Published online Apr 27, 2022. doi: 10.4240/wjgs.v14.i4.362
Peer-review started: December 8, 2021
First decision: January 8, 2022
Revised: February 24, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: April 27, 2022
Processing time: 136 Days and 19.5 Hours
Schwannomas, also known as neurinomas, are benign tumors derived from Schwann cells. Gastrointestinal schwannomas are rare and are most frequently reported in the stomach. They are usually asymptomatic and are difficult to diagnose preoperatively; however, endoscopy and imaging modalities can provide beneficial preliminary diagnostic data. There are various surgical options for management. Here, we present a case of a large gastric schwannoma (GS) managed by combined laparoscopic and endoscopic surgery.
A 28-year-old woman presented with a 2-mo history of epigastric discomfort and a feeling of abdominal fullness. On upper gastrointestinal endoscopy and endoscopic ultrasonography, a hypoechogenic submucosal mass was detected in the gastric antrum: It emerged from the muscularis propria and projected intraluminally. Computed tomography showed a nodular lesion (4 cm × 3.5 cm), which exhibited uniform enhancement, on the gastric antrum wall. Based on these findings, a preliminary diagnosis of gastrointestinal stromal tumor was established, with schwannoma as a differential. Considering the large tumor size, we planned to perform endoscopic resection and to convert to laparoscopic treatment, if necessary. Eventually, the patient underwent combined laparoscopic and gastroscopic surgery. Immunohistochemically, the resected specimen showed positivity for S-100 and negativity for desmin, DOG-1, α-smooth muscle actin, CD34, CD117, and p53. The Ki-67 index was 3%, and a final diagnosis of GS was established.
Combined laparoscopic and endoscopic surgery is a minimally invasive and effective treatment option for large GSs.
Core Tip: Gastric schwannomas (GSs) do not have specific clinical and endoscopic characteristics. Therefore, preoperative diagnosis may be difficult, and they can be misdiagnosed as gastrointestinal stromal tumors. In addition, while laparoscopic resection is possible, it is difficult to determine the location of intraluminal tumors. In contrast, endoscopic resection is only suitable for small submucosal tumors. Here, we present a case of a GS excised using laparoscopic-gastroscopic cooperative surgery. Additionally, we performed a literature review on computed tomography findings and surgical interventions used in the management of gastrointestinal stromal tumors and GSs.
- Citation: He CH, Lin SH, Chen Z, Li WM, Weng CY, Guo Y, Li GD. Laparoscopic-assisted endoscopic full-thickness resection of a large gastric schwannoma: A case report. World J Gastrointest Surg 2022; 14(4): 362-369
- URL: https://www.wjgnet.com/1948-9366/full/v14/i4/362.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i4.362
Schwannomas are neurogenic tumors that emerge from Schwann cells. The most common site of a gastric schwannoma (GS) is the stomach, followed by the colon and rectum[1]. They usually arise from the muscular layer, with no specific clinical and endoscopic characteristics, and can frequently be misdiagnosed as gastrointestinal stromal tumors (GISTs), which are more common[2].
A GS can be managed by various surgical options, which have their advantages and disadvantages. Here, we report a case of a GS that was resected using combined gastroscopic and laparoscopic surgery.
A 28-year-old woman presented with a 2-mo history of epigastric discomfort and a feeling of abdominal fullness.
Two months before presentation, the patient developed epigastric discomfort, which was accompanied by a sensation of abdominal fullness. She did not experience abdominal pain, melena, and vomiting and exhibited no other symptoms of discomfort.
The patient was a non-smoker and did not drink alcohol. She reported no known food or drug allergies. Additionally, she had no history of blood transfusion or prior surgical procedure.
The patient reported no significant family history.
Clinical data on admission were as follows: Body temperature, 36 °C; blood pressure, 120/84 mmHg; heart rate, 80 beats/min; and respiratory rate, 16 breaths/min. The abdomen appeared flat and soft, and the patient did not experience any abdominal tenderness or rebound pain.
Routine blood tests, liver and kidney function tests, and electrolyte assay revealed no marked irregularities, and tumor markers were also negative.
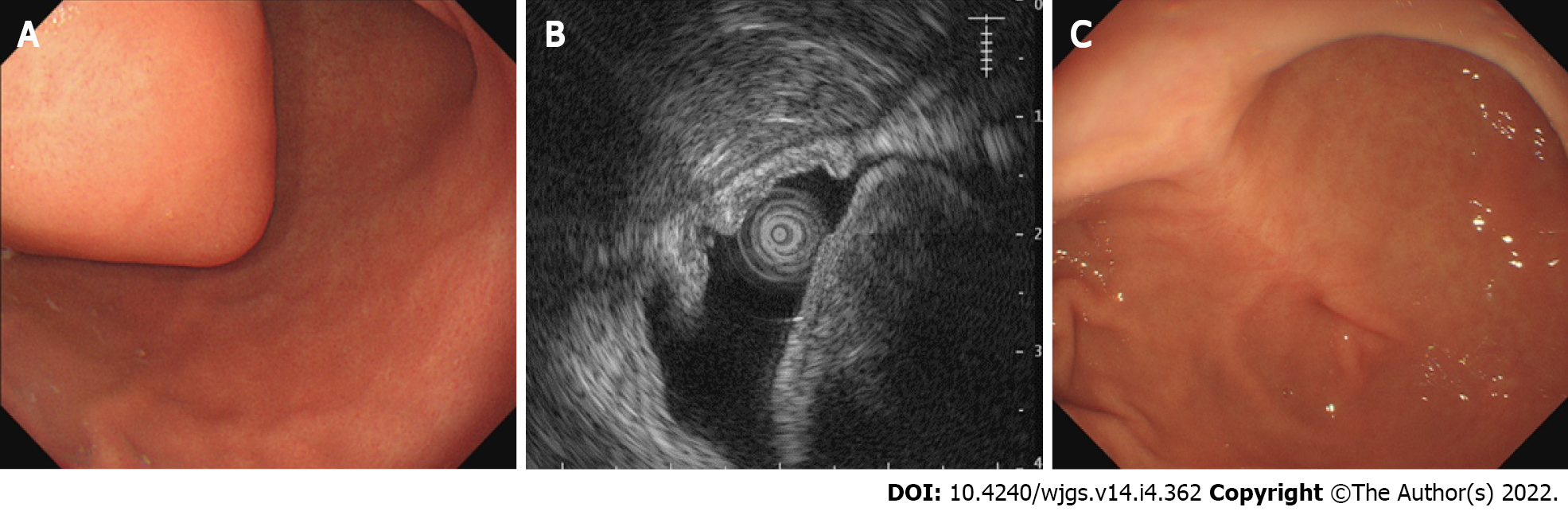
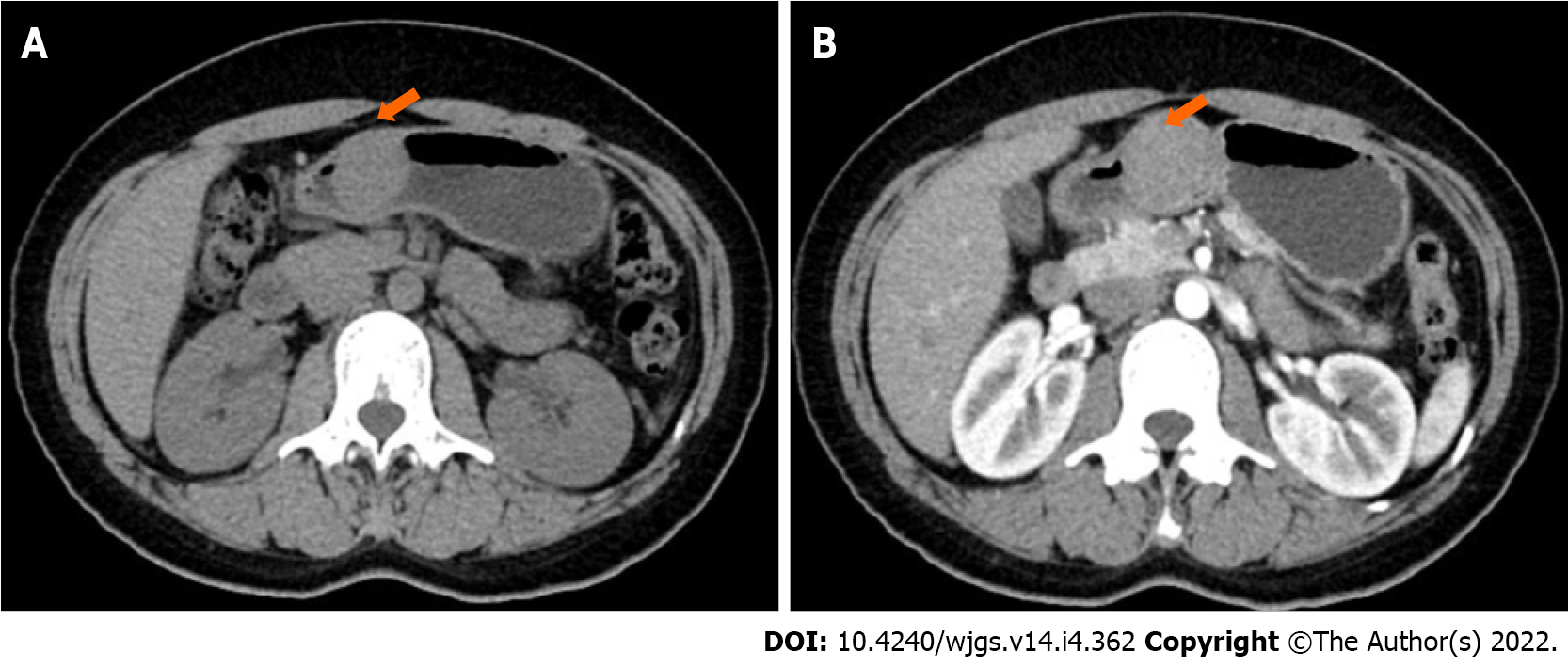
On upper gastrointestinal (GI) endoscopy and endoscopic ultrasonography (EUS), we detected a hypoechogenic submucosal mass, which arose from the muscularis propria and projected into the lumen, in the gastric antrum (Figure 1). Computed tomography (CT) images revealed a nodular lesion (4.5 cm × 4 cm) showing homogeneous enhancement on the gastric antrum wall (Figure 2).
A working diagnosis of GIST was established, with schwannoma as a differential.
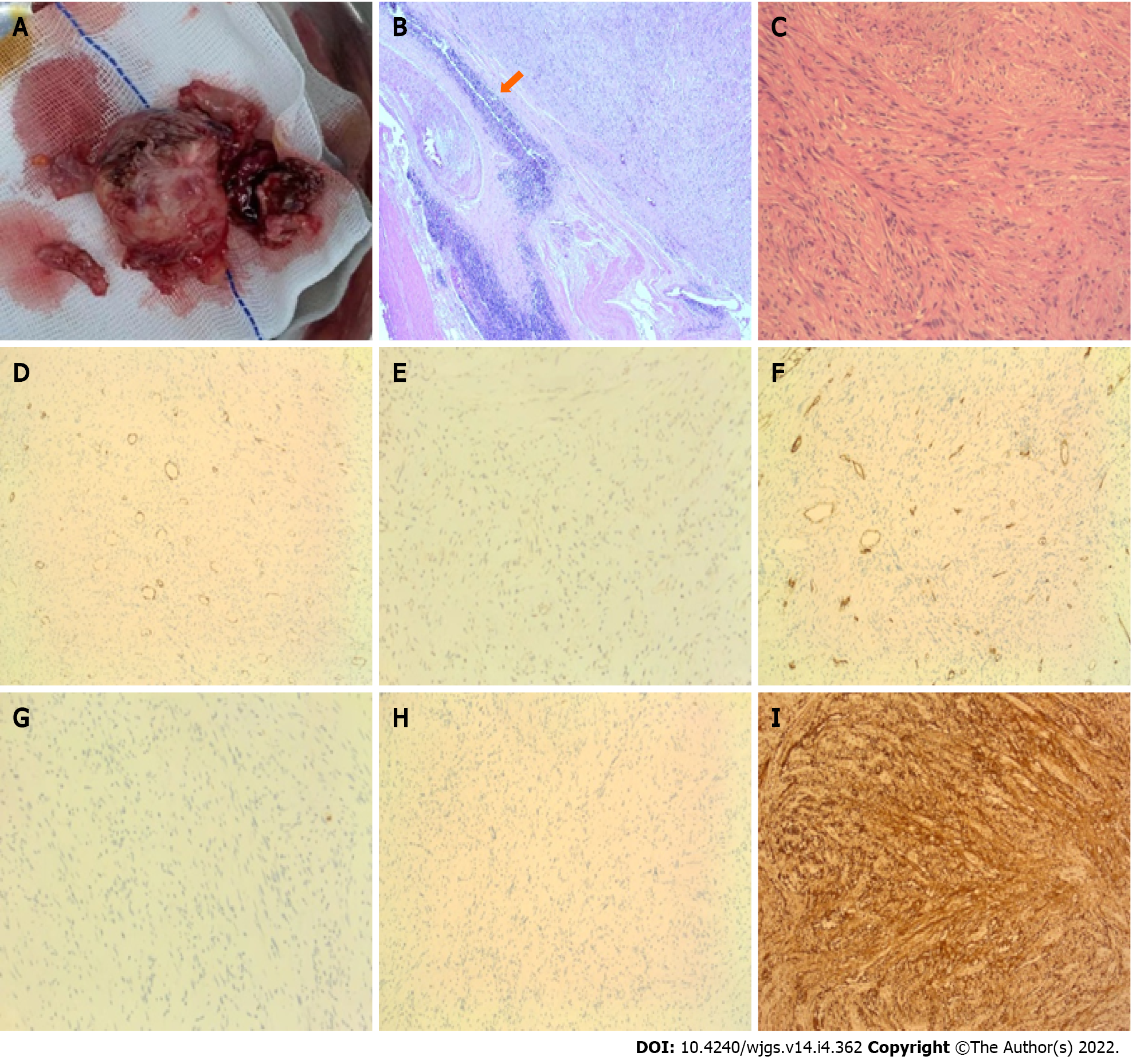
Histopathological examination confirmed that the tumor was localized within the gastric muscularis propria. The tumor was well circumscribed and comprised fusiform cells. Immunohistochemically, it showed S-100 (+), 3% Ki-67 index, desmin (-), DOG-1 (-), α-smooth muscle actin (-), CD34 (-), CD117 (-), and P53 (-). Accordingly, a final diagnosis of a GS was established (Figure 3).
First, endoscopic resection was performed: Endoscopic full-thickness resection (EFTR) was conducted under general anesthesia with endotracheal intubation. A smooth submucosal lesion measuring 5 cm in diameter was observed on the anterior wall of the gastric antrum. We marked the edge of the lesion, injected a solution of methylene blue and saline into the mucosa, and subsequently excised the tumor gradually using a hook knife. Bleeding was minimal and easily controlled with electric hemostatic forceps. Following a successful EFTR, a large full-thickness defect was left on the gastric wall. A supplementary laparoscopic surgery was conducted considering the large defect size and difficulties with endoscopic closure and tumor extraction via the esophagus. The patient was placed in a supine position, and a tiny arc-shaped incision was made under the umbilicus. Next, the abdominal cavity was punctured using a pneumoperitoneum (PP) needle and filled with CO2 gas to generate a peak pressure of 1.59 kPa. The PP needle was then removed. Subsequently, a cannula needle was used to puncture the abdominal cavity. The inner core of the cannula was removed, and the needle was placed into a laparoscope. Two trocar punctures were made on the left and right sides of the abdomen using the open technique. A defect measuring 5.5 cm × 5 cm was detected on the anterior wall of the gastric antrum, approximately 2 cm from the pylorus, and surrounded by small amounts of bloody fluid. The large excised tumor measuring 5 cm × 4 cm dropped into the abdominal cavity and was placed in an extraction pouch, which was subsequently removed via the main surgical incision. The edge of the defect on the stomach wall was trimmed using an ultrasonic knife. Subsequently, the wound was closed with a 3-0 slippery thread. Finally, we confirmed the absence of bleeding in the abdominal cavity, extracted the laparoscope, checked for appropriate retrieval of all instruments and gauze, and closed the incision and puncture sites with silk thread.
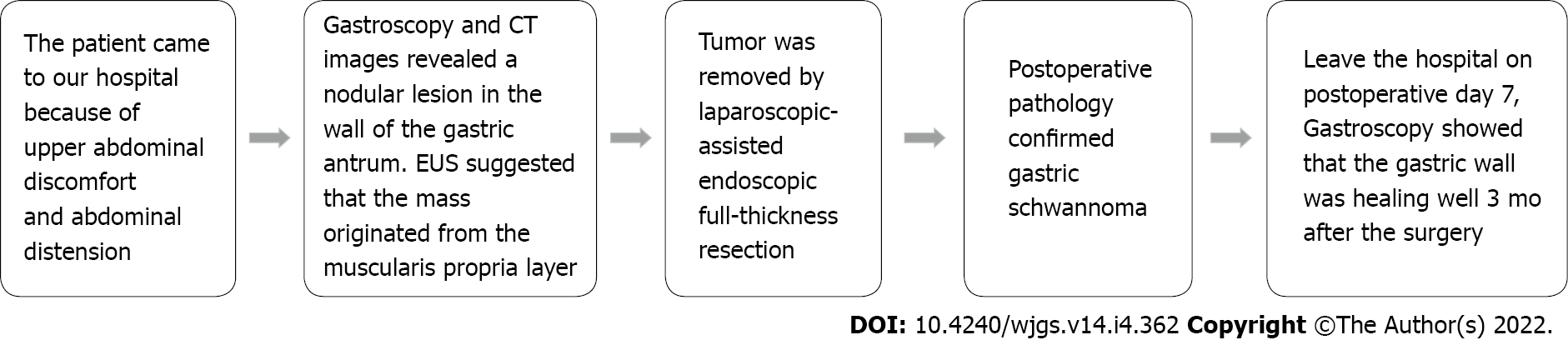
The patient recovered fully and was discharged on postoperative day 7, and a check-up was performed 3 mo after the surgery. Gastroscopy showed an improvement in the healing of the gastric wall. Figure 4 illustrates the timeline of the clinical course of the patient.
GI mesenchymal tumors comprise a wide range of spindle cell tumors, including GISTs, leiomyomas, leiomyosarcomas, and schwannomas[3]. Furthermore, schwannomas are spindle cell mesenchymal tumors that originate from Schwann cells. GSs originate from the gastrointestinal neural plexus. Most GSs are benign, and only a few malignant cases have been reported in the literature[4,5]. Schwannomas are generally asymptomatic in affected patients; however, they may cause abdominal discomfort, pain, or digestive symptoms in some cases. A palpable mass may be detected if the tumor is large and exophytic. Dysphagia and obstipation are possible symptoms when the lesions originate from the esophagus or rectum, respectively. Bleeding may occur if deep ulcerations are present[6,7].
GISTs are the most prevalent mesenchymal tumors of the GI tract, and 60%–70% of cases occur in the stomach. They are similar to GSs in terms of age of onset, clinical manifestations, and gross and histological appearance; however, the prognoses differ. Generally, schwannomas are mostly benign and have a good prognosis, while 10%–30% of cases of GIST are malignant[3]. Therefore, it is essential to distinguish between a GS and GIST and to develop a targeted treatment plan. The diagnostic workup for gastric tumors mainly includes upper GI endoscopy, CT, magnetic resonance imaging, and intracavitary (endoscopic) ultrasound. On endoscopy, both GS and GIST present as elevated submucosal lesions with a firm consistency. On EUS, a GS usually shows a hypoechogenic lesion originating from the muscularis propria[8]. Reports on EUS assessment show that round shape, definite borders, heterogeneous hypoechogenicity or isoechogenicity, and lack of cystic alteration and calcification are crucial markers for GS diagnosis. In contrast, on EUS, a GIST usually shows a hypoechoic or anechoic and slightly heterogeneous tumor. Hyperechogenicity is a potential sign of malignancy. GISTs are usually observed in the third or fourth layer of the gastric wall and rarely in the second layer[8]. Unlike GISTs, on CT, schwannomas appear to be uniform, significantly contrast-enhancing tumors with no evidence of hemorrhage, necrosis, cystic alteration, or calcification[9]. Despite these differences, establishing accurate preoperative diagnoses of GSs and GISTs is challenging.
In this patient, the tumor was detected on abdominal CT and was initially thought to be a GIST. Gastroscopic and EUS findings were not contradictory; therefore, the tumor was misdiagnosed as a GIST until a correct diagnosis was established based on the tumor’s immunohistochemical profile.
A GS rarely presents with specific clinical features and imaging characteristics. Therefore, preoperative diagnosis is challenging, and definitive diagnosis can only be established after careful pathological examination of the resected specimen. Given these challenges, surgical resection is the optimal treatment approach. Local extirpation, wedge resection, and partial, subtotal, or total gastrectomy are all acceptable approaches. Laparoscopic techniques can also be employed[10].
Submucosal gastric tumor therapies have greatly advanced in recent years, thereby enabling a more frequent use of minimally invasive endoscopic techniques, such as snare polypectomy, endoscopic submucosal dissection, and EFTR. Some studies have shown that EFTR is safe and effective for schwannomas and other tumors originating from the muscularis propria[11,12]. However, for larger GSs, endoscopic resection should not be indicated without careful consideration because we believe that this could increase the risk of surgery and the incidence of postoperative complications.
Although laparoscopic resection can be used to treat GSs, it is difficult to precisely locate tumors within the gastric lumen with a laparoscope from the serosal surface alone. Consequently, a large portion of the stomach wall may be removed, leading to gastric deformity and outlet obstruction. Laparoscopic endoscopic cooperative surgery (LECS) was first introduced by Hiki et al[13] as a surgical intervention for GISTs and is currently classified as “classical LECS.” LECS is superior to laparoscopic or robot-assisted wedge resection and partial resection because the gastric serosa resection area is substantially reduced, which lowers the possibility of post-surgical gastric deformity and reduces the negative impact on patients’ quality of life[14]. Subsequently, Mitsui et al[15] developed another non-exposure technique, known as “non-exposure endoscopic wall-inversion surgery” (NEWS), that can prevent contamination and tumor dissemination into the peritoneal cavity. Only a few studies[13,15-19] have previously reported GS resection using LECS and NEWS (Table 1). Shoji et al[20] reported that LECS or NEWS is suitable for submucosal tumors measuring less than 5 cm in diameter. In this case, because the diameter of the gastric tumor reached 5 cm, we considered that endoscopic treatment alone might be complicated by difficulties in closing the gastric wall defect after tumor excision and removing the specimen through the esophagus. Therefore, after discussing with the patient, we decided to remove the tumor endoscopically, and if difficulties arose, laparoscopy would be performed. Accordingly, we could excise the tumor completely without removing a large part of the gastric wall while causing minimal trauma and ensuring safety. The tumor was removed using a gastroscope. The large defect in the gastric wall after tumor resection was difficult to close; therefore, suturing was performed laparoscopically. This combined surgery resulted in complete tumor excision and prevented wound expansion. Although our procedure differed from classical LECS in terms of surgical details, the goal of treatment was still to achieve complete resection of the lesion and avoid the expansion of the incision. Postoperative patient management included gastric acid inhibition, fluid replacement, dietary restriction, and nutritional support. The patient was mobile on postoperative day 1. She recovered completely and was discharged from the hospital 1 wk after surgery. Considering the outcomes of this case, we believe that laparoscopic-assisted endoscopic full-thickness resection can reduce the risk of endoscopic surgery and simultaneously achieve precise resection of lesions, which should be evaluated in future studies.
| Ref. | Gender | Age (yr) | No. of cases | Tumor size (cm) | Pathology | Treatment | Hospital stay (d) |
| Eom et al[16] | 3 Males; 11 Females | Median 61.0 IQR (51.0–66.8) | 14 | Median 2.6 IQR (2.3–3.7) | 9 GISTs, 2 GS, 3 Leiomyomas | LECS | Median 5.0 IQR (4.0– 5.5) |
| Mahawongkajit et al[17] | Female | 50 | 1 | 2.1 | GS | NEWs | NR |
| Sugiyama et al[18] | Female | 49 | 1 | 1.7 | GS | NEWS | 5 |
| Matsuda et al[19] | 47 Males; 53 Females | mean ± SD: 59.8 ± 13.2 | 100 | mean ± SD: 3.09 ± 1.06 | 75 GISTs; 11 GS; 6 Leiomyomas; 5 Ectopic pancreas; 2 Neuroendocrine tumor; 1 Lymphangioma | LECS | mean ± SD: 8.4 ± 10.2 |
| Mitsui et al[15] | Males | 58 | 1 | 2.4 × 2.3 × 1.9 | GS | NEWS | 7 |
| Hiki et al[13] | 7 Females | Range 34–66 | 7 | mean ± SD: 4.6 ± 0.3 | 6 GISTs 1 GS | LECS | mean ± SD: 7.4 ± 8.1 |
GSs are uncommon and generally mostly benign. Despite advances in endoscopic and imaging techniques, accurate preoperative diagnosis of a GS is difficult to establish. Final diagnosis requires histopathological and immunohistochemical examinations. Surgical resection is the optimal treatment option, and the emergence of techniques, such as EFTR, has greatly increased the possibility of minimally invasive removal of small tumors. For larger GSs, combined laparoscopic and gastroscopic surgery is recommended for tumor resection.
The authors thank Jian-Liang Wu and Li-Wei Sun for assisting in the preparation of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Kung WM, Taiwan; Sharaf MM, Syria; Sperti C, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Hong HS, Ha HK, Won HJ, Byun JH, Shin YM, Kim AY, Kim PN, Lee MG, Lee GH, Kim MJ. Gastric schwannomas: radiological features with endoscopic and pathological correlation. Clin Radiol. 2008;63:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Hsu WH, Wu TS, Hsieh MS, Kung YM, Wang YK, Wu JY, Yu FJ, Kuo CH, Su YC, Wang JY, Wu DC, Hu HM. Comparison of Endoscopic Submucosal Dissection Application on Mucosal Tumor and Subepithelial Tumor in stomach. J Cancer. 2021;12:765-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 4. | Loffeld RJ, Balk TG, Oomen JL, van der Putten AB. Upper gastrointestinal bleeding due to a malignant Schwannoma of the stomach. Eur J Gastroenterol Hepatol. 1998;10:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Takemura M, Yoshida K, Takii M, Sakurai K, Kanazawa A. Gastric malignant schwannoma presenting with upper gastrointestinal bleeding: a case report. J Med Case Rep. 2012;6:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, Wang J, Zhu XZ. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Lin CS, Hsu HS, Tsai CH, Li WY, Huang MH. Gastric schwannoma. J Chin Med Assoc. 2004;67:583-586. [PubMed] |
| 8. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005;184:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Basso N, Rosato P, De Leo A, Picconi T, Trentino P, Fantini A, Silecchia G. Laparoscopic treatment of gastric stromal tumors. Surg Endosc. 2000;14:524-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Zhai YQ, Chai NL, Li HK, Lu ZS, Feng XX, Zhang WG, Liu SZ, Linghu EQ. Endoscopic submucosal excavation and endoscopic full-thickness resection for gastric schwannoma: five-year experience from a large tertiary center in China. Surg Endosc. 2020;34:4943-4949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 12. | Jain D, Mahmood E, Desai A, Singhal S. Endoscopic full thickness resection for gastric tumors originating from muscularis propria. World J Gastrointest Endosc. 2016;8:489-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 14. | Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 15. | Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K, Seto Y. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Eom BW, Kim CG, Kook MC, Yoon HM, Ryu KW, Kim YW, Rho JY, Kim YI, Lee JY, Choi IJ. Feasibility of Non-Exposure Simple Suturing Endoscopic Full-Thickness Resection in Comparison with Laparoscopic Endoscopic Cooperative Surgery for Gastric Subepithelial Tumors: Results of Two Independent Prospective Trials. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 17. | Mahawongkajit P, Chanswangphuvana P. Laparoscopy-assisted endoscopic full-thickness resection of upper gastrointestinal subepithelial tumors: A single-center early experience. Mol Clin Oncol. 2020;12:461-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Sugiyama T, Ebi M, Ochiai T, Kurahashi S, Saito T, Onishi K, Yamamoto K, Inoue S, Adachi K, Yoshimine T, Yamaguchi Y, Tamura Y, Izawa S, Hijikata Y, Funaki Y, Ogasawara N, Sasaki M, Kasugai K. Gastric schwannoma with high accumulation on fluorodeoxyglucose-positron emission tomography resected by non-exposed endoscopic wall-inversion surgery. Clin J Gastroenterol. 2020;13:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T, Yamaguchi T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. 2016;84:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Shoji Y, Takeuchi H, Goto O, Tokizawa K, Nakamura R, Takahashi T, Wada N, Kawakubo H, Yahagi N, Kitagawa Y. Optimal minimally invasive surgical procedure for gastric submucosal tumors. Gastric Cancer. 2018;21:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |