Published online May 27, 2022. doi: 10.4240/wjgs.v14.i5.409
Peer-review started: December 30, 2021
First decision: March 10, 2022
Revised: March 12, 2022
Accepted: April 26, 2022
Article in press: April 26, 2022
Published online: May 27, 2022
Processing time: 145 Days and 23 Hours
Repeated liver resection is an effective treatment for recurrent hepatocellular carcinoma (HCC). However, few studies have compared the outcome of laparoscopic repeat hepatectomy (LRH) and open repeat hepatectomy (ORH) for recurrent HCC, and few of those have included cirrhotic patients.
To compare short-term and long-term outcomes of cirrhotic patients with LRH and ORH for recurrent HCC.
We retrospectively analysed the clinical records retrieved from a prospectively collected database of all patients who underwent hepatectomy for post-hepatectomy recurrent HCC at our institute between May 2006 and June 2021. Cases of recurrent HCCs larger than 7 cm were excluded. Patient demographics, operative details, perioperative outcomes, pathologic details, disease-free survival (DFS), and overall survival (OS) data of LRH and ORH were compared.
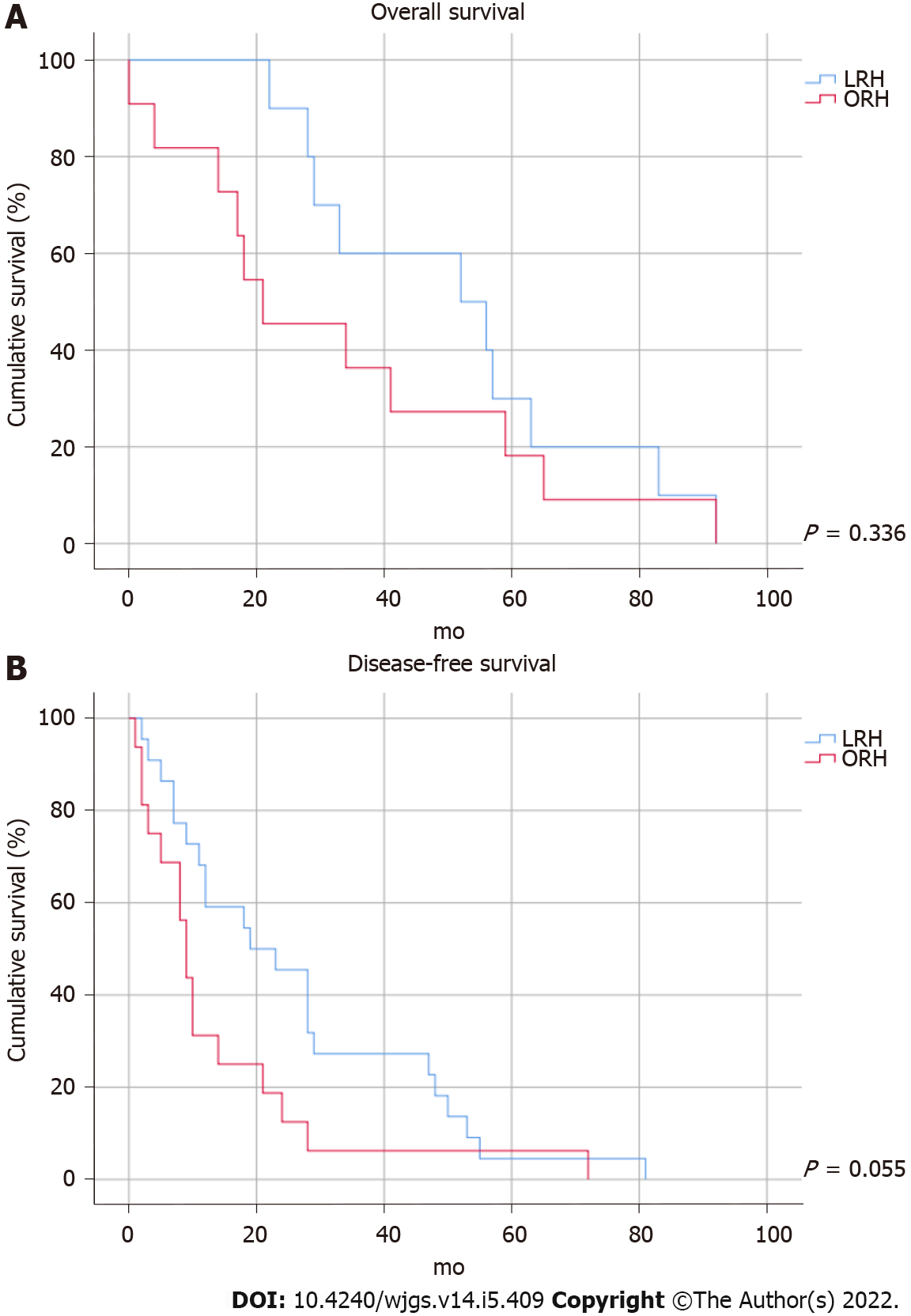
Data from 29 patients with LRH and 22 with ORH were compared. The LRH group showed significantly better outcomes for blood loss (median 300 mL vs 750 mL, P = 0.013) and length of hospital stay (median 5 d vs 7 d, P = 0.003). The 1-, 3- and 5-year OS rates in the LRH group were 100.0%, 60.0% and 30.0%, respectively; the corresponding rates in the ORH group were 81.8%, 36.4% and 18.2% (P = 0.336). The 1-, 3- and 5-year DFS rates in the LRH group were 68.2%, 27.3% and 4.5%, respectively; the corresponding rates in the ORH group were 31.3%, 6.3% and 6.3% (P = 0.055). There were no significant differences in overall and DFS between the two groups.
Laparoscopic re-resection should be considered for patients presenting with recurrent HCC less than or equal to 7 cm after previous hepatectomy.
Core Tip: Laparoscopic liver re-resection for recurrent hepatocellular carcinoma had similar oncological outcomes compared with open surgery, even in patients with cirrhosis. Laparoscopic re-resection should be considered for all patients suitable for liver re-resection for recurrent hepatocellular carcinoma.
- Citation: Cheng KC, Ho KM. Laparoscopic vs open liver re-resection for cirrhotic patients with post-hepatectomy hepatocellular carcinoma recurrence: A comparative study. World J Gastrointest Surg 2022; 14(5): 409-418
- URL: https://www.wjgnet.com/1948-9366/full/v14/i5/409.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i5.409
Hepatocellular carcinoma (HCC) can be cured by liver resection[1]. Although, the oncological outcome of liver resection is frequently jeopardized by tumour recurrence, with a reported 5-year recurrence rate of 50%-70%[2-4], and intrahepatic recurrence accounts for approximately 80% of postoperative recurrences[2]. Repeated liver resection has been demonstrated to be an effective treatment for recurrent HCC, and has low morbidity and mortality[5-7]. However, owing to multiple liver metastases, reduced liver function, and poor general health, less than 30% of patients with recurrences can undergo recurrent resection[8].
Laparoscopic liver resection (LLR) has emerged as a valuable treatment option for HCC during the last decade. LLR has a shorter operative time, less blood loss, shorter hospital stay, and lower overall morbidity than open liver resection, along with comparable disease-free and overall survival (OS)[9-15]. However, because of the development of adhesions, altered anatomy, the establishment of collateral circulation, reduced liver function, and loss of liver parenchyma following the prior surgery, laparoscopic repeat hepatectomy (LRH) is technically more complex than primary resection. Patients with HCC are likely to suffer from liver cirrhosis and portal hypertension resulting from underlying hepatitis B or C infection, and intraoperative haemorrhage and haemostasis associated with abnormal primary haemostasis are a challenge even for surgeons experienced in LLR[16,17]. Furthermore, these patients are more likely to develop postoperative complications like pleural effusion, chest infection, ascites, portal vein thrombosis, kidney failure and liver failure after hepatectomy[18,19].
Few retrospective studies have compared the outcome of LRH and open repeat hepatectomy (ORH) for recurrent HCC, and few of those have included cirrhotic patients[20-29]. This study aimed to compare the short-term and long-term outcomes of cirrhotic patients with post-hepatectomy HCC recurrence and undergoing LRH or ORH.
This study was approved by the Hong Kong Hospital Authority Research Ethics Committee (Kowloon Central/Kowloon East; Ref. KC/KE-21-0278/ER-4). The clinical records of all patients undergoing hepatectomy for post-hepatectomy recurrent HCC at our institute from May 2006 to June 2021 were retrieved and retrospectively analyzed from a prospectively collected database. Patients with radiological features typical of recurrent HCC of less than or equal to 7 cm in size on contrast-enhanced computed tomography or magnetic resonance imaging were included. All patients received the same perioperative care and evaluation protocols. Functional liver reserve for major hepatectomy was assessed by indocyanine green retention at 15 min and computed tomography liver volumetry. The criteria for LLR and open hepatectomy were previously described[30]. The same team of hepatobiliary surgeons performed all the operations. Liver resection was described using the Brisbane 2000 terminology[31].
Patient demographics and preoperative characteristics included in the analysis were the date and extent of the previous operation, date of recurrence, liver function tests, and serum alpha-fetoprotein (AFP) levels. Operative details, including the operative time, extent of liver resection, operative approach, volume of blood loss, and blood transfusion requirements, were collected. Short-term outcomes included operative factors (operative time, use of Pringle manoeuvre, blood loss, blood transfusion, and conversion) and postoperative factors (length of hospital stay, resection margin, and complications). Long-term outcomes included OS and disease-free survival (DFS). Major hepatectomy was defined as resection of three or more Couinaud liver segments. Cirrhosis was diagnosed by histology findings. Perioperative outcomes included 30-d mortality and Clavien-Dindo complications[32]. International Study Group of Liver Surgery criteria were used to define post-hepatectomy liver failure and bile leakage[33,34]. The number of tumors, the size of the largest tumor nodule, and the resection margin were all derived from the specimens' histological information. The presence of tumor cells within 1 mm of the transection line was classified as a positive resection margin.
Blood tests for liver function, AFP, chest X-ray, and abdominal computed tomography scan with contrast, or ultrasonography of the liver if contrast injection was contraindicated, were all part of the patient's follow-up routine. Patients were checked every three months for the first two years after surgery and then every six months after that. If a patient missed an appointment, they were actively contacted for follow-up. Recurrence was reported as the date of radiological recurrence. A multidisciplinary team of surgeons, radiologists, and oncologists chose subsequent treatments, such as re-resection, microwave or radiofrequency ablation (RFA), transarterial chemo-embolisation, or systemic therapy.
All hepatectomies, except for lesions near important vascular structures, aimed to achieve a gross resection margin of 1 cm, and intraoperative ultrasonography was performed. A right subcostal incision with an upper midline extension was used for open liver re-resections. Hepatic parenchymal transection was performed with a Cavitron Ultrasonic Surgical Aspirator (Olympus, Tokyo, Japan). Haemostasis was achieved by electrocautery or suture. For laparoscopic procedures, patients were placed in a Lloyd-Davies position (right side up for posterosuperior lesions). The chief surgeon stood between the patient’s legs and two assistants stood at the patient’s left side. The open Hasson technique was used to introduce the first trocar and pneumoperitoneum was established at a pressure of 12 mmHg. Depending on the tumor site, four working ports were inserted with direct vision after introducing the flexible laparoscope. Harmonic Scalpel (Ethicon, Somerville, NJ, United States) was used to accomplish adhesiolysis. The procedure was then followed by intraoperative ultrasonography. For major or segmental liver resections, the extrahepatic Glissonian method was used to control hepatic inflow, liver parenchymal transection was accomplished with Harmonic Scalpel, and haemostasis was achieved by bipolar diathermy, clips, or sutures. Resected specimens were placed in plastic bags and removed using a Pfannenstiel incision or the extension of one of the ports. In both laparoscopic and open surgery, the Pringle manoeuvre was used selectively in cases with excessive bleeding, and drains were placed only when indicated. Intraoperative RFA was occasionally used for small lesions deep within the liver parenchyma and was carried out using a Cooltip RFA system (Medtronic, Minneapolis, MN, United States) by either the surgeon or interventional radiologist.
Statistical analysis was performed with SPSS version 26 (IBM Corp., Armonk, NY, United States). Mann-Whitney U test was used to compare differences between the values of quantitative variables and Pearson chi-squared or Fisher's exact test was used to compare categorical variables. Survival analysis was analysed by the Kaplan-Meier method and differences were compared using the log-rank test. Statistical significance was set at P ≤ 0.05.
During the study period, 52 patients had liver resection for recurrent HCC following an initial curative liver resection at our center. There were no missing data. One patient with a 7.5-cm diameter tumour and ORH was excluded, and the remaining 29 patients with LRH and 22 patients with ORH were included. Of the 29 LRH patients, 18 had one previous liver resection and 11 had two or more (Table 1). The demographic and clinicopathological characteristics are shown in Table 2. Between-group differences in baseline characteristics, including age, sex, cirrhosis, hepatitis B carrier status, liver function, AFP level, tumour size, number, and location, type of resection, and concurrent ablation, were not significant. Preoperative bilirubin was higher in the LRH (median 17 mmol/L) than in the ORH (13 mmol/L) group (P = 0.007). The median tumour size was 1.75 cm in the LRH group and 2.75 cm in the ORH group. There was one hepatitis C patient in the ORH group and none in the LRH group.
| Previous liver resection | LRH, n = 29 | ORH, n = 22 | P value |
| Approach | 0.395 | ||
| Laparoscopic | 14 (48.3) | 8 (36.4) | |
| Open | 15 (51.7) | 14 (63.6) | |
| Type of resection | 0.224 | ||
| Major | 24 (82.8) | 15 (68.2) | |
| Minor | 5 (17.2) | 7 (31.8) | |
| Tumour location, segment | 0.780 | ||
| II, III, IV, V, VI | 16 (55.2) | 13 (59.1) | |
| VII, VIII | 13 (44.8) | 9 (40.9) | |
| Number of previous hepatectomy | 0.478 | ||
| 1 | 17 (58.6) | 16 (72.7) | |
| 2 | 9 (31.0) | 5 (22.7) | |
| 3 | 2 (6.9) | 0 (0.0) | |
| 4 | 0 (0.0) | 1 (4.5) | |
| 5 | 1 (3.4) | 0 (0.0) | |
| Microscopic lymphovascular invasion | 1.000 | ||
| No | 20 (69.0) | 15 (68.2) | |
| Yes | 4 (13.8) | 4 (18.2) | |
| Not assessed | 5 (17.2) | 3 (13.6) |
| Characteristic | LRH, n = 29 | ORH, n = 22 | P value |
| Age | 64 (57.5-67.5) | 65.5 (59.75-69.25) | 0.607 |
| Sex | 0.688 | ||
| Male | 25 (86.2) | 20 (90.9) | |
| Female | 4 (13.8) | 2 (9.1) | |
| Cirrhosis on histology | 19 (65.5) | 13 (59.1) | 0.638 |
| HBsAg-positive | 27 (93.1) | 20 (90.9) | 1.000 |
| Albumin in g/L | 39 (36-41) | 36 (34-40) | 0.109 |
| Total bilirubin in µmol/L | 17 (13-20) | 13 (10-16) | 0.007 |
| International normalized ratio | 1.1 (1.05-1.20) | 1.085 (1.055-1.148) | 0.587 |
| Platelet count as × 109/L | 123 (99-173) | 161.5 (115.25-201.00) | 0.092 |
| Alpha-fetoprotein in IU/mL | 11 (4.25-288.00) | 17 (4.0-174.5) | 0.814 |
| Type of resection | 0.055 | ||
| Sub-segmentectomy | 19 (65.5) | 8 (36.4) | |
| Segmentectomy | 5 (17.2) | 2 (9.1) | |
| Left lateral sectionectomy | 2 (6.9) | 1 (4.5) | |
| Right bisegmentectomy | 1 (3.4) | 4 (18.2) | |
| Left hepatectomy +/− extended | 1 (3.4) | 1 (4.5) | |
| Right hepatectomy +/− extended | 1 (3.4) | 5 (22.7) | |
| Central bisectionectomy | 0 (0.0) | 1 (4.5) | |
| Intraoperative ablation | 1 (3.4) | 2 (9.1) | 0.571 |
| Tumour size in cm | 0.054 | ||
| < 1 | 2 (6.9) | 1 (4.5) | |
| ≥ 1-2 | 15 (51.7) | 7 (31.8) | |
| ≥ 2-3 | 5 (17.2) | 3 (13.6) | |
| ≥ 3-4 | 5 (17.2) | 2 (9.1) | |
| ≥ 4-5 | 1 (3.4) | 8 (36.4) | |
| ≥ 5 | 1 (3.4) | 1 (4.5) | |
| Number of tumours | 0.295 | ||
| Single | 25 (86.2) | 16 (72.7) | |
| Multiple | 4 (13.8) | 6 (27.3) | |
| Tumour location, segment | 0.491 | ||
| I | 2 (6.9) | 0 (0.0) | |
| II, III, IV, V, VI | 14 (48.3) | 9 (40.9) | |
| VII, VIII | 13 (44.8) | 13 (59.1) |
Operative outcomes are shown in Table 3. Blood loss (median 300 mL vs 750 mL, P = 0.013) and length of hospital stay (median 5 d vs 7 d, P = 0.003) were significantly better in the LRH group. One patient in the ORH group who underwent right anterior sectionectomy died within 30 d after the operation because of chest infection, sepsis, and multiorgan failure. All other complications were successfully treated by conservative measures or interventional radiological drainage. There were six conversions from laparoscopic to open surgery. Three were owed to insecure margins, two due to dense adhesions from previous open surgery, and one due to profuse bleeding from the hepatic vein.
| Outcome | LRH, n = 29 | ORH, n = 22 | P value |
| Operative time in min | 250 (177.5-320.5) | 300.5 (223.00-378.75) | 0.224 |
| Pringle manoeuvre used | 2 (6.9) | 3 (13.6) | 0.641 |
| Blood loss in mL | 300 (200-700) | 750 (300-1450) | 0.013 |
| Blood transfusion | 6 (20.7) | 8 (36.4) | 0.214 |
| Conversion | 6 (20.7) | ||
| Hospital stay in d | 5 (4-7) | 7 (5.75-11.50) | 0.003 |
| Resection margin in mm | 7.25 (5.00-13.25) | 4.25 (1.00-8.25) | 0.073 |
| Positive margin | 2 (7.1) | 2 (9.1) | 0.801 |
| Complications | 3 (10.3) | 6 (27.3) | 0.150 |
| Chest infection | 0 | 1 | |
| Pleural effusion | 1 | 3 | |
| Arrhythmia | 2 | 0 | |
| Bile leak | 0 | 2 | |
| Liver failure | 0 | 0 | |
| UTI | 0 | 1 | |
| Intra-abdominal infection | 1 | 1 | |
| Clavien-Dindo severity of complications | |||
| IIIa | 1 (1.1) | 5 (22.7) | 0.073 |
| IIIb | 0 | 0 | |
| IV | 0 | 0 | |
| V | 0 (0.0) | 1 (4.5) | 0.431 |
Median follow-up was 54 mo (interquartile range 28-85 mo). No patients were lost to follow-up. OS and DFS are shown in Figure 1. The 1-, 3- and 5-year OS rates were 100.0%, 60.0% and 30.0% in the LRH group and 81.8%, 36.4% and 18.2% in the ORH group, respectively. Except for the single case of 30-d postoperative mortality mentioned above, all patients died of malignant cachexia. The 1-, 3- and 5-year DFS were 68.2%, 27.3% and 4.5% in the LRH group and 31.3%, 6.3% and 6.3% in the ORH group, respectively. Differences in overall (P = 0.336) and DFS (P = 0.055) between the two groups were not significant.
Although the benefits of LLR over open liver resection in terms of improved short-term postoperative outcomes and equivalent oncological outcomes are well established[9-15], the importance of LLR in recurrent HCC has yet to be determined. The short-term benefits of LRH were established in this trial, including decreased blood loss, a shorter hospital stay, and oncological results were comparable to ORH.
The presence of abdominal adhesions makes re-resection more challenging. Menzies and Ellis[35] observed that 93% of patients with past laparotomy had intra-abdominal adhesions in a prospective analysis, and their findings were corroborated in an autopsy investigation by Weibel et al[36], who detected adhesions in 67% of cases with prior abdominal surgery. For surgeons doing laparoscopic liver resection, dense or highly vascularized adhesions, particularly those around the hepatic hilum or major vessels, remain a significant challenge. However, optical magnification during laparoscopic re-resection increases the precision of dissection, and the pneumoperitoneum tightens the adhesion bands, making the dissection and adhesiolysis easier. LLR may also decrease the formation of adhesions and injury to the liver parenchyma, collateral arteries, and surrounding structures, allowing for further resections[37,38]. In this retrospective study, although adhesion scoring was not documented, the conversion rate was higher than reported in our previously reported series of primary LLR patients (20% vs 10%)[15,30]. Two of the conversions to open surgery were because of adhesions related to previous open surgery. The conversions illustrate the impact of adhesions on liver resection.
In this series, 62.7% of the patients had a histological diagnosis of cirrhosis, and more than 90% were hepatitis B carriers. Even for cirrhotic patients with recurrent HCC, LRH was safe and feasible, and it had a superior short-term outcome than ORH. Over a decade ago, Belli et al[39] suggested that laparoscopic liver re-resection was only indicated for HCC in patients with well-compensated Child-Pugh class A chronic liver disease without signs of severe portal hypertension, a single exophytic or subcapsular HCC located in the left (segments II, III, or IVb) or right (segments V or VI) liver and a maximum size of 4 cm to 5 cm. Increased experience and advances in technology have extended the indications for laparoscopic hepatectomy. After a previous hepatectomy, intrahepatic recurrence in the liver remnant might benefit from LRH with less blood loss and a shorter hospital stay.
RFA has been recommended as an alternative to repeat liver resection for recurrent HCC. A recent meta-analysis by Liu et al[40] found that 1-, 3- and 5-year OS and 1-year DFS rates following repeated liver resection for recurrent HCC were similar to those achieved by RFA in patients who satisfied the Milan criteria (i.e. maximal diameter of a single tumour ≤ 5 cm, or ≤ 3 tumours ≤ 3 cm each). Repeated liver resection was superior to RFA in 3- and 5-year DFS, but if the tumour size for RFA was not limited, 3- and 5-year OS and 1-, 3- and 5-year DFS were better with repeated liver resection than with RFA. RFA should therefore be reserved for patients with small deep-seated tumors that meet the Milan criteria, and liver re-resection should be the first-line treatment for subcapsular or massive tumors.
There were a few study limitations. First, it was a retrospective analysis, and there were missing data on the adhesion scores after the first hepatectomy. Second, only 51 patients had repeated hepatectomy during the study period. The small sample size was prone to type 2 errors. Third, we conducted only univariate analysis, which is subject to confounding factors. For confounder control, Cox regression or propensity score matching should be considered. However, our sample size was too small for such an analysis. Fourth, we included patients with hepatectomies between 2006 and 2021. Surgical instruments and techniques have improved throughout time, despite the fact that all of the operations were performed by the same group of devoted hepatobiliary surgeons.
Larger studies, or even randomized controlled trials, are needed to further understand the role of LRH in the treatment of recurrent HCC. Documentation of the adhesion score upon repeated hepatectomy would allow an analysis of the benefits of laparoscopic surgery on the formation of adhesions.
Laparoscopic liver re-resection for recurrent HCC was associated with less blood loss and shorter hospital stays than open surgery, even in patients with cirrhosis. According to the long-term assessment, overall and DFS was similar between the two groups. Laparoscopic re-resection should be considered for patients who have undergone previous hepatectomy and present with recurrent HCC of less than or equal to 7 cm in size. Regardless, more extensive prospective trials are required to guide the optimal treatment choice for patients with recurrent HCC.
Recurrent hepatocellular carcinoma can be effectively treated with repeated liver resection (HCC). For recurrent HCC, few studies have compared the outcomes of laparoscopic repeat hepatectomy (LRH) with open repeat hepatectomy (ORH), and even fewer have included cirrhotic patients.
Currently, there is a lack of evidence of the effectiveness of LRH for the treatment of recurrent HCC in cirrhotic patients.
This study aimed to compare the short-term and long-term outcomes for cirrhotic patients with LRH and ORH for recurrent HCC. The study was intended to provide insights on performing LRH for cirrhotic patients with recurrent HCC.
A prospectively collected database identified all patients undergoing repeat hepatectomy for recurrent HCC between May 2006 and June 2021. Recurrent HCC with tumours > 7 cm were excluded. Patient demographics, operative details, perioperative outcomes, pathologic details, disease-free survival (DFS) and overall survival (OS) associated with LRH and ORH were compared.
Cirrhosis was histologically diagnosed in 62.7% of our patients and more than 90% were hepatitis B carriers. Blood loss (median 300 mL vs 200 mL, P = 0.013) and length of hospital stay (median 5 d vs 7 d, P = 0.003) were significantly better in the LRH group. There were no significant differences in the 1-, 3- and 5-year OS and DFS rates between the LRH and ORH groups.
Even in patients with cirrhosis, laparoscopic liver resection for recurrent HCC was associated with decreased blood loss, a shorter hospital stay, and equivalent overall and DFS to open surgery.
Laparoscopic re-resection should be considered for patients with recurrent HCC of less than or equal to 7 cm in size that develop subsequent to a previous hepatectomy. However, larger studies or randomised controlled trials should be conducted to confirm the advantages of LRH for the management of recurrent HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, India; Mazzarella G, Italy S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, Kosuge T, Okada S, Takayasu K, Yamasaki S. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 276] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, Lo CM. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Nakajima Y, Ko S, Kanamura T, Nagao M, Kanehiro H, Hisanaga M, Aomatsu Y, Ikeda N, Nakano H. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg. 2001;192:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Sugimachi K, Maehara S, Tanaka S, Shimada M, Sugimachi K. Repeat hepatectomy is the most useful treatment for recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, Lo CM. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: A Propensity Analysis at a Single Center. Ann Surg. 2016;264:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 10. | Memeo R, de'Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, Azoulay D. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg. 2014;38:2919-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, Hatano E, Tanahashi Y, Mizuguchi T, Kamiyama T, Ikeda T, Tanaka S, Taniai N, Baba H, Tanabe M, Kokudo N, Konishi M, Uemoto S, Sugioka A, Hirata K, Taketomi A, Maehara Y, Kubo S, Uchida E, Miyata H, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 12. | Xiang L, Li J, Chen J, Wang X, Guo P, Fan Y, Zheng S. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103:1895-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscarà C, Scotti M, Romito R, Mariani L, Mazzaferro V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Komatsu S, Brustia R, Goumard C, Perdigao F, Soubrane O, Scatton O. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surg Endosc. 2016;30:1965-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Ho KM, Cheng KC, Chan FK, Yeung YP. Laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: A propensity case-matched analysis of the long-term survival. Ann Hepatobiliary Pancreat Surg. 2021;25:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Violi F, Leo R, Vezza E, Basili S, Cordova C, Balsano F. Bleeding time in patients with cirrhosis: relation with degree of liver failure and clotting abnormalities. C.A.L.C. Group. Coagulation Abnormalities in Cirrhosis Study Group. J Hepatol. 1994;20:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1210] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 18. | Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27:2592-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, Ohira G, Nakajima T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2013;20:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Chan AC, Poon RT, Chok KS, Cheung TT, Chan SC, Lo CM. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg. 2014;38:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, Tan W, Zhang L. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc. 2017;31:4790-4798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Hallet J, Sa Cunha A, Cherqui D, Gayet B, Goéré D, Bachellier P, Laurent A, Fuks D, Navarro F, Pessaux P; French Colorectal Liver Metastases Working Group, Association Française de Chirurgie. Laparoscopic Compared to Open Repeat Hepatectomy for Colorectal Liver Metastases: a Multi-institutional Propensity-Matched Analysis of Short- and Long-Term Outcomes. World J Surg. 2017;41:3189-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Noda T, Eguchi H, Wada H, Iwagami Y, Yamada D, Asaoka T, Gotoh K, Kawamoto K, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M. Short-term surgical outcomes of minimally invasive repeat hepatectomy for recurrent liver cancer. Surg Endosc. 2018;32:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Ome Y, Hashida K, Yokota M, Nagahisa Y, Yamaguchi K, Okabe M, Kawamoto K. The feasibility and efficacy of pure laparoscopic repeat hepatectomy. Surg Endosc. 2018;32:3474-3479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Goh BKP, Syn N, Teo JY, Guo YX, Lee SY, Cheow PC, Chow PKH, Ooi LLPJ, Chung AYF, Chan CY. Perioperative Outcomes of Laparoscopic Repeat Liver Resection for Recurrent HCC: Comparison with Open Repeat Liver Resection for Recurrent HCC and Laparoscopic Resection for Primary HCC. World J Surg. 2019;43:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Inoue Y, Fujii K, Ishii M, Kagota S, Tomioka A, Hamamoto H, Osumi W, Tsuchimoto Y, Terasawa T, Ogura T, Masubuchi S, Yamamoto M, Imoto A, Asai A, Komeda K, Fukunishi S, Hirokawa F, Goto M, Tanaka K, Okuda J, Higuchi K, Uchiyama K. Laparoscopic Repeat Hepatic Resection for the Management of Liver Tumors. J Gastrointest Surg. 2019;23:2314-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | van der Poel MJ, Barkhatov L, Fuks D, Berardi G, Cipriani F, Aljaiuossi A, Lainas P, Dagher I, D'Hondt M, Rotellar F, Besselink MG, Aldrighetti L, Troisi RI, Gayet B, Edwin B, Abu Hilal M. Multicentre propensity score-matched study of laparoscopic versus open repeat liver resection for colorectal liver metastases. Br J Surg. 2019;106:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Onoe T, Yamaguchi M, Irei T, Ishiyama K, Sudo T, Hadano N, Kojima M, Kubota H, Ide R, Tazawa H, Shimizu W, Suzuki T, Shimizu Y, Hinoi T, Tashiro H. Feasibility and efficacy of repeat laparoscopic liver resection for recurrent hepatocellular carcinoma. Surg Endosc. 2020;34:4574-4581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Chan FK, Cheng KC, Yeung YP. Laparoscopic liver resection: lessons learnt after 100 cases. Hong Kong Med J. 2014;20:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002;4:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24842] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 33. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1727] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 34. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1413] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 35. | Menzies D, Ellis H. Intestinal obstruction from adhesions--how big is the problem? Ann R Coll Surg Engl. 1990;72:60-63. [PubMed] |
| 36. | Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg. 1973;126:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 297] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Gutt CN, Oniu T, Schemmer P, Mehrabi A, Büchler MW. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004;18:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Machairas N, Papaconstantinou D, Stamopoulos P, Prodromidou A, Garoufalia Z, Spartalis E, Kostakis ID, Sotiropoulos GC. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer Res. 2018;38:3181-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Belli G, Cioffi L, Fantini C, D'Agostino A, Russo G, Limongelli P, Belli A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc. 2009;23:1807-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Liu J, Zhao J, Gu HAO, Zhu Z. Repeat hepatic resection VS radiofrequency ablation for the treatment of recurrent hepatocellular carcinoma: an updated meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |