95.1) | 52 (53.6) | 45 (46.4) | 0.664 |
| Bowel resection site, n (%) | ||||
| Jejunum | 28 (27.5) | 12 (42.9) | 16 (57.1) | 0.302 |
| Ileum | 82 (80.4) | 48 (58.5) | 34 (41.5) | 0.041a |
| Colon | 42 (41.2) | 18 (42.9) | 24 (57.1) | 0.132 |
| Rectum | 2 (2.0) | 0 (0.0) | 2 (100.0) | 0.219 |
| Bowel resection length (cm) (mean ± SD) | ||||
| Small intestine | 65.39 ± 58.86 | 59.84 ± 48.80 | 71.64 ± 68.43 | 0.314 |
| Colon | 14.23 ± 23.93 | 11.88 ± 24.30 | 16.88 ± 23.47 | 0.294 |
| Total | 78.85 ± 58.36 | 70.41 ± 48.18 | 88.52 ± 67.43 | 0.117 |
Reduced EF, defined as an EF determined by echocardiography of less than 50% at the time of initial hospitalization, was not common in either group, and the EF did not differ significantly between survivors and nonsurvivors. NOMI (95.1%) was the most frequent etiology of acute mesenteric ischemia, followed by arterial thrombosis (4.9%). The etiology of acute mesenteric ischemia did not differ significantly between survivors and nonsurvivors. The ileum (80.4%) was the most common resection site, followed by the colon (41.2%), jejunum (27.5%), and rectum (2.0%). The frequency of resection in the ileum were significantly higher in survivors than in nonsurvivors (58.5% vs 41.5%, respectively; P = 0.041); however, the Cox regression analysis revealed that bowel resection not involving the colon was more powerful in predicting survival (see later text). The average total bowel resection lengths were 78.8 ± 58.36 cm and 65.39 ± 58.86 and 14.23 ± 23.93 cm in the small intestine and colon, respectively. The length of bowel resection did not differ significantly between the groups.
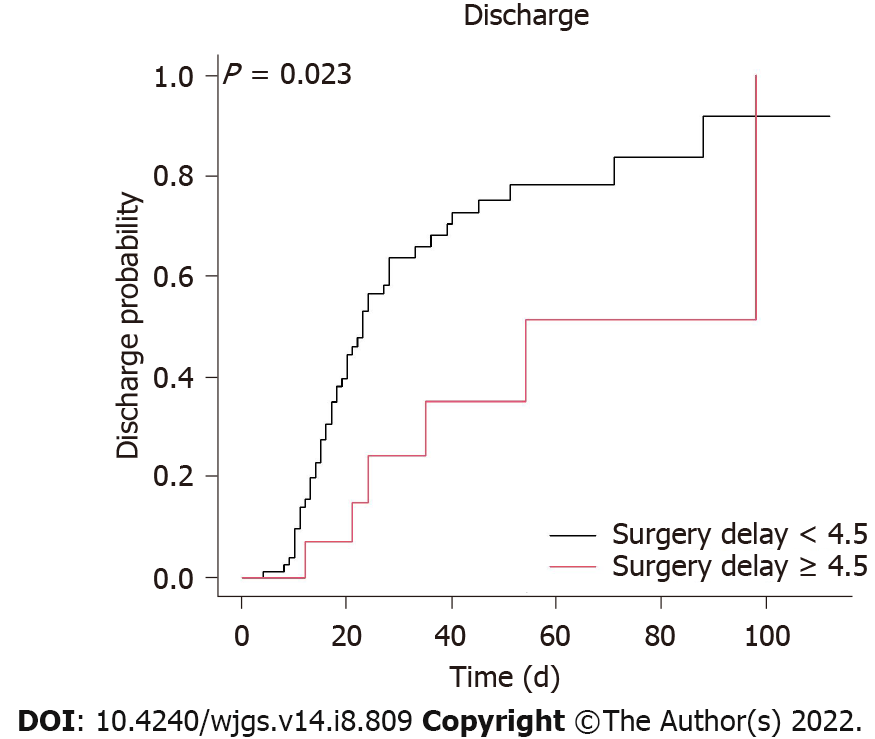
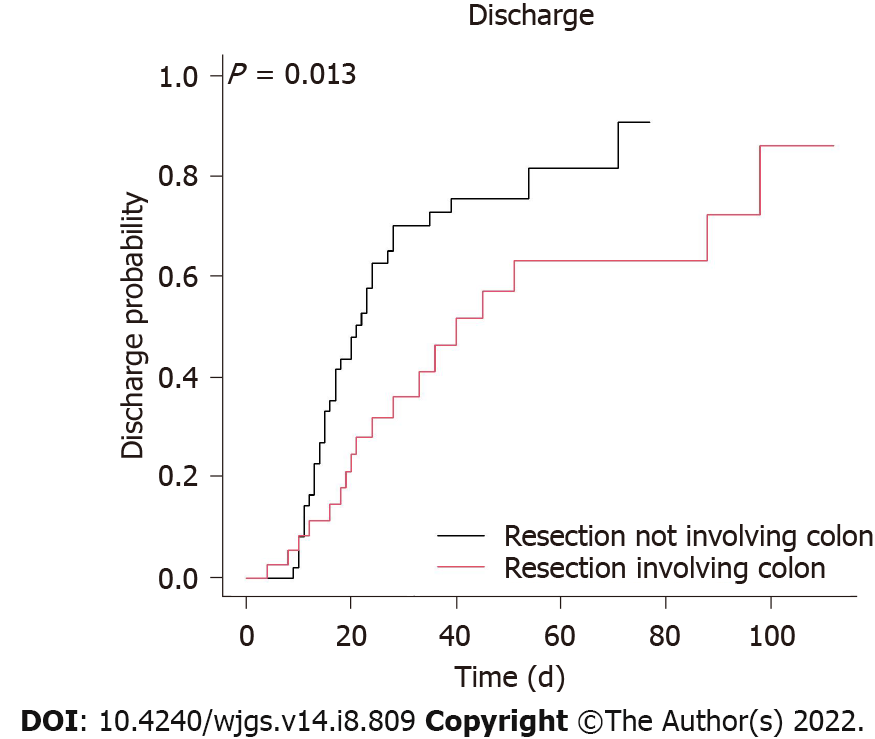
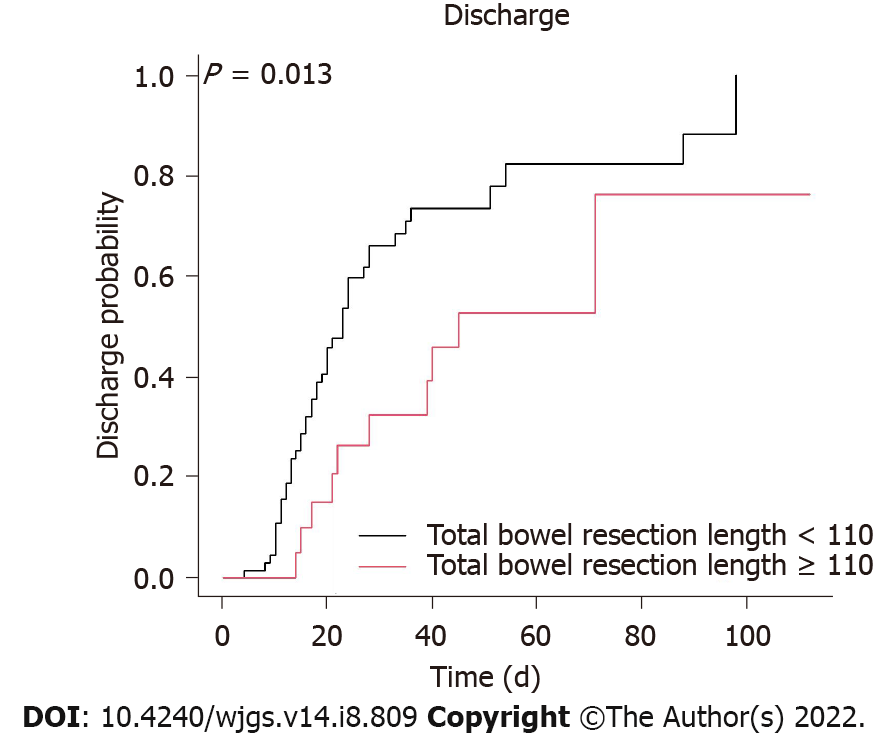
A Cox regression analysis was used to identify important in-hospital protective factors (Table 3). In the univariate analysis, our results demonstrated that a surgery delay < 4.5 d (HR 2.63, 95%CI 1.11 to 6.25; P = 0.028) (Figure 1), no shock (HR 2.86, 95%CI 1.49 to 5.26; P = 0.001), a higher potassium level on day 1 of hospitalization (HR 1.44, with a 95%CI 1.13 to 1.83; P = 0.003), no resection of the colon (HR 2.08, 95%CI 1.15 to 3.85; P = 0.015) (Figure 2), and a total bowel resection length < 110 cm (HR 2.33, 95%CI 1.18 to 4.76; P = 0.015) (Figure 3) were correlated with survival. After 1 wk of hospitalization, patients with a lower WBC count (HR 0.93, 95%CI 0.88 to 0.98; P = 0.006), lower neutrophil count (HR 0.96, 95%CI 0.93 to 0.99; P = 0.005), higher lymphocyte count (HR 1.06, 95%CI 1.01 to 1.11; P = 0.030), and lower CRP level (HR 0.99, 95%CI 0.99 to 1.00; P = 0.009) also had better in-hospital outcomes. After multivariate adjustment, only higher potassium levels on day 1 of hospitalization (HR 1.78, 95%CI 1.25 to 2.54; P = 0.001), a lower neutrophil count (HR 0.92, 95%CI 0.84 to 1.00; P = 0.038) 1 wk after admission, no resection of the colon (HR 2.70, 95%CI 1.05 to 7.14; P = 0.039), and a total bowel resection length < 110 cm (HR 3.85, 95%CI 1.41 to 11.11; P = 0.009) were independently associated with survival.
| Variable | Protective measurement univariate | Protective measurement multivariate |
| Hazard ratio (95%CI) | Hazard ratio (95%CI) | |
| Surgery delay < 4.5 d | 2.63 (1.11-6.25)a | 2.70 (0.69-10.0) |
| No shock | 2.86 (1.49-5.26)a | 1.67 (0.33-8.33) |
| Potassium level in hospital on day 1 | 1.44 (1.13-1.83)a | 1.78(1.25-2.54)a |
| WBC count in hospital on day 7 | 0.93 (0.88-0.98)a | 0.94 (0.85-1.03) |
| Neutrophil count in hospital on day 7 | 0.96 (0.93-0.99)a | 0.92 (0.84-1.00)a |
| Lymphocyte count in hospital on day 7 | 1.06 (1.01-1.11)a | 0.89 (0.76-1.04) |
| CRP level in hospital on day 7 | 0.99 (0.99-1.00)a | 0.99 (0.99-1.00) |
| No resection of colon | 2.08 (1.15-3.85)a | 2.70 (1.05-7.14)a |
| Total resection length < 110 cm | 2.33 (1.18-4.76)a | 3.85 (1.41-11.11)a |
This retrospective study assessed differences between survivors and nonsurvivors among patients with acute mesenteric ischemia who underwent chronic dialysis in terms of in-hospital survival, as previous reports are limited. The univariate analysis revealed that a surgery delay < 4.5 d, no shock, no resection of the colon, a total bowel resection length < 110 cm, and improved hemogram and biochemistry data 1 wk after admission were significantly associated with a better in-hospital prognosis. There were no differences in age, sex or baseline comorbidities between the survivors and nonsurvivors. According to the multivariate analysis, with respect to in-hospital survival, a higher potassium level on day 1 of hospitalization, a lower neutrophil level after 1 wk of admission, no resection of the colon, and a total bowel resection length < 110 cm were associated with higher in-hospital survival. Our results emphasize the importance of early diagnosis and early surgical intervention in chronic dialysis patients with mesenteric ischemia.
The relevant literature reports in-hospital mortality rates of 45% to 73%[2,5,6,10], and a similarly high in-hospital mortality rate (46.6%) was observed in this study. Previous investigations reported that early surgical intervention was associated with better survival. Duran et al[11] reviewed 54 nondialysis patients with acute mesenteric ischemia who underwent open surgery and found that the mortality rate was related to surgery time (from admission to surgery), with 27% mortality in the < 12-h group, 20% mortality in the 12-24-h group, and 50% mortality in the > 24-h group. In chronic dialysis patients, Charra et al[15] found that the 1-mo mortality rate was limited to 15% when 75% of patients were surgically treated in the first 24 h. Similarly, Bender et al[2] observed an increased mortality rate (85.7%, 6 of 7) when surgery was delayed for more than 24 h after the onset of abdominal pain compared with no mortality (100%, 4 of 4) when the interval was within this critical period. Among 11 chronic dialysis patients with mesenteric ischemia, Picazo et al[10] demonstrated that only 3 (27%) who underwent surgery less than 8 h from the time of their arrival at the emergency room survived. In our work, higher mortality was associated with a longer surgery delay, defined as the time from the onset of signs and symptoms to operation (57%, 8 of 14 in the ≥ 4.5-d group vs 47.1%, 42 of 89 in the < 4.5-d group). There are three possible explanations for the slightly longer surgery delay in our work compared with those in other studies. First, the definitions of surgery delay differ among studies. Second, since surgical risk is higher in chronic dialysis patients than in nondialysis patients, most physicians prefer to administer supportive treatment first, including gastrointestinal decompression, aggressive intravascular volume resuscitation, hemodynamic monitoring and support, correction of electrolyte abnormalities, pain control, and initiation of broad-spectrum antibiotics, which may prolong the time of surgery delay. Third, chronic bowel ischemia due to atherosclerosis is prominent in chronic dialysis patients; thus, mesenteric ischemia may be more tolerable in this population than in nondialysis patients, which may explain the longer surgery delay among chronic dialysis patients. Although a short surgery delay was not significantly associated with survival after multivariate adjustment, the protection afforded by a short surgery delay may have been masked or confounded by other factors, such as total bowel resection length, potassium level, or site of operation. The present work reported an important finding: a shorter surgery delay is associated with better survival and the acceptable surgery delay may be longer among chronic dialysis patients than among nondialysis patients.
Tran et al[16] analyzed 212 patients undergoing surgery for acute mesenteric ischemia with a predominant etiology of embolism or in situ thrombosis and found that the time to revascularization was associated with predicted 30-d and all-cause 2-year mortality, total bowel resection length and postoperative short-bowel syndrome. They emphasized that early and routine vascular surgery consultation and definitive revascularization may mitigate outcomes of patients suspected to have acute mesenteric ischemia. However, in the present study, all of our study population received bowel resection without documented revascularization procedures before or after intestinal resection. The reason for the lack of revascularization procedures may be that NOMI, rather than vascular occlusion, was the leading cause of acute mesenteric ischemia among the chronic dialysis patients.
Correlations with the bowel involvement site, bowel resection length, and survival have not been well described in chronic dialysis patients with acute mesenteric ischemia. A previous investigation showed a worse mesenteric ischemia prognosis when the colon was involved. Acosta-Merida et al[13] demonstrated a significantly higher mortality rate of mesenteric ischemia when the large bowel was involved (78% vs 22%), and Aliosmanoglu et al[12] also concluded that colon involvement had a negative effect on the mortality rate. Similarly, in the present study, we found that bowel resection not involving the colon independently predicted survival. One of the reasons for the higher mortality rate in these patients may be that more extensive resection is necessary, including colon resection. Second, colon continuity may be important. According to previous reports, short-bowel syndrome is unavoidable after resection if more than 70% of the small intestine or less than 100 cm of small bowel is left[17]. Since the colon has important digestive and absorption functions, additional resection of the ileocecal region or the colon increases the severity of short-bowel syndrome. Patients with a short small bowel and no colon are likely to require long-term parental nutrition and fluids; however, if more than half of the colon is brought into continuity, parental nutrition is less likely to be needed unless shorter than 50-cm jejunum remains[18]. A third explanation may involve the intense microbiologic flora in the colon, bacterial translocation, and systemic effects[12]. Our study found an independent protective effect of a total bowel resection length < 110 cm in this population, which has not been described previously. Based on the above findings, we emphasize the importance of bowel continuity and colon preservation in chronic dialysis patients with acute mesenteric ischemia; to maximally reduce the extent of bowel resection, early diagnosis and aggressive surgical intervention are important.
Watershed areas of circulation are more vulnerable to NOMI[19]. A higher frequency of involvement of the right colon and the cecum has been reported in dialysis patients[1,10,15,20]. This intestinal segment seems to be particularly susceptible to nonocclusive ischemia since natural collateral circulation struggles to keep up with tissue demands if the main arterial source is lost[21]. In addition, the right colonic vasa recta are longer and originate from a more distant site than those in the left colon, which may increase resistance to reperfusion after an ischemic insult from arterial hypotension[22]. However, in our study, the ileum (80.4%) was the intestinal segment most involved, followed by the colon (41.2%), likely due to hypoperfusion at the superior mesenteric artery level and often to severe episodes of arterial hypotension. NOMI has only rarely been reported to be associated with peritoneal dialysis, possibly due to the lower occurrence of abruptly hypotensive episodes[23]. Despite having a more stable blood pressure than patients on hemodialysis, peritoneal dialysis patients may experience severe hypotensive conditions with less symptoms. Contributing factors are inappropriate use of dialysate, resulting in excessive fluid removal; diuretics, and a very low-salt diet coupled with the tendency of dialysate to remove endogenous aldosterone, which is needed for adequate sodium absorption by the gastrointestinal tract[7]. An extremely high mortality rate among peritoneal dialysis patients with mesenteric ischemia has been reported (8 of 10 cases, 80%)[7]. In our study, consistent with a previous investigation, the mortality rate among peritoneal dialysis patients with acute mesenteric ischemia was even higher (7 of 8 cases, 87.5%). Since the presentation of NOMI is similar to that of peritonitis, the presence of peritonitis may mask the condition, and the key to a correct diagnosis is a high index of suspicion in predisposed patients. The high mortality rate is a reflection of the failure to recognize the syndrome at an early, treatable stage[24].
Whether the CRP level predicts in-hospital mortality in acute mesenteric ischemia patients is controversial. Yu et al[25] analyzed 12 dialysis patients with mesenteric ischemia and found comparable CRP levels among survivors and nonsurvivors. In contrast, Destek et al[26] demonstrated that the CRP level was significantly correlated with the total lengths of stay in the hospital and intensive care unit (ICU). Kaçer et al[27] found that the CRP/albumin ratio was a powerful predictor of in-hospital mortality in patients with acute mesenteric ischemia, and it was superior to the WBC count, neutrophil to lymphocyte ratio, and lactate level. In the present study, we found that a lower CRP level after 7 d of admission predicted better survival in these patients, but the protective effect was masked after multivariate adjustment, probably because of confounding by total bowel resection length. We suggest the close monitoring of CRP levels during hospitalization in treatment response monitoring.
Leukocytosis is a common finding among patients with mesenteric ischemia[2,5,6,28]. Yu et al[25] disclosed that not all dialysis patients with mesenteric ischemia had leukocytosis initially, but all deceased patients had leukocytosis; however, the difference was not statistically significant. In our work, we observed lower leukocyte counts at baseline (11.69 ± 5.49 k/μL vs 14.21 ± 6.74 k/μL; P = 0.041) and 1 wk after treatment (10.05 ± 5.04 k/μL vs 13.96 ± 8.19 k/μL; P = 0.004) in survivors. Improvement in leukocytosis after 1 wk of treatment significantly predicted better survival, but the protective effect was masked after multivariate adjustment, possibly due to confounding by other factors, such as total bowel resection length. We suggest monitoring leukocyte levels during hospitalization and treatment response monitoring.
Shock is also a common clinical feature in dialysis patients with mesenteric ischemia. In a literature review, shock developed in 27%-60%[1,10] of dialysis patients with mesenteric ischemia at the time of diagnosis, and septic shock was the main cause of early death[1]. Schoenberg et al[29] found that the mortality rate of systemic inflammatory response syndrome ranged from 6% to 7% and that of septic shock exceeded 50% in an ICU population. Unsurprisingly, the frequency of shock was higher among nonsurvivors in this study. Univariate analysis revealed that no shock during hospitalization, which was associated with milder disease activity, was associated with higher in-hospital survival, but the protective effect disappeared after multivariate adjustment.
Diamond et al[28] demonstrated that hyperkalemia (6 of 12), metabolic acidosis (10 of 12), and leukocytosis (8 of 12) were the most consistently noted laboratory findings in dialysis patients with mesenteric ischemia; however, these data are difficult to interpret in dialysis patients since some of them are already increased due to uremia itself and/or due to the time elapsed from the last dialysis session[30]. In chronic dialysis patients, hyperkalemia beginning at a serum potassium level ≥ 5.7 mEq/L was associated with all-cause mortality, and mortality risk estimates increased ordinally through ≥ 6.0 mEq/L[31]. Paradoxically, in our work, both the univariate and multivariate analyses demonstrated a protective value of a higher potassium level on the first day of hospitalization. However, the mean potassium level was still within the normal range among survivors and nonsurvivors in our study, which may explain the paradox, and we suggest keeping the potassium level within the normal range in this population.
Cardiac diseases, such as congestive heart failure, cardiac arrhythmia, low cardiac output states, recent myocardial infarction, and severe valvular cardiac disease, are acknowledged risk factors for acute mesenteric ischemia[32], but the prognostic value of heart failure has not been elucidated in chronic dialysis patients. In our work, there were no significant differences in left ventricular EF among survivors and nonsurvivors.
This study has several limitations. First, this was a retrospective study at a single medical center that enrolled predominantly Asian patients; thus, its findings may not apply to the general population. Second, since this study involved a single center, the number of considered cases was limited, reducing the capacity to detect significance with respect to some variables. Third, only chronic dialysis patients were enrolled, and the in-hospital outcomes of mesenteric ischemia in chronic dialysis patients and nondialysis patients were not compared. Therefore, further study is needed. Fourth, the quick Sepsis-related Organ Failure Assessment (qSOFA) score, with a cutoff value ≤ 3, was found to be a reliable predictor of survival in NOMI patients treated with conservative management[33]. We did not analyze the qSOFA score in the present work, and further study of the prognostic value of the qSOFA score in NOMI patients treated with surgery is needed. Fifth, frequent and severe hypotension when receiving dialysis occurred more commonly in patients who developed bowel ischemia[34], but in this work, we did not analyze the impact of blood pressure on in-hospital mortality. Further investigation is warranted. Nevertheless, this work provides important information about protective factors for survival in patients with mesenteric receiving chronic dialysis.
Outcomes of acute mesenteric ischemia in chronic dialysis patients were poor, and only 53.3% of these patients survived the index hospitalization. A surgery delay less than 4.5 d, no shock during admission, bowel resection not involving the colon, and a total bowel resection length < 110 cm were associated with better in-hospital survival. This study emphasizes that early diagnosis and prompt surgical intervention in chronic dialysis patients with acute mesenteric ischemia are beneficial.
Mesenteric ischemia is significantly more common in end-stage kidney disease patients undergoing chronic dialysis than in the general population and is associated with high morbidity and mortality. However, reports on prognostic factors in this population are limited.
Reports on prognostic factors in chronic dialysis patients with acute mesenteric ischemia are lacking.
The aim of this retrospective study was to identify the protective factors for mesenteric ischemia in chronic dialysis patients to promote earlier initiation of aggressive therapy in this targeted population and improve their poor prognosis.
One hundred and three chronic dialysis patients with surgically confirmed acute mesenteric ischemia in a tertiary medical center over 14 years were retrospectively analyzed. Cox regression and Kaplan-Meier analysis were used for prognostic analysis by R statistical analysis software.
The in-hospital mortality rate among the 103 enrolled patients was 46.6%. Univariate analysis was performed to compare factors in survivors and nonsurvivors, with better in-hospital outcomes associated with a surgery delay (defined as the time from onset of signs and symptoms to operation) < 4.5 d, no shock, no resection of the colon, and a total bowel resection length < 110 cm. Following multivariate adjustment, resection not involving the colon (HR 2.70, 95%CI 1.05 to 7.14; P = 0.039), and a total bowel resection length < 110 cm (HR 4.55, 95%CI 1.43 to 14.29; P = 0.010) were significantly associated with survival.
A surgery delay < 4.5 d, no shock, no resection of the colon, and a total bowel resection length < 110 cm predicted better outcomes in chronic dialysis patients with acute mesenteric ischemia.
This study emphasizes that early diagnosis and prompt surgical intervention in chronic dialysis patients with acute mesenteric ischemia are beneficial.
The authors would like to thank the Research Services Center for Health Information at Chang Gung University for conducting the statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brillantino A, Italy; Kazmi SSH, Norway; Sateesh J, India S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Bassilios N, Menoyo V, Berger A, Mamzer MF, Daniel F, Cluzel P, Buisson C, Martinez F. Mesenteric ischaemia in haemodialysis patients: a case/control study. Nephrol Dial Transplant. 2003;18:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Bender JS, Ratner LE, Magnuson TH, Zenilman ME. Acute abdomen in the hemodialysis patient population. Surgery. 1995;117:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Cooke M, Sande MA. Diagnosis and outcome of bowel infarction on an acute medical service. Am J Med. 1983;75:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Liavag I. Diagnosis and treatment of acute mesenteric vascular insufficiency. Geriatrics. 1969;24:49-60. [PubMed] |
| 5. | John AS, Tuerff SD, Kerstein MD. Nonocclusive mesenteric infarction in hemodialysis patients. J Am Coll Surg. 2000;190:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Ori Y, Chagnac A, Schwartz A, Herman M, Weinstein T, Zevin D, Gafter U, Korzets A. Non-occlusive mesenteric ischemia in chronically dialyzed patients: a disease with multiple risk factors. Nephron Clin Pract. 2005;101:c87-c93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Archodovassilis F, Lagoudiannakis EE, Tsekouras DK, Vlachos K, Albanopoulos K, Fillis K, Manouras A, Bramis J. Nonocclusive mesenteric ischemia: a lethal complication in peritoneal dialysis patients. Perit Dial Int. 2007;27:136-141. [PubMed] |
| 8. | Valentine RJ, Whelan TV, Meyers HF. Nonocclusive mesenteric ischemia in renal patients: recognition and prevention of intestinal gangrene. Am J Kidney Dis. 1990;15:598-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Wilcox MG, Howard TJ, Plaskon LA, Unthank JL, Madura JA. Current theories of pathogenesis and treatment of nonocclusive mesenteric ischemia. Dig Dis Sci. 1995;40:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Picazo M, Cuxart M, Sans R, Sardá C, Expósito E. [Mesenteric ischemia in hemodialysis patients]. Nefrologia. 2008;28:198-202. [PubMed] |
| 11. | Duran M, Pohl E, Grabitz K, Schelzig H, Sagban TA, Simon F. The importance of open emergency surgery in the treatment of acute mesenteric ischemia. World J Emerg Surg. 2015;10:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Aliosmanoglu I, Gul M, Kapan M, Arikanoglu Z, Taskesen F, Basol O, Aldemir M. Risk factors effecting mortality in acute mesenteric ischemia and mortality rates: a single center experience. Int Surg. 2013;98:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Acosta-Merida MA, Marchena-Gomez J, Hemmersbach-Miller M, Roque-Castellano C, Hernandez-Romero JM. Identification of risk factors for perioperative mortality in acute mesenteric ischemia. World J Surg. 2006;30:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Sumbal R, Ali Baig MM, Sumbal A. Predictors of Mortality in Acute Mesenteric Ischemia: A Systematic Review and Meta-Analysis. J Surg Res. 2022;275:72-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Charra B, Cuche J, Ruffet M, Terrat JC, Beurlet J, Labrosse H, Vanel T, Calemard E, Chazot C, Vovan C. Segmental necrosis of ascending colon in haemodialysis patients. Nephrol Dial Transplant. 1995;10:2281-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tran LM, Andraska E, Haga L, Sridharan N, Chaer RA, Eslami MH. Hospital-based delays to revascularization increase risk of postoperative mortality and short bowel syndrome in acute mesenteric ischemia. J Vasc Surg. 2022;75:1323-1333.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Keller J, Panter H, Layer P. Management of the short bowel syndrome after extensive small bowel resection. Best Pract Res Clin Gastroenterol. 2004;18:977-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Adaba F, Rajendran A, Patel A, Cheung YK, Grant K, Vaizey CJ, Gabe SM, Warusavitarne J, Nightingale JM. Mesenteric Infarction: Clinical Outcomes After Restoration of Bowel Continuity. Ann Surg. 2015;262:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 20. | Berger A, Mamzer-Bruneel MF, Wind P, Cuenod CA, Buisson C, Cugnenc PH. Opaque enema CT scan allows early diagnosis of non-occlusive right colonic ischaemia in dialysis patients. Nephrol Dial Transplant. 1997;12:2179-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Landreneau RJ, Fry WJ. The right colon as a target organ of nonocclusive mesenteric ischemia. Case report and review of the literature. Arch Surg. 1990;125:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Steward JA, Rankin FW. Blood supply of the large intestine: its surgical considerations. Arch Surg. 1933;26:843-891. [DOI] [Full Text] |
| 23. | Liu HL, Huang JJ, Lan RR, Wang MC, Sung JM, Hsieh RY. Ischaemic bowel disease in patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 1999;14:2032-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Korzets Z, Ben-Chitrit S, Bernheim J. Nonocclusive mesenteric infarction in continuous ambulatory peritoneal dialysis. Nephron. 1996;74:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Yu CC, Hsu HJ, Wu IW, Lee CC, Tsai CJ, Chou CC, Wu MS. Factors associated with mortality from non-occlusive mesenteric ischemia in dialysis patients. Ren Fail. 2009;31:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Destek S, Yabacı A, Abik YN, Gül VO, Değer KC. Predictive and prognostic value of L-lactate, D-dimer, leukocyte, C-reactive protein and neutrophil/lymphocyte ratio in patients with acute mesenteric ischemia. Ulus Travma Acil Cerrahi Derg. 2020;26:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Kaçer İ, Çağlar A, Akıllı NB. The Prognostic Value of C-Reactive Protein/Albumin Ratio in Acute Mesenteric Ischemia. Am Surg. 2022;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Diamond SM, Emmett M, Henrich WL. Bowel infarction as a cause of death in dialysis patients. JAMA. 1986;256:2545-2547. [PubMed] |
| 29. | Schoenberg MH, Weiss M, Radermacher P. Outcome of patients with sepsis and septic shock after ICU treatment. Langenbecks Arch Surg. 1998;383:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Daugirdas JT, Blake P, Ing TS, Blagg C. Handbook of Dialysis, Fourth Edition. Philadelphia: Lippincott Williams and Wilkins, 2007: 482-483. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB. Serum Potassium Levels and Mortality in Hemodialysis Patients: A Retrospective Cohort Study. Am J Nephrol. 2016;44:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Toda Y, Komatsu S, Fukami Y, Saito T, Matsumura T, Osawa T, Kurahashi S, Uchino T, Kato S, Yasui K, Hanazawa T, Kaneko K, Sano T. Prognostic factors for the successful conservative management of nonocclusive mesenteric ischemia. World J Emerg Surg. 2022;17:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Seong EY, Zheng Y, Winkelmayer WC, Montez-Rath ME, Chang TI. The Relationship between Intradialytic Hypotension and Hospitalized Mesenteric Ischemia: A Case-Control Study. Clin J Am Soc Nephrol. 2018;13:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |