Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.1079
Peer-review started: January 26, 2021
First decision: March 29, 2021
Revised: April 3, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: September 27, 2021
Processing time: 234 Days and 23.8 Hours
Hepatic resection (HR) results in an inflammatory response that can be modified by perioperative steroid administration. However, it remains to be determined if this response's attenuation translates to a reduction in complications.
To evaluate if perioperative administration of steroids reduces complications following HR.
A systematic review of randomized controlled trials (RCTs) was conducted on PubMed, Embase, and Cochrane Central Register of Controlled Trials to evaluate the effect of perioperative steroid (compared to placebo or no intervention) use in patients undergoing HR. Clinical outcomes were extracted, and meta-analysis was performed.
8 RCTs including 590 patients were included. Perioperative steroid administration was associated with significant reduction in postoperative complications [odds ratios: 0.58; 95% confidence intervals (CI): 0.35-0.97, P = 0.04]. There was also improvement in biochemical and inflammatory markers, including serum bilirubin on postoperative day 1 [MD: -0.27; 95%CI: (-0.47, -0.06), P = 0.01], C-reactive protein on postoperative day 3 [MD: -4.89; 95%CI: (-5.83, -3.95), P < 0.001], and interleukin-6 on postoperative day 1 [MD: -54.84; 95%CI: (-63.91,
Perioperative steroids administration in HR may reduce overall complications, postoperative bilirubin, and inflammation. Further studies are needed to determine the optimal dose and duration and patient selection.
Core Tip: Hepatic resection results in an inflammatory response that can be modified by perioperative steroid administration. However, it remains to be determined if this response's attenuation translates to a reduction in complications. This systematic review compares eight randomized controlled trials including 590 patients. We found that perioperative steroid administration was associated with a significant reduction in postoperative complications. There was also improvement in biochemical and inflammatory markers.
- Citation: Hai HH, Aw P, Teng TZJ, Shelat VG. Perioperative steroid administration reduces overall complications in patients undergoing liver resection: A meta-analysis. World J Gastrointest Surg 2021; 13(9): 1079-1094
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/1079.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.1079
Stress from major surgery results in an inflammatory response secondary to cytokine and free radical release. This inflammatory response is essential for healing and restoring physiologic function; however, it can increase morbidity, mortality and worsen postoperative outcomes if excessive[1]. Modifying the inflammatory response by perioperative steroid administration could improve surgical outcomes. In a rabbit experiment conducted almost five decades ago, Santiago Delpín et al[2] demonstrated that preoperative methylprednisolone administration attenuated the inflammatory response secondary to hepatic inflow occlusion[2] and improved survival as compared to postoperative or no steroid administration and concluded that methylprednisolone protects liver during warm ischemia.
With a better understanding of liver anatomy, advances in surgical technology, and improvements in critical care, hepatic resection (HR) is accepted as a gold standard treatment for primary and metastatic liver cancers[3]. HR is major abdominal surgery with the potential for blood loss, tissue hypoperfusion, and acidosis[4]. Further, low central venous pressure anesthesia and hepatic inflow occlusion with resulting ischemia-reperfusion injury aggravate the inflammatory response[5,6]. Perioperative morbidity and mortality following elective HR, though has reduced, further improvements[7] are needed. Besides, inflammatory markers are increasingly shown to predict short-term perioperative and long-term oncologic outcomes following HR[8]. Thus, modulation of inflammatory response to improve surgical outcomes remains an unmet need in hepatic surgery. In a systematic review and meta-analysis on the benefit of preoperative steroid administration in patients undergoing HR, Yang et al[9] has shown a reduction in postoperative day 1 bilirubin, postoperative day 1 interleukin-6 (IL-6), and postoperative day 3 C-reactive protein (CRP) levels but no difference in liver failure, bile leak, infectious complications, wound complications and pleural effusion. It is unclear if the advantages of perioperative steroid immunomodulation are nullified by inherent risks associated with steroid therapy itself: Hyperglycemia, predisposition to infection, impairment of wound healing, and reactivation of the hepatitis virus[10]. Thus, it remains to be determined if an inflammatory response's attenuation translates to a reduction in perioperative complications, and more evidence is needed. We report an updated meta-analysis on the benefits of perioperative steroid administration in patients undergoing HR.
The search was conducted according to the PRISMA statement[11]. A literature search of published studies on perioperative use of steroids in PubMed, EMBASE, and Cochrane Library databases was independently conducted by two co-authors (Aw P and Hai HH). A combination of subject headings and text words were used as needed to define the use of steroids in liver resection. We employed the terms ("Liver surger*" OR "Liver resection*" OR "Resection of Liver" OR "Resection Liver" OR "HR*" OR Hepatectom* OR "Liver remov*" OR "Liver lobectom*" OR "Hepatic Lobectom*" OR "Lobectomy of Liver" OR "Hemihepatectom*") and ("Steroids" OR "Betamethasone" OR "Dexamethasone" OR "Prednisone" OR "Prednisolone" OR "Fluprednisolone" OR "Methylprednisolone" OR "Fluocortolone" OR "Paramethasone" OR "Cortisone" OR "Cortisol" OR "Hydrocortisone" OR "Fludrocortisone" OR "Hydroxycorticosteroids" OR "Glucocorticoids" OR "Corticosteroids").
Only prospective randomized controlled trials (RCTs), either open-label, single or double-blinded, were included in this study. Inclusion criteria were major or minor HR, regardless of pathology. Studies that involved perioperative prophylactic administration of steroids in improving the outcome of HR and which contained a control group (placebo or no intervention) were included. For studies to be included, the outcomes measured should include the following postoperative liver function tests: Bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), or prothrombin time. When there was a duplication of data, the most recent and comprehensive study was included. Articles not written in English or Mandarin language were excluded. The co-author (Aw P) is a native Mandarin language speaker and translated the Mandarin report into English. We excluded case series and non-human studies. Identified studies were assessed independently by two co-authors (Aw P and Hai HH) for potential inclusion, initially by title and abstract. After the initial screening, the full text was obtained and reviewed in its entirety. Any conflict was resolved by consensus or in consultation with the senior author (Shelat VG). References of included studies were screened to identify for additional study.
From each included study, two co-authors (Aw P and Hai HH) independently extracted the following information: Publication details (first author, year of publication), study characteristics (study design, intervention and control group sample, gender, age, type of resection), and study outcomes (postoperative outcomes, morbidity, and mortality). A meta-analysis was performed with data available on postoperative outcomes following perioperative steroids administration using Review Manager Version 5.3 (Cochrane Collaboration). Odds ratios (OR) and 95% confidence intervals (CI) were calculated using the Cochrane Mantel Haenszel method test based on the random effects model or dichotomous data, while continuous data were calculated using weighted mean differences and 95%CI. The level of statistical significance was set at P < 0.05. To assess heterogeneity between the studies, I2 was calculated to estimate the variability among the included studies. An I2 of > 50% suggested considerable heterogeneity. Studies that presented data in graphical form were omitted as it was not possible to extract the data accurately and attempts to contact the authors of the original studies were unsuccessful. Data excluded are postoperative day 1 (POD1) AST and ALT in the study by Hayashi et al[12] and POD1 Bilirubin and ALT from the study by Donadon et al[13].
The primary outcomes of interest included the clinical outcomes–postoperative morbidity, length of hospital stay, and mortality. Where possible, data on specific subcategories of complications (hepatobiliary, pulmonary, and infectious complications) were extracted and analyzed. The secondary outcomes of interest included serological outcomes - postoperative liver function parameters (bilirubin, AST, ALT, prothrombin time) and postoperative inflammatory markers (CRP, IL-6).
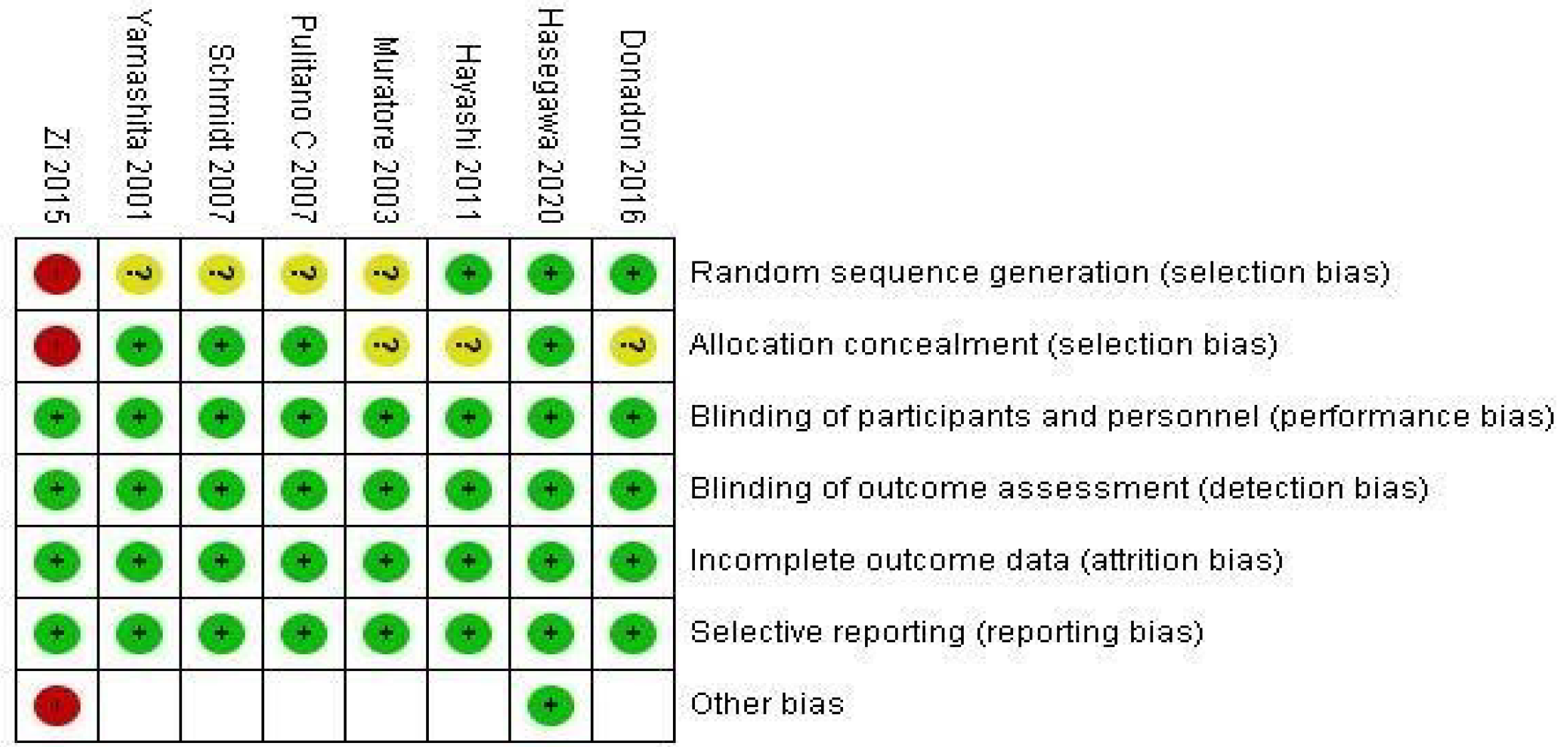
Two co-authors (Aw P and Hai HH) independently assessed and checked the included studies to ensure consistency and check for bias risk. The various risks of bias assessment were done using Review Manager Version 5.3. The biases involving random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias, were considered. The GRADE approach[14] was used to evaluate the quality of the body of evidence for each outcome measure.
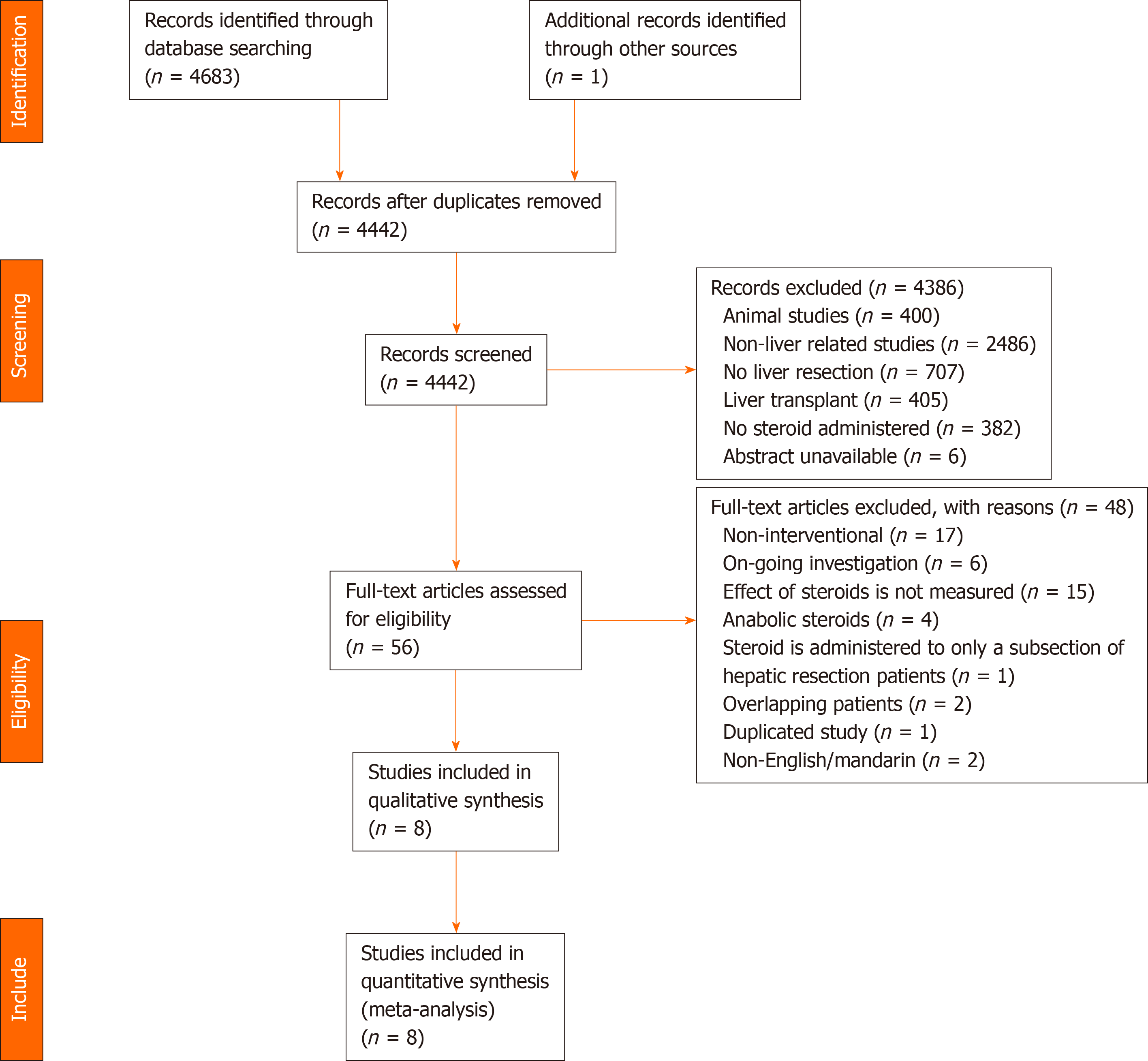
A systematic search identified 4684 studies, of which 242 duplicate studies were excluded. Subsequent screening of title and abstract was performed independently by two authors (Aw P and Hai HH), and 61 studies were identified for full-text evaluation. Finally, eight prospective RCTs involving 590 patients were included in this meta-analysis (Table 1 and Table 2). A detailed diagram showing the search process is shown in Figure 1. In total, 296 patients were randomized into the intervention group, consisting of various regimens of perioperative administration of steroids, and 294 patients into the control group (Table 3).
| Ref. | Number | Age | Sex (M/F) | Pathology | |||||
| Steroid | Control | Steroid | Control | Steroid | Control | Steroid | Control | ||
| Donadon et al[13], 2016 | 16 | 16 | 65 (27-80)1 | 63 (22-77)1 | 10/6 | 9/7 | Metastasis | 6 | 12 |
| HCC | 6 | 2 | |||||||
| Cholangiocarcinoma | 4 | 0 | |||||||
| Others | 0 | 2 | |||||||
| Hasegawa et al[15], 2020 | 50 | 50 | 67 (59-74)2 | 68 (62–75)2 | 30/20 | 31/19 | Metastasis | 21 | 14 |
| Hayashi et al[12], 2011 | 102 | 98 | 69 (39-81)1 | 70 (35-82)1 | - | - | Metastasis | 32 | 23 |
| HCC | 63 | 66 | |||||||
| Cholangiocarcinoma | 6 | 5 | |||||||
| Others | 1 | 4 | |||||||
| Muratore et al[20], 2003 | 25 | 28 | 65.4 ± 10.83 | 64.1 ± 11.73 | 8/17 | 11/17 | - | - | - |
| Pulitanò et al[17], 2007 | 37 | 36 | 63 (31-85)4 | 61.8 (21-78)4 | 23/14 | 22/14 | Metastasis | 16 | 14 |
| HCC | 12 | 14 | |||||||
| Cholangiocarcinoma | 5 | 4 | |||||||
| Hemangioendothelioma | 1 | 1 | |||||||
| Benign | 3 | 3 | |||||||
| Schmidt et al[18], 2007 | 10 | 10 | 575 | 655 | 3/7 | 4/6 | HCC | 2 | 1 |
| Metastasis | 4 | 4 | |||||||
| Cholangiocarcinoma | 0 | 2 | |||||||
| Benign | 4 | 3 | |||||||
| Yamashita et al[16], 2001 | 17 | 16 | 60.3 ± 1.86 | 56.8 ± 3.96 | 4/13 | 11/5 | HCC | 13 | 8 |
| Metastasis | 0 | 3 | |||||||
| Cholangiocarcinoma | 0 | 1 | |||||||
| Living donor of transplant | 4 | 4 | |||||||
| Zi et al[19], 2015 | 40 | 39 | 57.55 ± 8.813 | 57.51 ± 10.323 | 23/17 | 17/22 | Malignant | 31 | 34 |
| Benign | 9 | 5 | |||||||
| Ref. | Type of resection | Duration of operation/min | Volume of blood loss/mL | Volume of liver resected/g | |||||
| Steroid | Control | Steroid | Control | Steroid | Control | Steroid | Control | ||
| Donadon et al[13], 2016 | Minor | 9 | 11 | 383.5 (235-546)1 | 351 (226-640)1 | 275 (100-1000)1 | 200 (0-700)1 | - | - |
| Major | 7 | 5 | |||||||
| Hasegawa et al[15], 2020 | Minor | 40 | 39 | 215 (170-294)2 | 233 (157-270)2 | 52 (29-149)2 | 34 (17-76)2 | - | - |
| Major | 10 | 11 | |||||||
| Hayashi et al[12], 2011 | Hemihepactectomy | 11 | 15 | 330 (165-834)1 | 316 (136-697)1 | 324 (5-1577)1 | 257 (10-1972)1 | 77 (4-1930)1 | 75 (6-1300)1 |
| Segmentectomy | 59 | 57 | |||||||
| Limited resection | 32 | 26 | |||||||
| Muratore et al[20], 2003 | Minor | 12 | 13 | - | - | 322.8 ± 261.43 | 294.6 ± 271.93 | - | - |
| Major | 13 | 15 | |||||||
| Pulitanò et al[17], 2007 | Right hepactectomy | 11 | 10 | 440 (220-480)1 | 408 (240-460)1 | 662 (300-800)1 | 621 (350-720)1 | 40.4 ± 203 (%) | 39.5 ± 183 (%) |
| Left hepatectomy | 6 | 8 | |||||||
| Extended right hepatectomy | 5 | 4 | |||||||
| Extended left hepatectomy | 2 | 2 | |||||||
| Bisegmentectomy | 6 | 4 | |||||||
| Segmentectomy | 4 | 5 | |||||||
| Wedge resection | 3 | 3 | |||||||
| Schmidt et al[18], 2007 | Segment II/III | 4 | 5 | 2224 | 2524 | 3404 | 7804 | - | - |
| Segment V-VIII | 6 | 5 | |||||||
| Yamashita et al[16], 2001 | Lobectomy or more | 5 | 6 | 338 ± 215 | 352 ± 145 | 892 ± 1065 | 822 ± 555 | 239 ± 505 | 187 ± 335 |
| Segmentectomy | 2 | 1 | |||||||
| Subsegmentectomy or less | 10 | 9 | |||||||
| Zi et al[19], 2015 | Major | 22 | 12 | 342.38 ± 129.733 | 353.21 ± 168.363 | 481.25 ± 415.983 | 496.15 ± 391.593 | - | - |
| Minor | 18 | 27 | |||||||
| Ref. | Study design | Country | Steroid protocol | Control protocol |
| Donadon et al[13], 2016 | RCT | Italy | Preoperative bolus infusion (5%) of 500 mg methylprednisolone in 250 mL glucose for 1 h prior to hepatectomy, then continuous infusion for 6 h | 250 mL glucose-5% as bolus and subsequent continuous infusion |
| Hasegawa et al[15], 2020 | RCT | Japan | Preoperative, up to 500 mg methylprednisolone in saline solution | Saline only |
| Hayashi et al[12], 2011 | RCT | Japan | Intraoperative 500 mg hydrocortisone immediately prior to hepatic-pedicle clamping 300 mg hydrocortisone on POD1 200 mg on POD 2, 100 mg on POD3 | 0 |
| Muratore et al[20], 2003 | RCT | Italy | Preoperative (30 min before surgery) 30 mg/kg per body weight IV methylprednisolone | 0 |
| Pulitanòet al[17], 2007 | RCT | Italy | Preoperative 500 mg methylprednisolone | 0 |
| Schmidt et al[18], 2007 | RCT | Germany | Preoperative (90 min before surgery) 30 mg/kg per body weight IV methylprednisolone | 50 mL IV saline |
| Yamashita et al[16], 2001 | RCT | Japan | Preoperative (2 h before surgery) 500 mg IV methylprednisolone | 0 |
| Zi et al[19], 2015 | RCT | China | Intraoperative 500 mg methylprednisolone prior to liver resection | Hepatectomy only |
The steroids used (Table 3) included methylprednisolone[13,15-20] and hydrocortisone[12], with varying methods of administration. All except one study[12] administered the steroid regime preoperatively, with Hayashi et al[12] administering steroids intraoperatively immediately before hepatic-pedicle clamping and subsequent steroid administration on postoperative days 1-3. We elected not to exclude the study by Hayashi et al[12] merely due to the difference in choice of steroid as the dose and duration were appropriately modified for equipotency for efficacy.
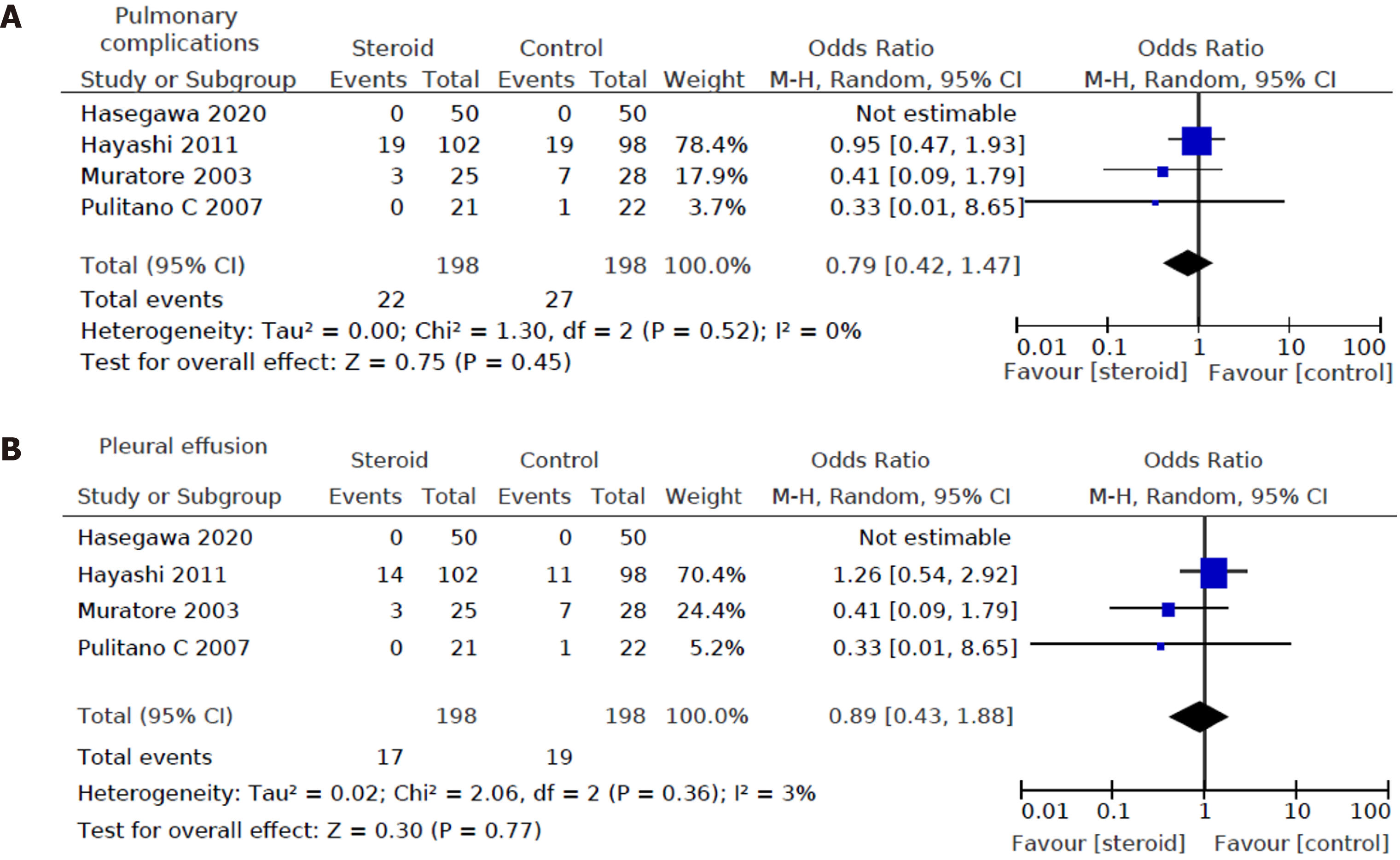
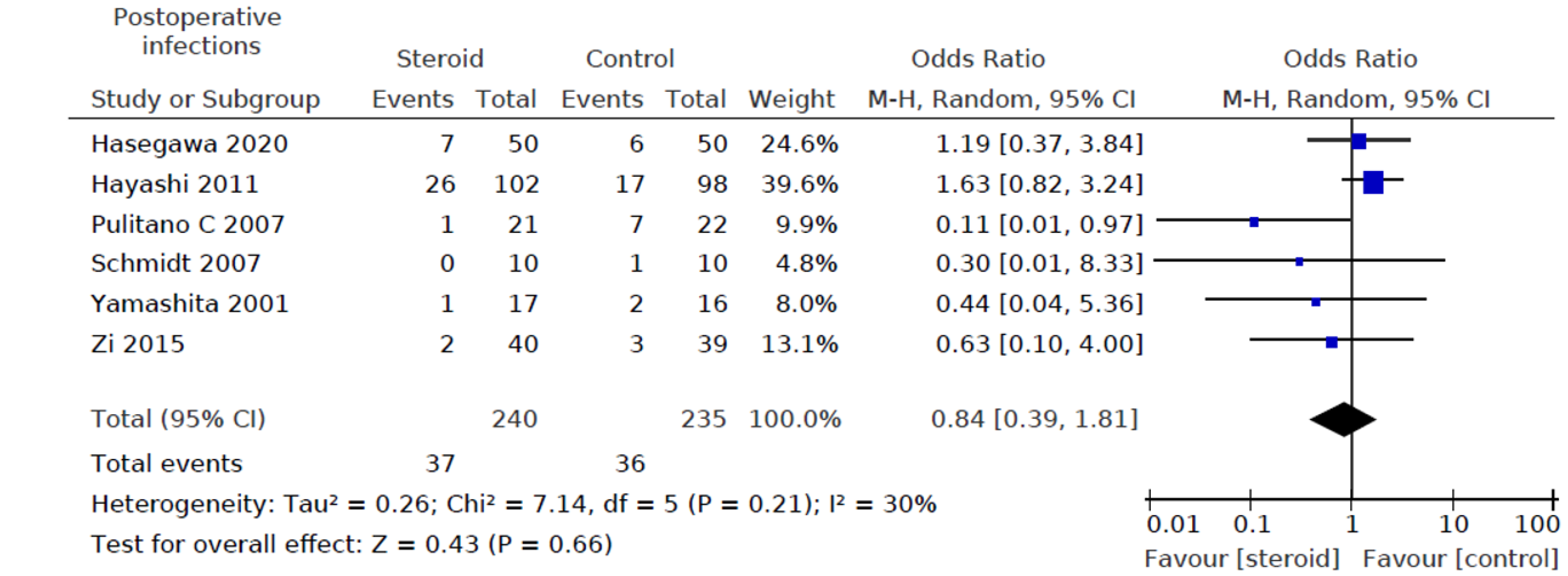
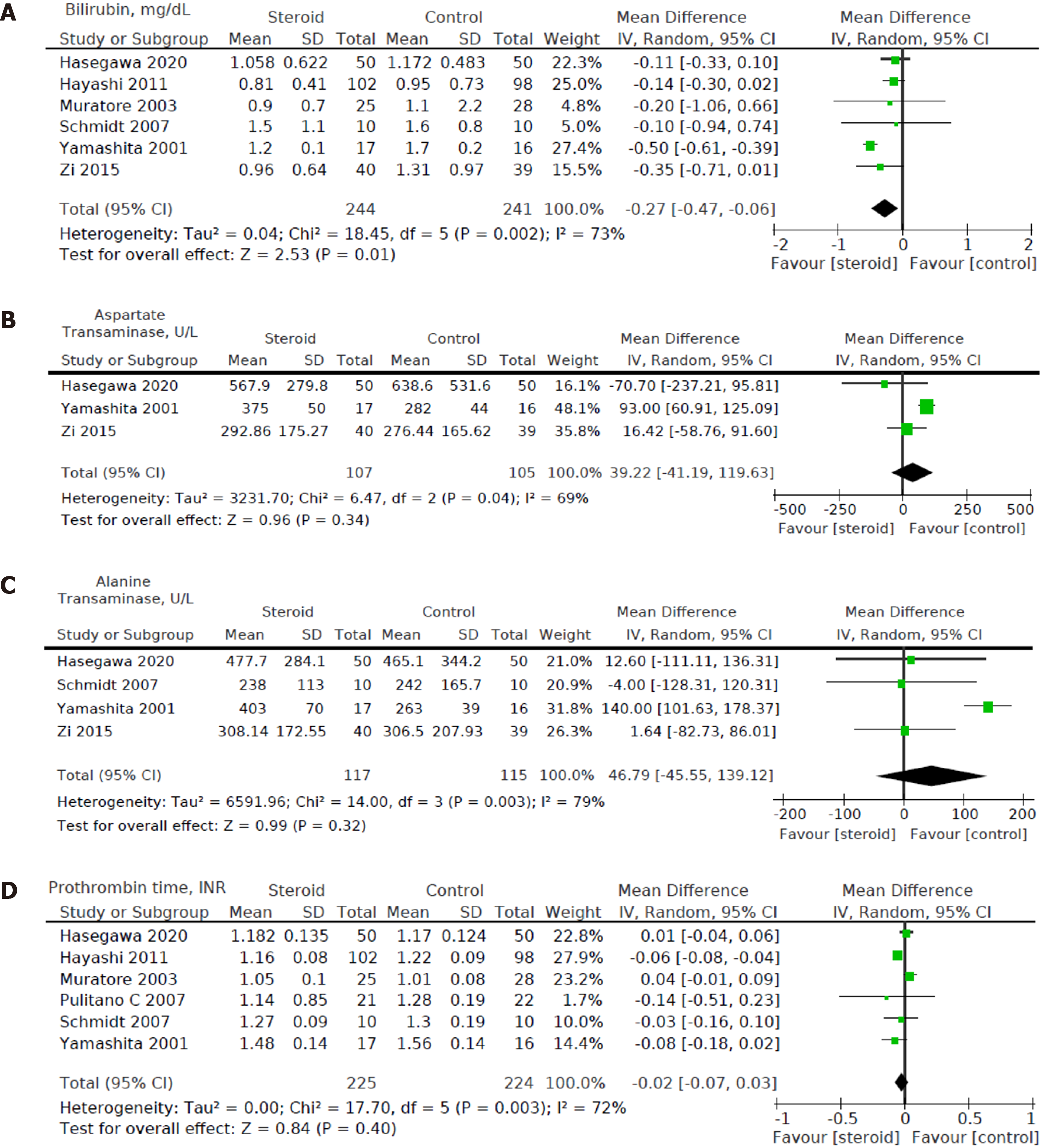
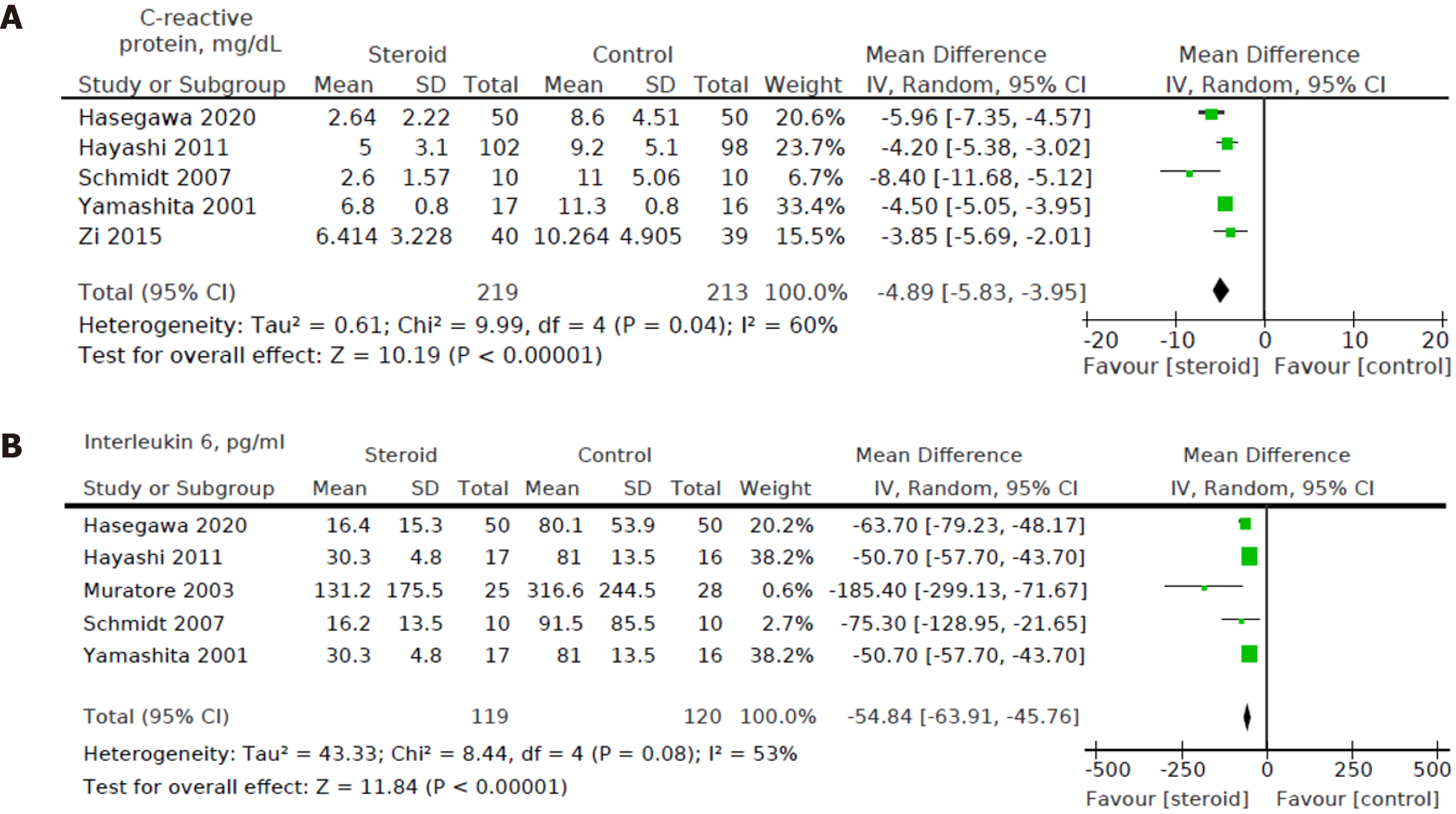
Heterogeneity of the primary outcomes and secondary outcomes was analyzed. Of note, there was statistically significant heterogeneity in the length of hospital stay (I2 60%, P = 0.02). Other primary outcomes did not show statistically significant heterogeneity-overall morbidity (I2 = 35%, P = 0.15), hepatobiliary complications (I2 = 0%, P = 0.95), liver dysfunction (I2 = 0%, P = 0.98), bile leakage (I2 = 0%, P = 0.89), pulmonary complications (I2 40%, P = 0.52), pleural effusion (I2 3%, P = 0.36), surgical site infection (I2 = 40%, P = 0.21), ascites (I2 = 0%, P = 0.81), and hemorrhage (I2 = 0%, P = 0.99). All of the secondary outcomes showed statistically significant heterogeneity except POD1 IL-6 Levels (I2 53%, P = 0.08). The heterogeneity for secondary outcomes was as follows: POD1 bilirubin (I2 = 73%, P < 0.001), POD1 AST (I2 = 69%, P = 0.04), POD1 ALT (I2 = 79%, P = 0.003), POD1 prothrombin (I2 = 72%, P = 0.003), and postoperative day 3 (POD3) CRP (I2 = 60%, P = 0.04). Due to the small number of studies, a funnel plot analysis was not conducted. The studies included in this systematic review were assessed with a summary of the Risk of Bias assessment shown in Figure 2. Biases assessed were detailed previously under the "Material and Methods" section.
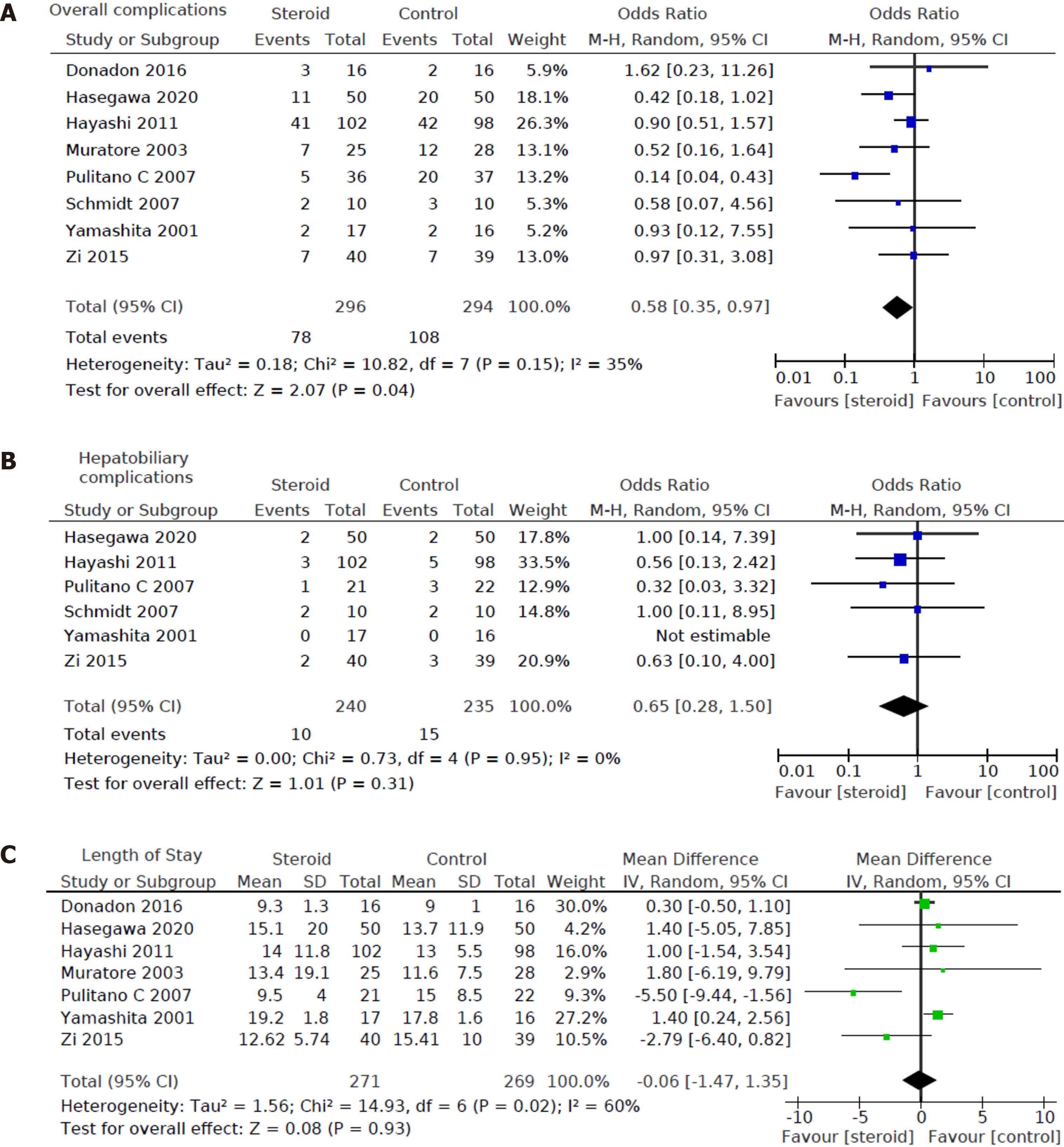
All 8 studies including a total of 590 patients (296 in the steroids group and 294 in the control group) reported postoperative morbidity. Overall morbidity was lower in the steroid group compared to the control group [OR: 0.58, 95%CI: (0.35, 0.97), P = 0.04] (Figure 3A). Six out of eight studies[12,15-19] including a total of 475 patients (240 in the steroids group and 235 in the control group) reported on hepatobiliary complications, and no statistically significant difference was found between the two groups [OR: 0.65; 95%CI: (0.28, 1.50); P = 0.31] (Figure 3B). 7 out of 8 studies[12,13,15-17,19,20] including a total of 540 patients (271 in the steroid group and 269 in the control group) reported length of hospital stay, and this was not different between the steroid group and the control group [MD: -0.06, 95%CI: (-1.47, 1.35), P = 0.93] (Figure 3C).
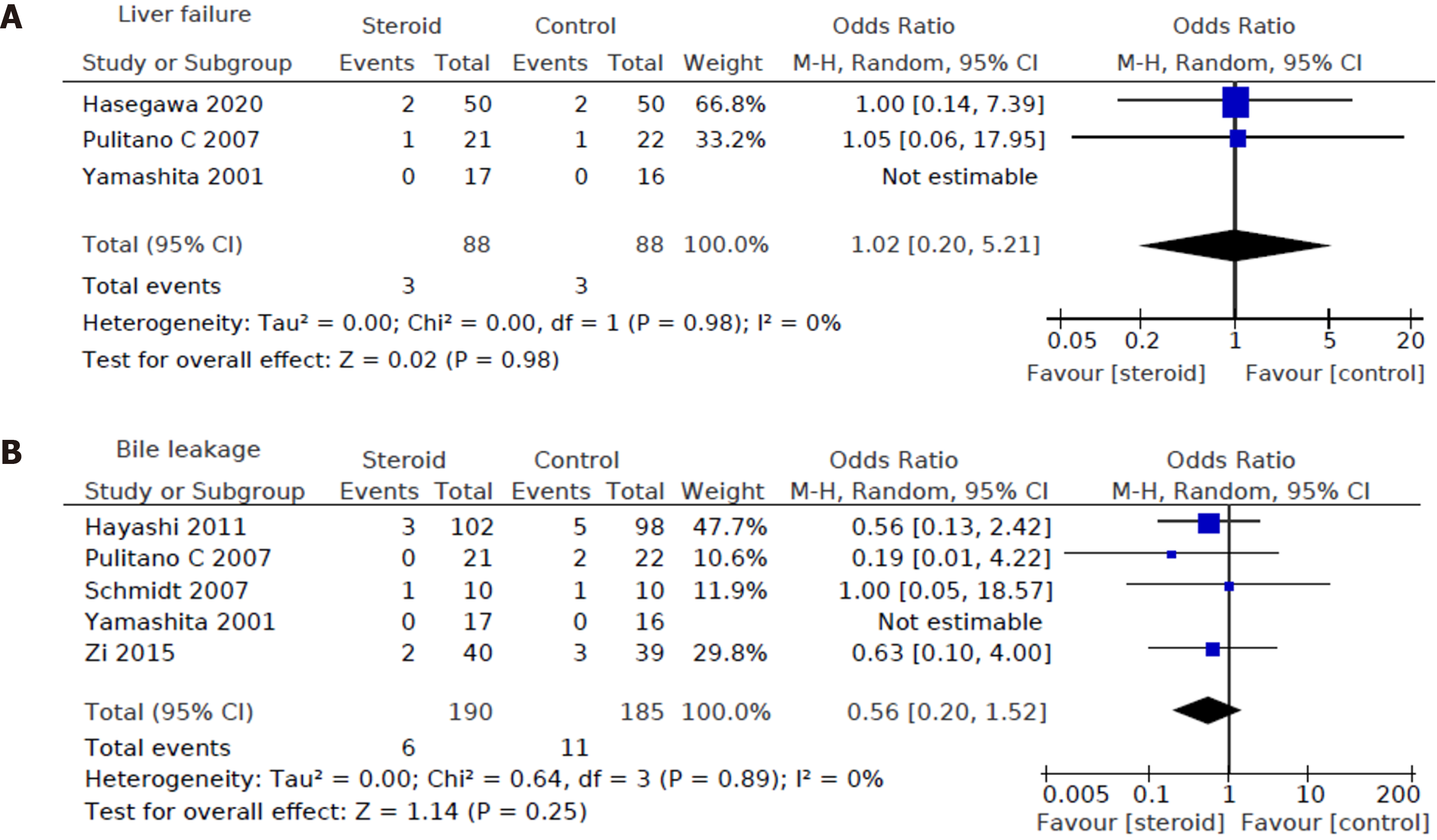
Regarding specific hepatobiliary complications, three out of eight studies reported on liver dysfunction or failure[15-17] and five out of eight studies reported on bile leakage[12,16-19]. No statistically significant difference could be found between the two groups in both liver dysfunction or failure [OR: 1.02, 95%CI: (0.20, 5.21), P = 0.98] (Figure 4A) and bile leakage (OR: 0.56, 95%CI: (0.20, 1.52), P = 0.25) (Figure 4B).
Four out of eight studies[12,15,17,20], including a total of 396 patients (198 in the steroids group and 198 in the control group), reported pulmonary complications. For pulmonary complications, no significant difference was detected between the two groups (OR: 0.79, 95%CI: (0.42, 1.47), P = 0.45) (Figure 5A). There was also no significant difference in pleural effusion between the two groups (OR: 0.89, 95%CI: (0.43, 1.88), P = 0.77) (Figure 5B).
Six out of eight studies[12,15-19] including a total of 475 patients (240 in the steroids group and 235 in the control group) reported on postoperative infection rates. There was no statistically significant difference between the two groups (OR: 0.84, 95%CI: (0.39, 1.81), P = 0.66) for infectious complications (Figure 6).
Lastly, five out of eight studies[12,13,15,16,19] including a total of 444 patients (225 in the steroids group and 219 in the control group) reported on postoperative mortality. Mortality was defined as 90-d mortality in Donadon et al[13] and Hasegawa et al[15]. The remaining three authors did not define mortality. There were no postoperative deaths reported.
Six out of eight studies[12,15,16,18-20] including a total of 485 patients (244 in the steroids group and 241 in the control group) reported bilirubin levels on POD1. We found a statistically significant decrease in POD1 bilirubin levels in the steroid group compared to the control group [MD: -0.27, 95%CI (-0.47, -0.06), P = 0.01] (Figure 7A).
Three out of eight studies[15,16,19] including a total of 212 patients (107 in the steroids group and 105 in the control group) reported AST on POD1. For POD1 AST levels, no statistically significant difference was found between the intervention group and control group [MD: 39.22, 95%CI: (-41.19, 119.63), P = 0.34] (Figure 7B).
Four out of eight studies[15,16,18,19] including a total of 232 patients (117 in the steroids group and 115 in the control group) reported ALT on POD1. For POD1 ALT levels, no statistically significant difference was found between the intervention group and control group [MD: 46.79, 95%CI: (-45.55, 139.12), P = 0.32] (Figure 7C).
Six out of eight studies[12,15-18,20] including a total of 449 patients (225 in the steroids group and 224 in the control group) reported data on prothrombin time on POD1. For POD1 prothrombin time, no statistically significant difference was found between perioperative administration of steroids and postoperative prothrombin time [MD: -0.02, 95%CI: (-0.07, 0.03), P = 0.40] (Figure 7D).
Five out of eight studies[12,15,16,18,19] including a total of 432 patients (219 in the steroids group and 213 in the control group) reported CRP levels on POD3. We found a statistically significant decrease in POD3 CRP levels in the steroids group compared to the control group [MD: -4.89, 95%CI: (-5.83, -3.95), P < 0.001] (Figure 8A).
Five out of eight studies[12,15,16,18,20] including a total of 239 patients (119 in the steroids group and 120 in the control group) reported POD1 IL-6 levels. We found a statistically significant decrease in the POD1 IL-6 levels between the intervention group and control group [MD: -54.84, 95%CI: (-63.91, -45.76), P < 0.001] (Figure 8B).
Steroids were introduced in medicine for almost a century, and their use has evolved with an enhanced understanding of human physiology, pharmacokinetics, and critical care principles. There is substantial evidence for continuing perioperative "stress dose" steroids in patients who are already taking steroids as part of chronic disease management. Recently, the anti-inflammatory and immune-modulating benefits of steroids are exploited to reduce postoperative nausea and vomiting (PONV) and pain. PONV is associated with general anesthesia and implicated in causing aspiration pneumonia, wound complications, psychological distress, and prolonged hospital length of stay; and thus, perioperative steroid use remains attractive to improve clinical outcomes. In a systematic review including 17 clinical trials, Karanicolas et al[21] reported that prophylactic dexamethasone decreases the incidence of PONV after laparoscopic cholecystectomy relative to placebo [RR: 0.55, 95%CI: (0.44, 0.67)][21], and the effect was dose-dependent and independent of the use of other anti-emetics. However, there are concerns that the hyperglycemia and immunosuppressive effect of corticosteroids may increase the risk of infectious complications, delay wound healing, and increase hospital length of stay[22].
Further, in patients undergoing HR, coagulation disturbance, and ischemia-reperfusion injury may impact clinical outcomes. The studies reporting perioperative steroid use in patients undergoing HR have shown inconsistent benefits. In a meta-analysis including six prospective RCTs and 411 patients undergoing HR, Yang et al[9] has reported that preoperative administration of steroid promotes the recovery of liver function and inhibits the inflammatory response without increasing postoperative complications[9]. However, there was no benefit in reducing overall morbidity [OR: 0.57, 95%CI: (0.27, 1.17), P = 0.13]. This updated meta-analysis includes 43.6% more patient sample (590 vs 411) and shows that perioperative steroid administration reduces overall morbidity. Also, POD1 bilirubin and IL-6 and POD3 CRP levels were lower in patients receiving perioperative steroids. However, perioperative steroid administration did not reduce individual hepatobiliary specific (liver dysfunction and bile leak), pulmonary or infectious complications. The effect of reduction in cumulative overall morbidity could be explained due to small and non-significant reductions in individual organ-specific complications.
Patients undergoing HR are routinely monitored postoperatively with serologic investigations including lactate, blood gas analysis, renal function, liver function, coagulation screen, and full blood count. Elevated serum bilirubin predicts postoperative liver dysfunction and is an important determinant of perioperative outcomes[3,23]. Serum bilirubin is dependent on the prehepatic load of heme, hepatic function, and biliary excretory function. International Study Group of Liver Surgery[24] proposed an elevated international normalized ratio together with hyperbilirubinemia on or after POD5 to predict post-hepatectomy liver failure. Similarly, in a study of 775 patients, Balzan et al[25] showed that a prothrombin time less than 50% of normal combined with serum bilirubin > 50 μmol/L on POD5 predicted mortality. Our results show that perioperative steroid administration was associated with lower serum bilirubin at POD1 but did not reduce liver dysfunction incidence, consistent with the previous report by Yang et al[9]. This could be due to few possible explanations. The definition of liver dysfunction or failure is heterogeneous in various included studies. In addition, POD1 bilirubin is not a reliable predictor of liver dysfunction as liver regeneration occurs over time, and bilirubin trends over the next few days are more critical than POD1 Levels. Lastly, CRP levels that fall too quickly on POD1 are associated with a higher risk of postoperative liver failure[26].
AST/ALT are markers of hepatocellular injury, and their levels tend to peak during the early postoperative period[27]. The levels following HR are influenced by hepatic ischemia and the duration of surgery[28]. Our results show that perioperative steroid modulation does not attenuate AST/ALT elevation, consistent with previous reports[9,20]. In a prospective RCT including 53 patients undergoing HR, Muratore et al[20] reported that preoperative administration of methylprednisolone (30 mg/kg 30 min before HR) reduced serum IL-6 levels in all patients. However, the effect on AST/ALT was mostly observed in patients with chronic liver disease[20]. Heterogeneity in patient demography and clinic profile, duration of surgery, blood loss, and anesthetic care may mask the effect of steroids on AST/ALT. Asians have a higher body fat percentage than Caucasians of similar body mass indices[29], which may lead to higher AST and ALT levels.
Inflammatory biomarkers are simple, cheap, readily available, and are increasingly reported to predict short-term perioperative and long-term oncologic outcomes in patients undergoing HR[8]. IL-6 is an acute phase cytokine transiently released in response to tissue injury and infections. Elevated IL-6 is associated with postoperative morbidity[5,17]. Previous studies have linked postoperative cytokines with a higher risk of complications in patients undergoing lung and abdominal surgery, there being a direct relationship between lower cytokine levels and reduced postoperative complications[1]. IL-6 levels also aid risk stratification of postoperative complications[6].
Interestingly, our study revealed a reduction in overall morbidity but not hepatobiliary complications after steroids. This is consistent with previous reports of thoracic and abdominal surgeries. In the context of HR, Hoffmann et al[5] showed that IL-6 has a pivotal role in postoperative hepatic regeneration, and patients with a deficient IL-6 response have impaired liver regeneration. Thus, the attenuation of IL-6 response by perioperative steroid administration could negatively influence hepatic regeneration and paradoxically increase liver dysfunction. This needs to be further investigated.
CRP is an acute-phase protein synthesized by the liver, and it depends on IL-6 regulation[30]. CRP upregulates pro- and anti-inflammatory cytokines, enhances phagocytosis, and increases the expression of endothelial adhesion molecules. CRP has a utility to monitor and predict postoperative complications and perioperative sepsis. De Jong et al[31] compared perioperative changes in CRP in 24 patients undergoing HR with nine patients undergoing laparotomy only due to unresectable tumors. They observed that CRP response was more significant in the laparotomy group than the HR group, and CRP response returned to normal by POD4[31]. Our study found that the use of steroids lowers the POD1 CRP levels. Thus, the CRP response's blunting could negate the beneficial effects of steroid administration, especially in patients with HR. It is possible that the effect may be different in minor resections with adequate future liver remnant and major resections with diminished capacity of the liver functional reserve, and further studies are warranted. POD1 CRP has a high negative predictive value for postoperative morbidity, which is consistent with our study[32].
Overall, we found that the perioperative administration of steroids did not increase the incidence of ascites, pleural effusion, and hepatobiliary complications following HR. Similarly, the length of hospital stay was also not altered by the administration of steroids. This is akin to the conclusions drawn by Yang et al[9] and Richardson et al[33]. However, our analysis showed that the use of steroids did not increase the risk of infectious complications. This is corroborated by the conclusion drawn from a meta-analysis, including 379 patients by Li et al[34]. There are currently trials being conducted to precisely evaluate the effect of high dose corticosteroids on postoperative complications[35]. The results of these trials can influence future surgical practice. Rapid increases in postoperative pro-inflammatory cytokines are associated with increased risk of postoperative complications and poorer prognosis; however, postoperative cytokines profile is diverse and nuanced[36]. Increased values of some cytokines such as IL-6 and tumor necrosis factor-α improve hepatic regeneration[37], while decreased CRP levels could either mean liver dysfunction due to reduced hepatic production or association with reduced overall postoperative morbidity. Also, laparoscopic surgery is known to reduce the inflammatory response, and the impact of perioperative steroids could be diverse in patients with open vs laparoscopic liver resection. Hasegawa et al[15] showed that corticosteroids help to suppress inflammation following laparoscopic liver resection[15].
Further studies need to be done to elucidate the relationship between the different corticosteroid therapies and the degree of effect on different inflammatory cytokines, which might impact open or laparoscopic HR outcomes. This is especially so as the studies included have different steroid administration methods, using steroids of different potency and dosage. While no significant difference in postoperative complications is noted between laparoscopic and open HR in general, perioperative steroids could impact the incidence of complications[38]. Zi et al[19] illustrate different specific instances in which perioperative steroids would be useful in HR, such as when enteral feeding is required. Early enteral feeding is shown to reduce inflammation and overall morbidity following HR[39].
Our meta-analysis has inherent strengths and limitations. The inclusion of two additional studies[15,19] with an increase in 43.6% of sample size enhanced our updated meta-analysis's statistical power. We only included prospective RCTs in our meta-analysis to reduce heterogeneity and increase the reliability and validity of results. Since the studies publishing statistically significant results are more likely to be published in English[40], the exclusion of non-English articles may affect the conclusions' reliability as population factors could be a compounding factor, an issue we have attempted to overcome in our study. However, due to various steroid regimes and different authors reporting different primary and secondary outcomes, we still found significant heterogeneity in many reported variables. The International Study Group on Liver Surgery needs to make recommendations to standardize the perioperative use of steroids in patients undergoing HR liver resection as type, dose, and time of administration of perioperative steroids could impact clinical outcomes. We omitted the data points for variables presented in graphical form as we were unable to contact the authors of the original studies to extract raw data, which could introduce bias. Lastly, there was also a lack of long-term follow-up data, and thus, the impact on survival outcomes is not established. This meta-analysis also reveals the limitations of RCTs. RCTs are traditionally considered higher in the hierarchy of medical scientific evidence. Only one study reported morbidity outcomes using an established classification system, thus limiting the clinical utility of morbidity data[13]. As evidenced in our meta-analysis, all the included RCTs did not report single mortality in patients undergoing HR, where in clinical practice, this is unlikely. RCTs are conducted in a controlled environment with strict inclusion-exclusion criteria with close monitoring and oversight by the study team, and results of RCTs are not generalizable in routine clinical practice, and this needs to be considered by all clinicians who read our results. Lastly, this study also highlights the fact that despite 90 d mortality outcomes are considered as essential key performance indicators for HR, even RCTs continued to be conducted without reporting this essential metric. In conclusion, perioperative steroid administration reduces overall morbidity in patients undergoing HR.
Hepatic resection (HR) results in an inflammatory response that can be modified by perioperative steroid administration. However, it remains to be determined if this response's attenuation translates to a reduction in complications.
Stress from major surgery results in an inflammatory response secondary to cytokine and free radical release. This inflammatory response is essential for healing and restoration of physiologic function; however, it can increase morbidity, mortality, and worsen postoperative outcomes if excessive. Modifying the inflammatory response by perioperative steroid administration could improve surgical outcomes. Besides, inflammatory markers are increasingly shown to predict short-term perioperative and long-term oncologic outcomes following HR. Thus, modulation of inflammatory response to improve surgical outcomes remains an unmet need in hepatic surgery.
To evaluate if perioperative administration of steroids reduces complications following HR.
A systematic review of randomized controlled trials (RCTs) was conducted on PubMed, Embase, and Cochrane Central Register of Controlled Trials to evaluate the effect of perioperative steroid (compared to placebo or no intervention) use in patients undergoing HR. Clinical outcomes were extracted, and meta-analysis was performed.
Eight RCTs including 590 patients were included. Perioperative steroid administration was associated with significant reduction in postoperative complications [odds ratios: 0.58; 95% confidence intervals (CI): (0.35, 0.97), P = 0.04]. There was also improvement in biochemical and inflammatory markers, including serum bilirubin on postoperative day 1 [MD: -0.27; 95%CI: (-0.47, -0.06), P = 0.01], C-reactive protein on postoperative day 3 [MD: -4.89; 95%CI: (-5.83, -3.95), P < 0.001], and IL-6 on postoperative day 1 [MD: -54.84; 95%CI: (-63.91, -45.76), P < 0.001].
Perioperative steroids administration in HR may reduce overall complications, postoperative bilirubin, and inflammation. Further studies are needed to determine the optimal dose and duration, and patient selection.
The International Study Group on Liver Surgery needs to make recommendations to standardize the perioperative use of steroids in patients undergoing HR liver resection as type, dose, and time of administration of perioperative steroids could impact clinical outcomes. Lastly, there was also a lack of long-term follow-up data, and thus, the impact on survival outcomes is not established.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki T, Bredt LC, Karamarkovic AR S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 402] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Santiago Delpín EA, Figueroa I, López R, Vázquez J. Protective effect of steroids on liver ischemia. Am Surg. 1975;41:683-685. [PubMed] |
| 3. | Madhavan S, Shelat VG, Soong SL, Woon WWL, Huey T, Chan YH, Junnarkar SP. Predicting morbidity of liver resection. Langenbecks Arch Surg. 2018;403:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Jerin A, Pozar-Lukanovic N, Sojar V, Stanisavljevic D, Paver-Erzen V, Osredkar J. Balance of pro- and anti-inflammatory cytokines in liver surgery. Clin Chem Lab Med. 2003;41:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hoffmann K, Nagel AJ, Tanabe K, Fuchs J, Dehlke K, Ghamarnejad O, Lemekhova A, Mehrabi A. Markers of liver regeneration-the role of growth factors and cytokines: a systematic review. BMC Surg. 2020;20:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EM, Noordzij PG. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann Surg. 2016;263:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Chan KS, Chia CLK, Ng FKL, Seow WHJ, Leong DY, Shelat VG. Impaired Handgrip Strength Does Not Predict Postoperative Morbidity in Major Hepatobiliary Surgery. J Surg Res. 2020;256:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma-neutrophil to lymphocyte ratio. Ann Transl Med. 2020;8:912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Yang L, Zhang Z, Kong J, Wang W. Systematic Review and Meta-Analysis of the Benefit and Safety of Preoperative Administration of Steroid in Patients Undergoing Liver Resection. Front Pharmacol. 2019;10:1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Polderman JAW, Farhang-Razi V, van Dieren S, Kranke P, DeVries JH, Hollmann MW, Preckel B, Hermanides J. Adverse side-effects of dexamethasone in surgical patients - an abridged Cochrane systematic review. Anaesthesia. 2019;74:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8877] [Article Influence: 554.8] [Reference Citation Analysis (0)] |
| 12. | Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama H, Higaki T. Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg. 2011;253:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Donadon M, Molinari AF, Corazzi F, Rocchi L, Zito P, Cimino M, Costa G, Raimondi F, Torzilli G. Pharmacological Modulation of Ischemic-Reperfusion Injury during Pringle Maneuver in Hepatic Surgery. A Prospective Randomized Pilot Study. World J Surg. 2016;40:2202-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1168] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 15. | Hasegawa Y, Nitta H, Takahara T, Katagiri H, Kanno S, Umemura A, Akiyama Y, Iwaya T, Otsuka K, Sasaki A. Glucocorticoid use and ischemia-reperfusion injury in laparoscopic liver resection: Randomized controlled trial. Ann Gastroenterol Surg. 2020;4:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Sugimachi K. Effects of preoperative steroid administration on surgical stress in hepatic resection: prospective randomized trial. Arch Surg. 2001;136:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 17. | Pulitanò C, Aldrighetti L, Arru M, Finazzi R, Catena M, Guzzetti E, Soldini L, Comotti L, Ferla G. Preoperative methylprednisolone administration maintains coagulation homeostasis in patients undergoing liver resection: importance of inflammatory cytokine modulation. Shock. 2007;28:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Schmidt SC, Hamann S, Langrehr JM, Höflich C, Mittler J, Jacob D, Neuhaus P. Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg. 2007;14:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Zi X, Yao H, Qiu Y, Fu X, Mao L, Zhou T, Chen C. Effect of intraoperative methylprednisolone in combination with perioperative enteral nutrition support on recovery after hepatectomy. Zhonghua Linchuang Yingyang Zazhi. 2015;23:89-94. [DOI] [Full Text] |
| 20. | Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg. 2003;90:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH. The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Ann Surg. 2008;248:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1218] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 23. | Helling TS. Liver failure following partial hepatectomy. HPB (Oxford). 2006;8:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1729] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 25. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 26. | Rahman SH, Evans J, Toogood GJ, Lodge PA, Prasad KR. Prognostic utility of postoperative C-reactive protein for posthepatectomy liver failure. Arch Surg. 2008;143:247-53; discussion 253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Olthof PB, Huiskens J, Schulte NR, Wicherts DA, Besselink MG, Busch OR, Heger M, van Gulik TM. Postoperative peak transaminases correlate with morbidity and mortality after liver resection. HPB (Oxford). 2016;18:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Giovannini I, Chiarla C, Giuliante F, Vellone M, Ardito F, Sarno G, Nuzzo G. Analysis of the components of hypertransaminasemia after liver resection. Clin Chem Lab Med. 2007;45:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Szanto KB, Li J, Cordero P, Oben JA. Ethnic differences and heterogeneity in genetic and metabolic makeup contributing to nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2019;12:357-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Hurlimann J, Thorbecke GJ, Hochwald GM. The liver as the site of C-reactive protein formation. J Exp Med. 1966;123:365-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | de Jong KP, Hoedemakers RM, Fidler V, Bijzet J, Limburg PC, Peeters PM, de Vries EG, Slooff MJ. Portal and systemic serum growth factor and acute-phase response after laparotomy or partial hepatectomy in patients with colorectal liver metastases: a prognostic role for C-reactive protein and hepatocyte growth factor. Scand J Gastroenterol. 2004;39:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Späth C, Srinivasa S, Walsh M, Singh P, Rodgers M, Koea J. Role of post-operative serum C-reactive protein levels as a predictor of complications in upper gastrointestinal surgery. ANZ J Surg. 2019;89:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Richardson AJ, Laurence JM, Lam VW. Use of pre-operative steroids in liver resection: a systematic review and meta-analysis. HPB (Oxford). 2014;16:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Li N, Gu WL, Weng JF, Lin F, Zhu GH, Lu MQ, Cao J. Short-term administration of steroids does not affect postoperative complications following liver resection: Evidence from a meta-analysis of randomized controlled trials. Hepatol Res. 2015;45:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Steinthorsdottir KJ. Preoperative high dose steroids for liver resection: Effect on complications in the immediate postoperative period (STEREO). 2020. [cited 27 January 2021]. Available from: https://www.wuxuwang.com/cpsus/3a5298aa-a186-11ea-82b4-00163e0eafb3. |
| 36. | Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Mitsuhashi N, Sawada S, Takeuchi D, Ambiru S, Miyazaki M. Circulating cytokines, chemokines, and stress hormones are increased in patients with organ dysfunction following liver resection. J Surg Res. 2006;133:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1175] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 38. | Chen J, Li H, Liu F, Li B, Wei Y. Surgical outcomes of laparoscopic vs open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore). 2017;96:e6460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Warner SG, Jutric Z, Nisimova L, Fong Y. Early recovery pathway for hepatectomy: data-driven liver resection care and recovery. Hepatobiliary Surg Nutr. 2017;6:297-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Nunan D, Heneghan C, Spencer EA. Catalogue of bias: allocation bias. BMJ Evid Based Med. 2018;23:20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |