Published online May 27, 2021. doi: 10.4240/wjgs.v13.i5.406
Peer-review started: February 5, 2021
First decision: March 16, 2021
Revised: March 30, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: May 27, 2021
Processing time: 105 Days and 1.5 Hours
Several benign conditions such as chronic pancreatitis, autoimmune pancreatitis, and paraduodenal pancreatitis can present as mass lesions and may mimic pancreatic ductal adenocarcinoma (PDAC) clinically and radiologically. Thorough histologic examination with attention to certain morphologic features can assist in deciphering neoplastic from reactive, however small biopsies often remain a challenge. Variable histologic patterns in conventional PDAC may also confound the diagnosis of PDAC. Uncommon subtypes of pancreatic carcinoma such as adenosquamous and squamous cell carcinoma, colloid carcinoma, medullary carcinoma, hepatoid carcinoma and signet ring cell carcinoma necessitate excluding metastasis from other sites prior to rendering the diagnosis of pancreatic carcinoma. The use of immunohistochemical staining and molecular markers can aid in separating benign from malignant and PDAC from metastasis. PDAC expresses a few non-specific epithelial and mucin immunomarkers such as CK7, CK19, MUC1, MUC4 and MUC5AC. However, the only immunohistochemical marker that is specific for PDAC in the right clinical context is SMAD4. Loss of SMAD4 within atypical glands and ducts supports the diagnosis of PDAC in a limited sample. Unfortunately, this finding is seen only in 50% of PDAC cases. The identification of certain mutations can help support a diagnosis of PDAC when benign conditions are in the differential. At the molecular level, KRAS oncogene mutations are seen in approximately 93% of PDACs. Subsequent neoplastic progression is driven by additional mutations of tumor suppressor genes, such as CDKN2A, TP53, and SMAD4. Molecular markers can also provide an insight to the prognosis. For instance, the loss of SMAD4 is associated with a poor outcome whereas mutations in MLL, MLL2, MLL3, and ARID1A are associa
Core Tip: Multiple benign entities share clinical and radiological features with pancreatic ductal adenocarcinoma requiring histologic examination to render the final diagnosis. However, the diagnosis of well differentiated adenocarcinoma can be challenging in a limited sample. Certain morphologic features and molecular markers can be used to resolve this issue.
- Citation: Aldyab M, El Jabbour T, Parilla M, Lee H. Benign vs malignant pancreatic lesions: Molecular insights to an ongoing debate. World J Gastrointest Surg 2021; 13(5): 406-418
- URL: https://www.wjgnet.com/1948-9366/full/v13/i5/406.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i5.406
In the pancreas, several benign conditions such as chronic pancreatitis (CP), autoimmune pancreatitis (AIP), and paraduodenal pancreatitis (PDP) can present as mass lesions and mimic pancreatic ductal adenocarcinoma (PDAC) clinically and radiologically[1]. The diagnosis of PDAC is relatively straightforward in resection specimens as multiple sections with large cut surfaces are available for microscopic examination. However, in small biopsy specimens (e.g., core needle biopsy) and intraoperative frozen sections, distinguishing PDAC from mass-forming benign lesions can be extremely challenging. Misdiagnosis in these settings may lead to an unnecessary surgical resection of a benign condition with resultant surgery-related complications. Alternatively, if one is too conservative at the microscope a diagnosis of resectable PDAC may be delayed with potentially adverse oncologic outcomes[2-4]. The aim of this review is to describe the microscopic, immunohistochemical and molecular features that can help distinguish PDAC and benign mass-forming entities, especially CP. Also, we will review a few subtypes of non-conventional PDAC that may further confound the diagnosis with their differing morphologies.
Smoking and CP are well-established risk factors of PDAC[4,5]. Early stage PDAC can be asymptomatic. However, at an advanced stage, patients usually present with variable symptoms ranging from painless jaundice, anorexia, weight loss and abdominal pain to pancreatic endocrine and exocrine insufficiency[6]. On magnetic resonance imaging and computed tomography, PDAC is usually seen as hypointense mass. However, PDAC not uncommonly shows similar intensity with surrounding tissue. In these cases, other features such as structural deformity, abrupt obstruction of the ducts, obstruction of both common bile duct and pancreatic duct (also known as “double duct sign”) and atrophy proximal to the lesion, may be helpful in rendering a correct diagnosis[3].
PDAC is derived from the epithelium of the pancreatic ductal tree. “Differentiation” of a malignant tumor is the degree of histomorphologic resemblance between the neoplastic cells and their origin. Therefore, a well-differentiated PDAC shows duct-like glands recapitulating the benign pancreatic ducts. Less differentiated (moderately, poorly, to undifferentiated) PDACs show more disorganized growth compared to well-differentiated tumors, with sheet-like, nested or single cell patterns of architec
CP is a progressive form of pancreatic injury subsequent to inflammation, fibrosis and atrophy[4,7]. Risk factors of CP include alcoholism, mechanical obstruction of the biliary tract and hyperlipidemia. As stated above, CP can be a risk factor for PDAC. Conversely, PDAC may lead to CP in the background pancreas[4,5,7]. Therefore, coexistence of CP and PDAC is not a rare occurrence and distinguishing these two is crucial given the differing management and prognosis[8]. Unfortunately, there is a high degree of overlap in clinical signs and symptoms between CP and PDAC[4,7]. Radiologically, dilation of the main pancreatic duct and branches with diffuse atrophy and calcification would favor CP over PDAC. However, again, focal atrophy and duct dilation can also be seen in PDAC and may be misleading[7].
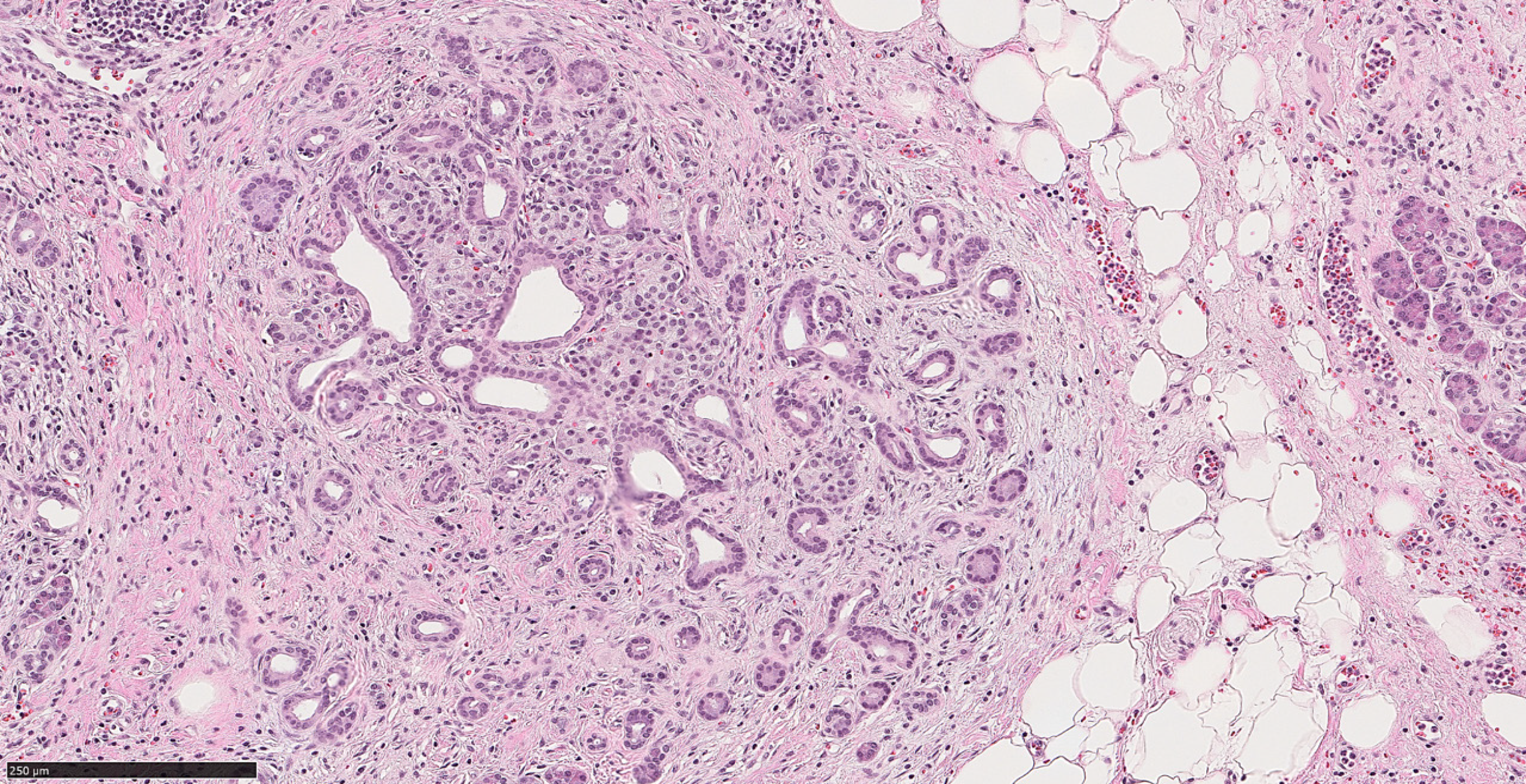
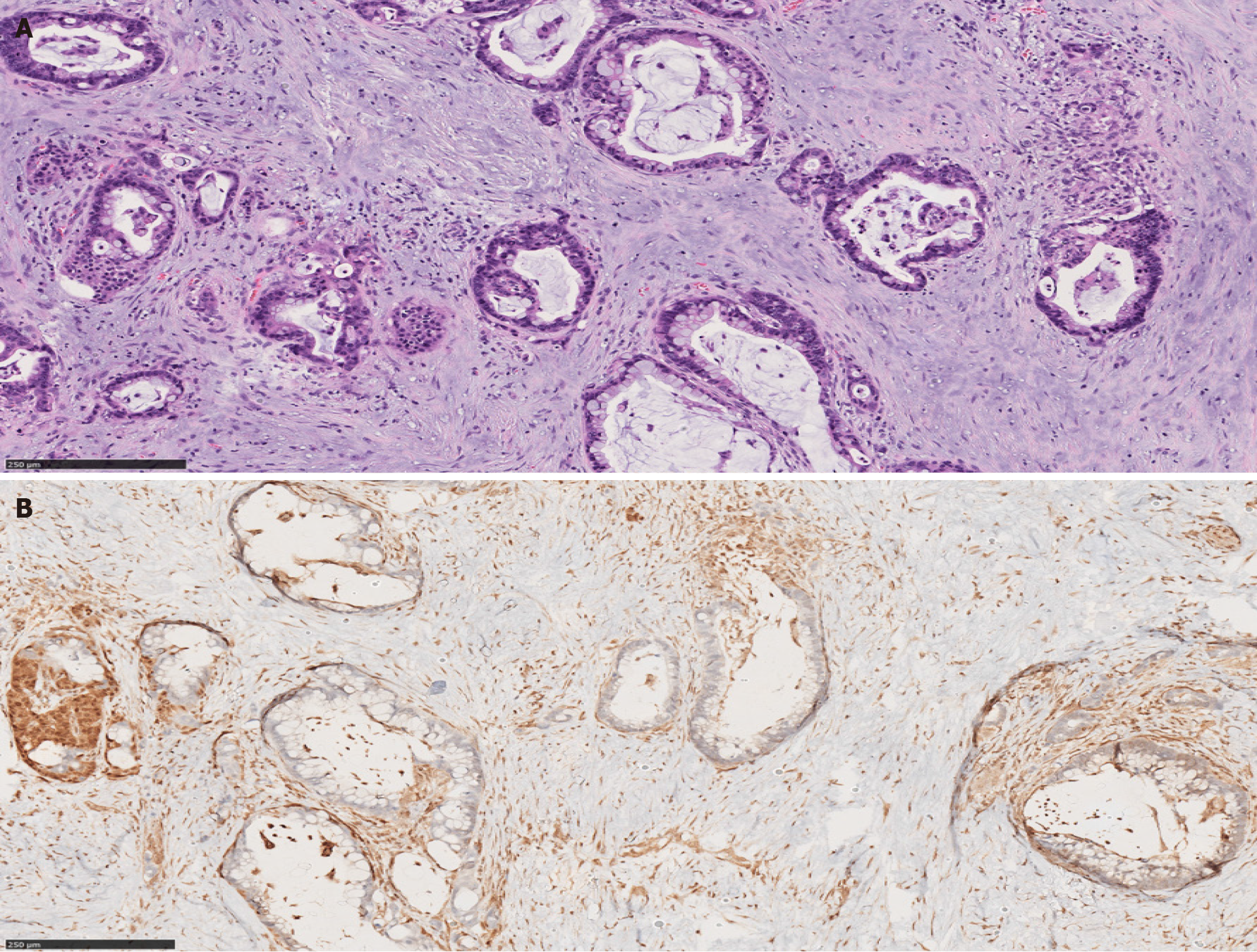
Microscopically, there is a striking morphologic resemblance between CP and well-differentiated PDAC that may lead to diagnostic challenges. In CP, benign acinar components frequently undergo atrophy. While their lobular architecture tends to be maintained, atrophic acini may resemble small ducts and the native small branch ducts may appear relatively prominent within the atrophic acini. Fibrosis in CP often encases the atrophic small duct-like acini and native small ducts. Consequently, those elements appear as ductular structures embedded in fibrotic stroma, mimicking well-differentiated invasive PDAC in desmoplastic stroma[8] (Figure 1).
The latest World Health Organization (WHO) classification of digestive system tumors listed relevant morphologic criteria in distinguishing PDAC and advanced CP. Architectural findings that are in favor of PDAC include the presence of irregular, ruptured, haphazard ducts in the immediate vicinity of neural structures or within the vessels[9]. Also, “naked” ducts in the adipose tissue favor PDAC over CP[10]. The presence of neutrophils and necrotic debris, rather than calculi and secretory plugs, is another histologic clue that supports the diagnosis of malignancy. Moreover, classic cytologic features of malignancy within the glands and ducts such as pleomorphism, nuclear hyperchromasia and increased mitotic rate support PDAC[9].
AIP is a rare form of CP. Type I AIP predominantly affects older males and type II affects younger patients without gender predilection[11-14]. Clinically, AIP commonly presents with painless obstructive jaundice and/or pancreatic mass similar to PDAC, thus is prone to misdiagnosis[11,12].
Two histologic patterns had been recognized and later designated as type I and type II, respectively[15,16]. Commonly found in the head of the pancreas, AIP is an important mimicker of PDAC, also commonly found in the head of the pan
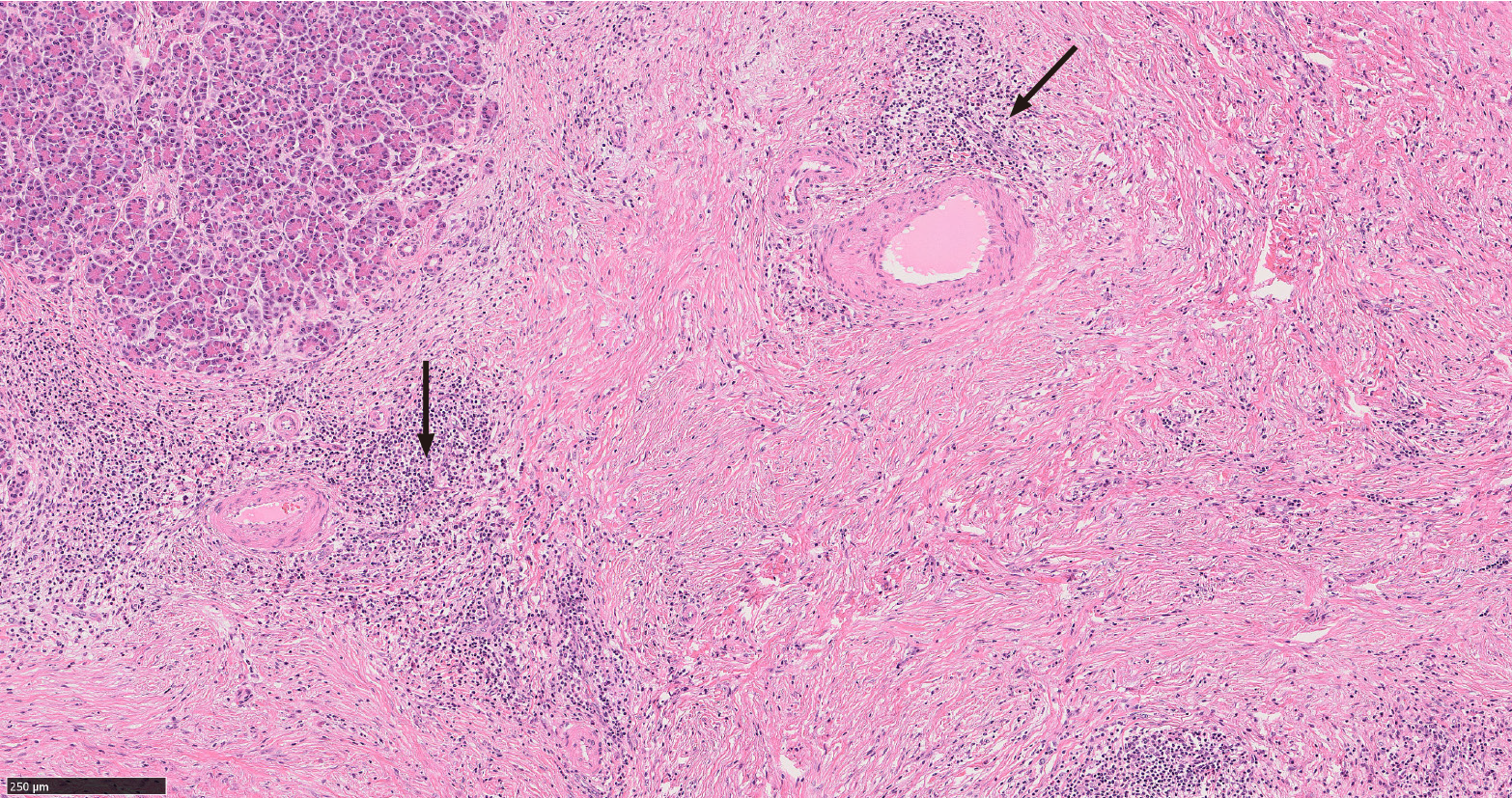
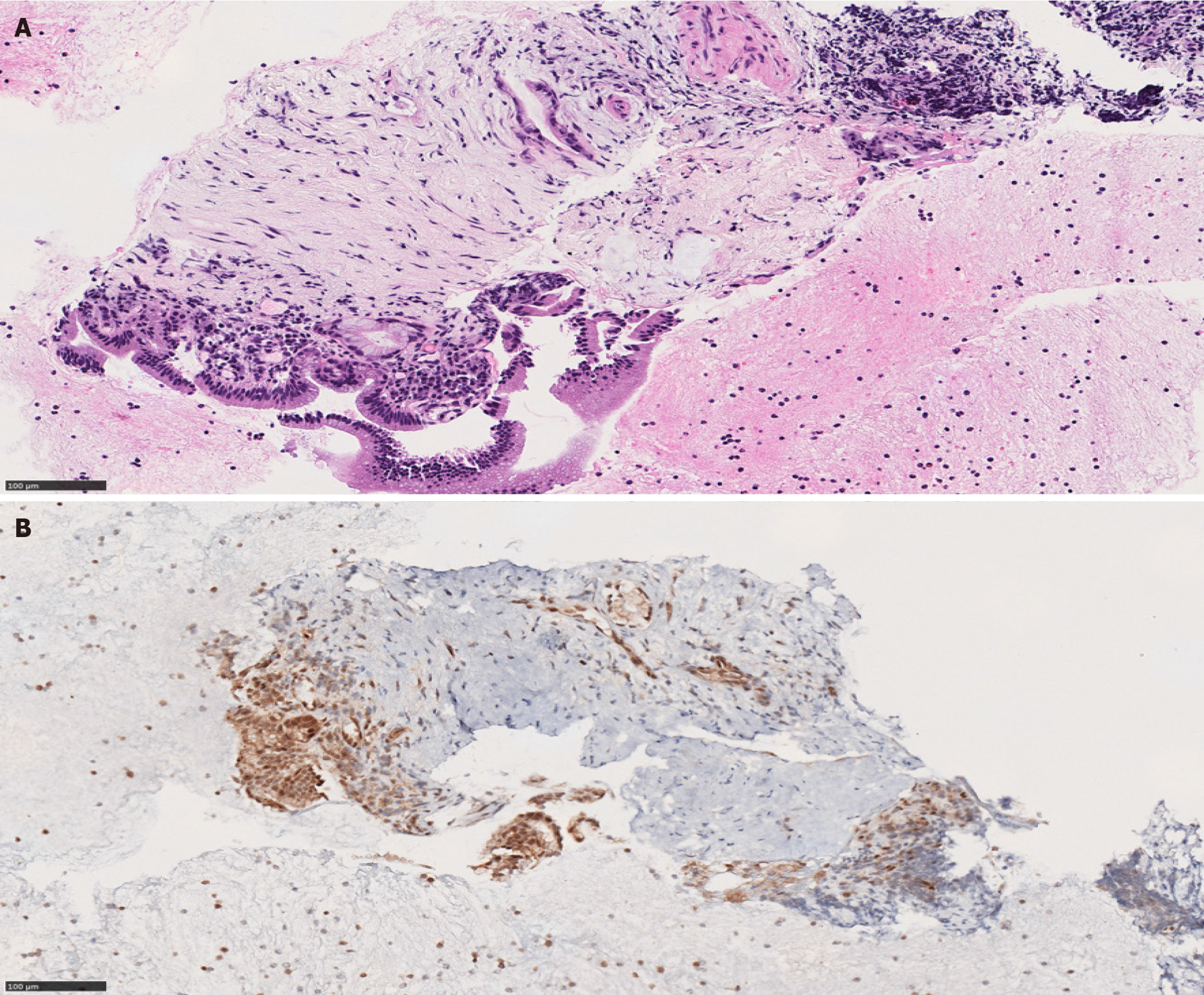
Type I and type II AIP have distinct histologic appearances. Type I is characterized by a dense lymphoplasmacytic infiltrate with an increased IgG4 expression, storiform fibrosis and obliterative phlebitis[16,18,19] (Figure 2). Type II AIP is seen in a younger age group compared to AIP type I. It is histologically characterized by the presence of granulocytes in the ductal epithelium with or without granulocytic involvement of the acini with low or absent IgG4 positive cells[16,18,19] (Figure 3).
The diagnostic approach of AIP is complex, thus an interdisciplinary approach combining clinical, laboratory, and radiological features and typical pathologic findings, when available, is warranted[14,18,20]. The HISORt criteria consisting of histology, imaging, serology, other organ involvement and response to therapy components are commonly used for a diagnosis of AIP by clinicians[7,18,20].
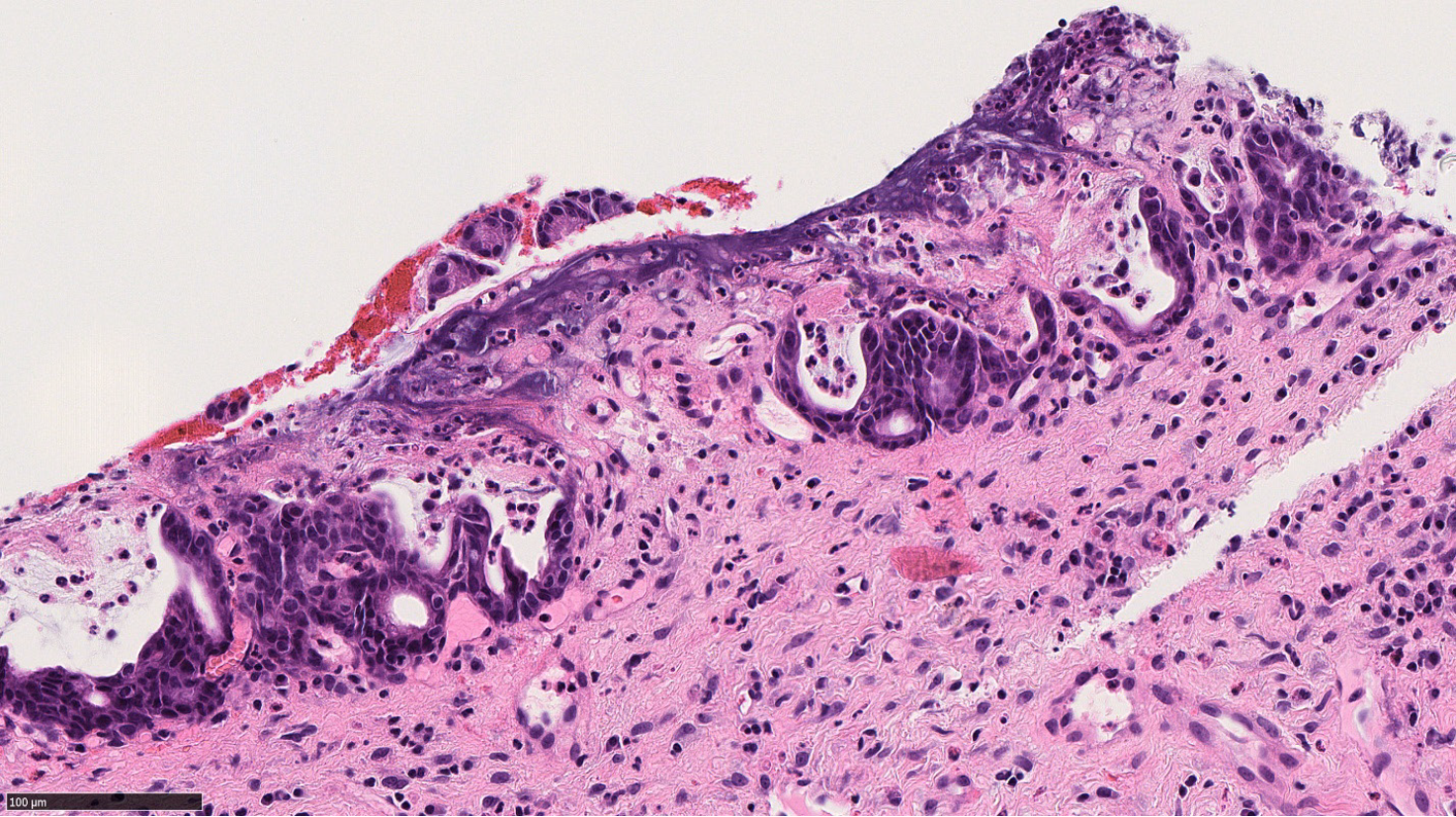
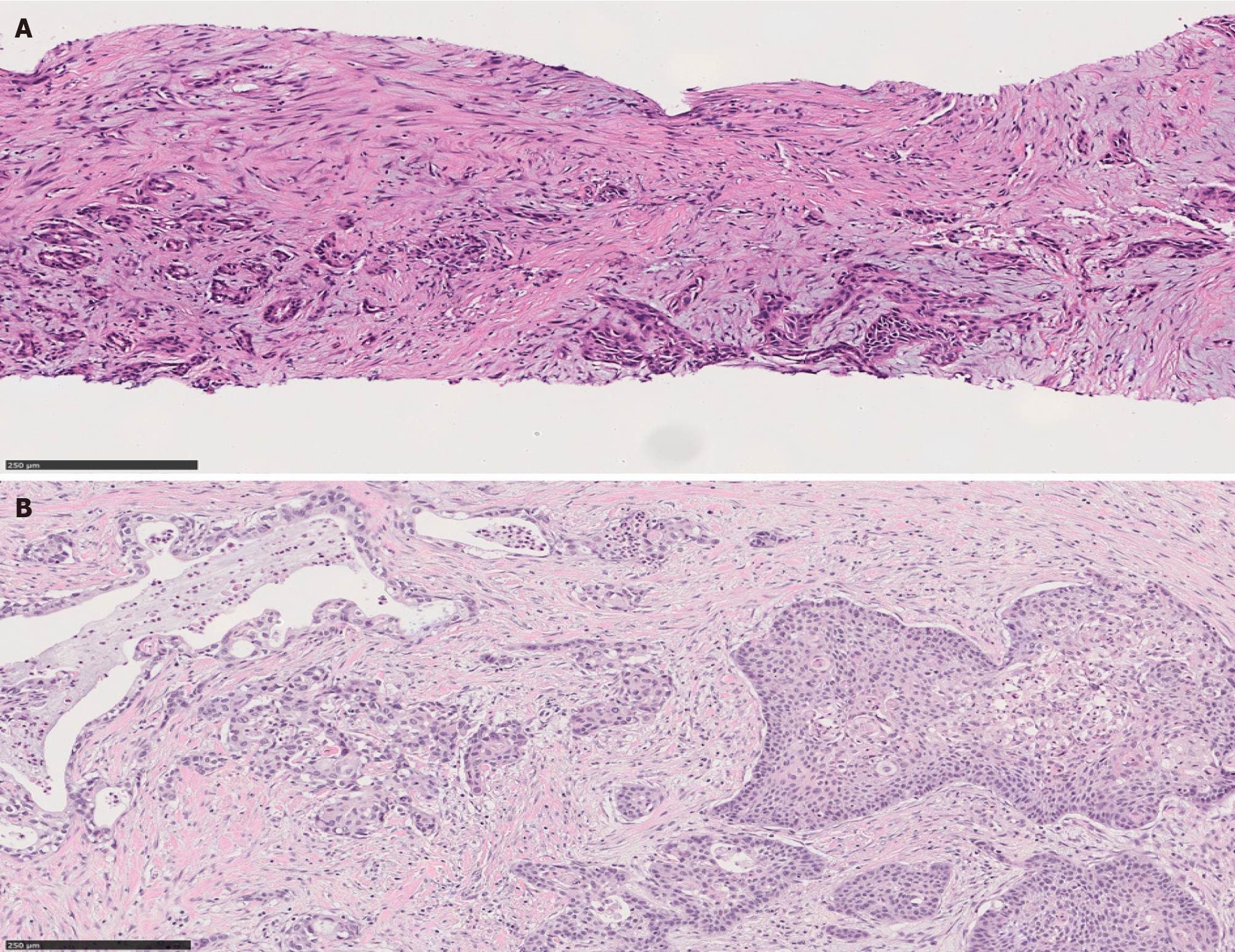
Also known as groove pancreatitis, PDP represents a localized type of CP affecting the region of pancreas between the pancreatic head, the duodenum, and the common bile duct [21,22]. The term PDP was introduced to include multiple pathologic entities such as cystic dystrophy of heterotopic pancreas and para-duodenal cysts[23]. Increased alcohol intake is a risk factor for PDP, which predominantly affects middle aged men[22,24]. Clinically PDP can manifest with a spectrum of symptoms including pain, vomiting, jaundice and weight loss[23,25].
Variable imaging modalities can be utilized to identify PDP. However, it is not uncommon to have an inconclusive radiologic diagnosis, with carcinoma of the pancreas as a differential diagnosis[25-27]. Duodenal wall thickening, Brunner gland hyperplasia and inflammation are typical features of PDP, in addition to proliferation of myofibroblasts, smooth muscle hyperplasia, edema and heterotopic pancreatic tissue (Figure 4). Cyst formation is also a common histologic finding[21,23-25].
A summary of distinctive morphologic features of PDAC and its benign mimics is outlined in Table 1.
| PDAC | CP | AIP | PDP |
| Perineural invasion; perivascular invasion; naked glands in adipose tissue; desmoplasia; anisonucleosis (4:1 nuclear size variation) | Intact lobular architecture; fibrosis; secretory plugs | Type I: Dense lymphoplasmacytic infiltrate with an increased IgG4 expression; storiform fibrosis; obliterative phlebitis. Type II: Granulocytes in the ductal epithelium; absent IgG4 positive cells. | Duodenal wall thickening; brunner gland hyperplasia; smooth muscle hyperplasia; heterotopic pancreatic tissue |
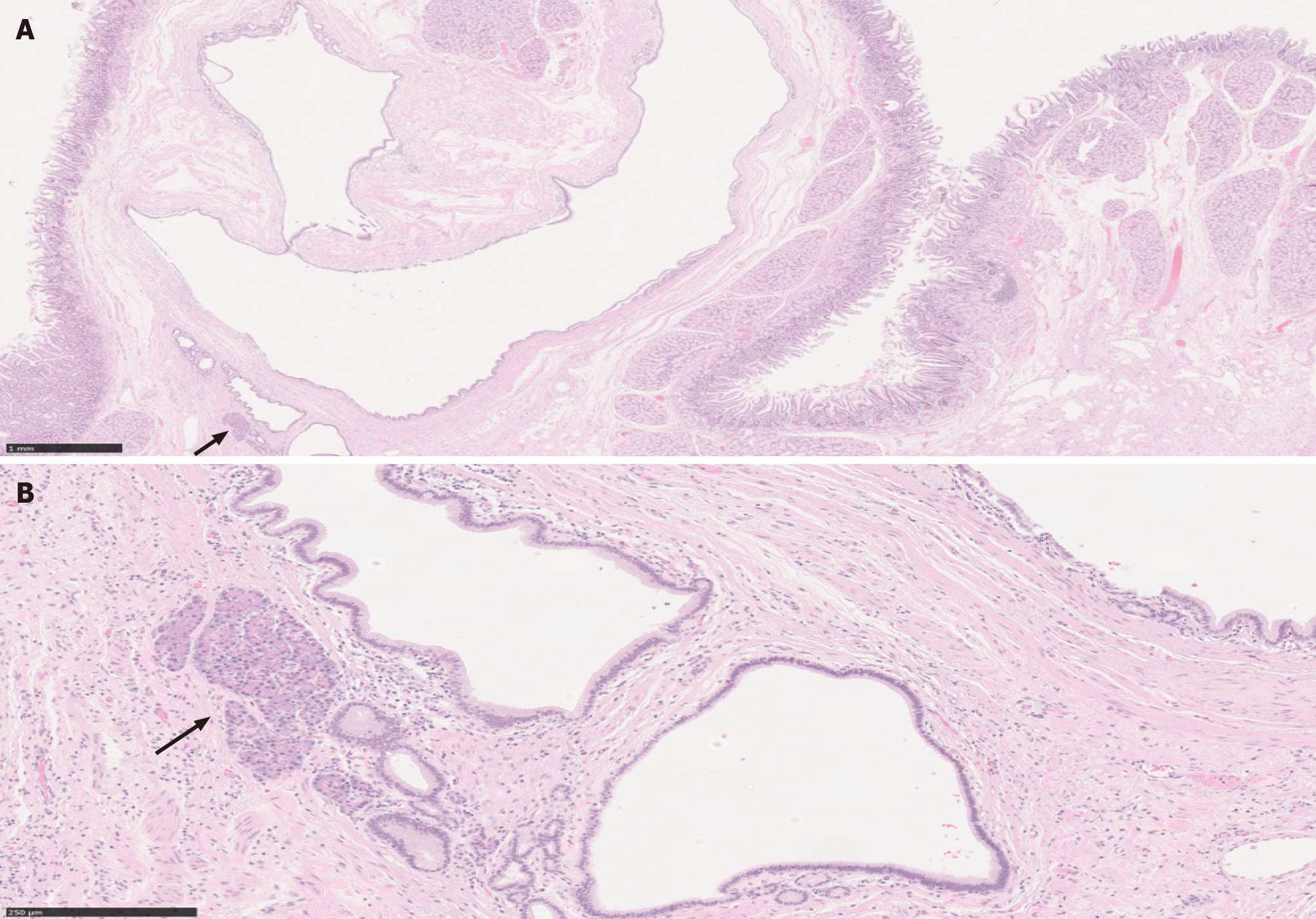
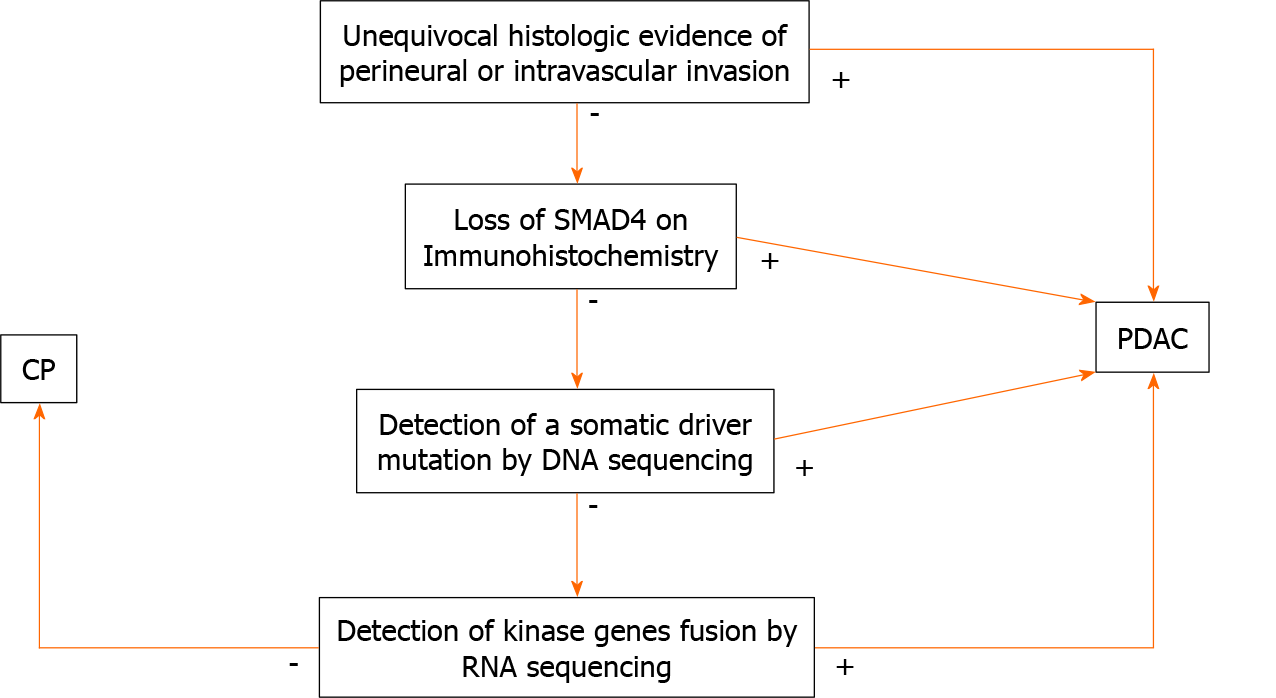
PDAC originates from ductal epithelium. Therefore, PDAC expresses a number of non-specific epithelial and mucin immunomarkers such as CK7, CK19, MUC1, MUC4 and MUC5AC. However, these markers can also be expressed in any benign ducts. Hence, none of these markers is useful in differentiating between PDAC and CP. The only immunohistochemical marker that confirms malignancy is SMAD4; loss of SMAD4 within atypical glands or ducts would be useful in making a diagnosis of PDAC in a limited sample. Unfortunately, this finding is seen only in 50% of PDAC cases (Figures 5 and 6). Consequently, positive (retained) SMAD4 staining within the glands does not exclude a diagnosis of PDAC[28-30].
Next generation sequencing (NGS) is a technique by which millions of nucleotide sequences are simultaneously deciphered and mutations or other genomic aberrations are detected. Using NGS, an entire human genome can be sequenced within a single day[31]. The Cancer Genome Atlas (TCGA) is a project that aims to discover then classify major cancer-causing genomic alterations called drivers. A few potential drivers of PDAC have been identified[32]. When a driver mutation is found in a pancreatic mass, a neoplastic process, such as PDAC, would be favored over CP.
According to the TCGA, KRAS oncogene mutations are seen in approximately 93% of PDACs. This mutation is usually seen early in the neoplastic process. Subsequent neoplastic progression is driven by mutations of tumor suppressor genes, such as CDKN2A, TP53, and SMAD4. Furthermore, genetic and epigenetic alterations lead to invasion and metastasis. These mutations are by far less frequent than KRAS mutation. For example, TP53 mutations and loss of SMAD4 are limited to approximately 50% of the cases[33-35]. The loss of SMAD4 is also associated with a poor outcome[36] whereas mutations in MLL, MLL2, MLL3, and ARID1A are associated with an impro
A multitude of other genetic alterations have been suggested to drive neoplasia in KRAS wild-type PDACs. These alterations are sub-grouped into three categories: (1) Tumors with an activated MAPK pathway associated with BRAF mutation; (2) Tumors with microsatellite instability due to defective DNA mismatch repair (MMR) featuring a high tumor mutational burden; and (3) Tumors with kinase gene fusions[38].
The following subtypes share a similar mutational profile with PDAC. Some of these may show additional pathognomonic or targetable mutations. Table 2 provides a summary of morphologic features and altered genes for PDAC subtypes.
| Subtype | Colloid | Medullary | Adenosquamous | Hepatoid | Signet ring cell |
| Morphologic features | More than 80% of extracellular mucin; focal signet-ring cells can be seen | Minimal glandular differentiation; syncytial growth pattern; abundant inflammatory infiltrate | More than 30% of squamous differentiation | More than 50% of hepatocellular differentiation | More than 80% of signet ring cell component |
| Altered genes | GNAS; ATM | MMR genes; POLE | SMAD4; CDKN2A | BAP1; Notch1 | NA |
Conventional PDAC may exhibit any of the morphologic patterns listed below; currently, these patterns are not classified as separate categories by the WHO[9].
Large duct pattern: Macroscopically, tumors with a large duct pattern show cysts (5 to 7 cysts) that are > 0.5 cm in diameter. These cysts may mimic intrapancreatic mucinous neoplasm (IPMN) radiologically and macroscopically. Microscopically, these neoplastic cysts are lined by atypical cuboidal epithelium with occasional papillary projections[39].
Foamy gland pattern: The foamy gland pattern poses a diagnostic challenge especially on frozen section as the cytoarchitectural features of malignancy are subtle in this particular pattern. Tumors are composed of glands resembling benign endocervical glands or gastric foveolar glands, with foamy (microvesicular) cytoplasm. Condensed brush border-like bands are noted at the luminal aspect of the glands, and the raisinoid, small nuclei are basally located[40].
Cystic papillary pattern: This pattern may be related to the large duct pattern. It is characterized by a papillary cystic architecture intermixed with intervening areas of conventional PDAC. The biologic behavior of PDAC with this pattern is similar to that of poorly differentiated adenocarcinoma[9,41].
Adenosquamous carcinoma is a tumor composed of both adenocarcinoma and squamous carcinoma components (Figure 7), with a poor prognosis. Some authors have suggested an arbitrary cut-off of 30% of the squamous component for the diagnosis[42-44]. The proportion of squamous component had no effect on overall survival in Voong et al[45]’s study. However, the presence of any degree of squamous differentiation in a PDAC appears to confer a worse outcome[44-46]. Pure squamous cell carcinomas of the pancreas are extremely rare, and a metastasis should always be ruled out before establishing this diagnosis[44,47]. At the molecular level, both adenosquamous and pure squamous carcinoma show the classic molecular alterations of PDAC[44,48-50].
More than 80% of extracellular mucin is required to establish the diagnosis of colloid carcinoma. Although focal signet-ring cells can be seen in this subtype, the overall prognosis of colloid carcinoma is more favorable than conventional PDAC[51-53]. Studies have shown similar molecular profiles between the colloid carcinoma and intestinal-type IPMN; both of which harbor somatic mutations in GNAS. Thus, intestinal-type IPMN is considered to be a precursor of this particular subtype of PDAC[54-57]. At the germline level, heterozygous mutations in the ATM gene seems to predispose patients to the development of the colloid subtype of PDAC[58]. Furthermore, germline ATM aberrations are shown to increase the efficacy of antineoplastic drugs that induce synthetic lethality such as platinum drugs and PARP inhibitors (e.g., Olaparib)[59,60].
The diagnosis of signet-ring cell carcinoma is rendered when more than 80% of the tumor demonstrates signet ring cells. This subtype is extremely rare in the pancreas therefore its prognosis is unknown. Similar to the other uncommon subtypes listed above, metastatic malignancies with a signet ring cell component, notably gastric and mammary carcinomas, must be excluded before such a diagnosis is rendered[61,62]. Due to its extreme rarity, molecular data on this subtype is currently unavailable in the literature[5].
The histologic hallmarks of this subtype include minimal glandular differentiation, a syncytial growth pattern and abundant inflammatory infiltrate[63]. MMR deficiency is common in this subtype (up to 20%) conferring a better response to immune checkpoint inhibitors and a slightly better prognosis compared to conventional PDAC[9,63,64]. Due to its rarity, molecular data are limited to some case reports. Kryklyva et al[65] recently reported a case of pancreatic medullary carcinoma without MMR deficiency. However, a somatic mutation in the POLE gene was noted resulting in a high tumor mutation burden and theoretically, increased neoantigen exposure with potential responsiveness to immune checkpoint inhibitor therapy.
The diagnosis of hepatoid PDAC requires that over 50% of the lesion shows hepatocellular differentiation[9]. This is another extremely rare subtype. Due to its extreme rarity, a metastatic hepatocellular carcinoma should always be considered first[66,67]. Data on the prognosis of this subtype are limited[9]. In the literature, molecular profiling of one case of pancreatic hepatoid carcinoma is available. This showed somatic mutations of BAP1 and NOTCH1. Both targets are considered not actionable by current precision immunotherapy[68].
While risk factors, clinical presentation and imaging studies can be helpful in the diagnosis of a mass lesion in the pancreas, there is a significant overlap between benign and malignant lesions. Therefore, histologic examination is a crucial compo
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aseni P, Chen Q, Sakamoto K, Zhao CF S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY
| 1. | Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020;20:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Adsay NV, Bandyopadhyay S, Basturk O, Othman M, Cheng JD, Klöppel G, Klimstra DS. Chronic pancreatitis or pancreatic ductal adenocarcinoma? Semin Diagn Pathol. 2004;21:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Hermanová M, Lenz J. Differential diagnosis of the chronic pancreatitis and the pancreatic ductal adenocarcinoma. Cesk Patol. 2012;48:135-140. [PubMed] |
| 4. | Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Ueda J, Tanaka M, Ohtsuka T, Tokunaga S, Shimosegawa T; Research Committee of Intractable Diseases of the Pancreas. Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: a multicenter retrospective analysis. Surgery. 2013;153:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Vareedayah AA, Alkaade S, Taylor JR. Pancreatic Adenocarcinoma. Mo Med. 2018;115:230-235. [PubMed] |
| 7. | Al-Hawary MM, Kaza RK, Azar SF, Ruma JA, Francis IR. Mimics of pancreatic ductal adenocarcinoma. Cancer Imaging. 2013;13:342-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Klöppel G, Adsay NV. Chronic pancreatitis and the differential diagnosis versus pancreatic cancer. Arch Pathol Lab Med. 2009;133:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Hruban RH, Adsay NV, Esposito I, Fukushima N, Furukawa T, Kloppel G, Maitra A, Notohara K, Offerhaus GJA, Ohike N, Pitman MB, Zamboni G. WHO classification of tumours of the digestive system. 5th ed. Lyon, France: International Agency for Research on Cancer (IARC), 2019: 322-332. |
| 10. | Bandyopadhyay S, Basturk O, Coban I, Thirabanjasak D, Liang H, Altinel D, Adsay NV. Isolated solitary ducts (naked ducts) in adipose tissue: a specific but underappreciated finding of pancreatic adenocarcinoma and one of the potential reasons of understaging and high recurrence rate. Am J Surg Pathol. 2009;33:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 440] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Crosara S, D'Onofrio M, De Robertis R, Demozzi E, Canestrini S, Zamboni G, Pozzi Mucelli R. Autoimmune pancreatitis: Multimodality non-invasive imaging diagnosis. World J Gastroenterol. 2014;20:16881-16890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Lopes Vendrami C, Shin JS, Hammond NA, Kothari K, Mittal PK, Miller FH. Differentiation of focal autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2020;45:1371-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Dite P, Novotny I, Dvorackova J, Kianicka B, Blaho M, Svoboda P, Uvirova M, Rohan T, Maskova H, Kunovsky L. Pancreatic Solid Focal Lesions: Differential Diagnosis between Autoimmune Pancreatitis and Pancreatic Cancer. Dig Dis. 2019;37:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 16. | Sugumar A, Klöppel G, Chari ST. Autoimmune pancreatitis: pathologic subtypes and their implications for its diagnosis. Am J Gastroenterol. 2009;104:2308-10; quiz 2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Klöppel G, Sipos B, Zamboni G, Kojima M, Morohoshi T. Autoimmune pancreatitis: histo- and immunopathological features. J Gastroenterol. 2007;42 Suppl 18:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1059] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 19. | Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, Liu X, Longnecker DS, Mino-Kenudson M, Notohara K, Rodriguez-Justo M, Srivastava A, Zamboni G, Zen Y. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42 Suppl 18:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Tezuka K, Makino T, Hirai I, Kimura W. Groove pancreatitis. Dig Surg. 2010;27:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Stolte M, Weiss W, Volkholz H, Rösch W. A special form of segmental pancreatitis: "groove pancreatitis". Hepatogastroenterology. 1982;29:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying "cystic dystrophy of heterotopic pancreas", "para-duodenal wall cyst", and "groove pancreatitis". Semin Diagn Pathol. 2004;21:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Raman SP, Salaria SN, Hruban RH, Fishman EK. Groove pancreatitis: spectrum of imaging findings and radiology-pathology correlation. AJR Am J Roentgenol. 2013;201:W29-W39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Kim JD, Han YS, Choi DL. Characteristic clinical and pathologic features for preoperative diagnosed groove pancreatitis. J Korean Surg Soc. 2011;80:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Addeo G, Beccani D, Cozzi D, Ferrari R, Lanzetta MM, Paolantonio P, Pradella S, Miele V. Groove pancreatitis: a challenging imaging diagnosis. Gland Surg. 2019;8:S178-S187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, Chen Z, Adsay NV. Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Tascilar M, Offerhaus GJ, Altink R, Argani P, Sohn TA, Yeo CJ, Cameron JL, Goggins M, Hruban RH, Wilentz RE. Immunohistochemical labeling for the Dpc4 gene product is a specific marker for adenocarcinoma in biopsy specimens of the pancreas and bile duct. Am J Clin Pathol. 2001;116:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Liu H, Shi J, Anandan V, Wang HL, Diehl D, Blansfield J, Gerhard G, Lin F. Reevaluation and identification of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med. 2012;136:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Wang HL, Kim CJ, Koo J, Zhou W, Choi EK, Arcega R, Chen ZE, Wang H, Zhang L, Lin F. Practical Immunohistochemistry in Neoplastic Pathology of the Gastrointestinal Tract, Liver, Biliary Tract, and Pancreas. Arch Pathol Lab Med. 2017;141:1155-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Slatko BE, Gardner AF, Ausubel FM. Overview of Next-Generation Sequencing Technologies. Curr Protoc Mol Biol. 2018;122:e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 473] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 32. | Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015;19:A68-A77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 2038] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 33. | Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185-203.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1390] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 34. | Ren B, Liu X, Suriawinata AA. Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions: Histopathology, Cytopathology, and Molecular Pathology. Am J Pathol. 2019;189:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 35. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA; Australian Pancreatic Cancer Genome Initiative; Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1995] [Article Influence: 199.5] [Reference Citation Analysis (1)] |
| 36. | Wang JD, Jin K, Chen XY, Lv JQ, Ji KW. Clinicopathological significance of SMAD4 loss in pancreatic ductal adenocarcinomas: a systematic review and meta-analysis. Oncotarget. 2017;8:16704-16711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Sausen M, Phallen J, Adleff V, Jones S, Leary RJ, Barrett MT, Anagnostou V, Parpart-Li S, Murphy D, Kay Li Q, Hruban CA, Scharpf R, White JR, O'Dwyer PJ, Allen PJ, Eshleman JR, Thompson CB, Klimstra DS, Linehan DC, Maitra A, Hruban RH, Diaz LA Jr, Von Hoff DD, Johansen JS, Drebin JA, Velculescu VE. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun. 2015;6:7686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 38. | Luchini C, Paolino G, Mattiolo P, Piredda ML, Cavaliere A, Gaule M, Melisi D, Salvia R, Malleo G, Shin JI, Cargnin S, Terrazzino S, Lawlor RT, Milella M, Scarpa A. KRAS wild-type pancreatic ductal adenocarcinoma: molecular pathology and therapeutic opportunities. J Exp Clin Cancer Res. 2020;39:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 39. | Kosmahl M, Pauser U, Anlauf M, Klöppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Adsay V, Logani S, Sarkar F, Crissman J, Vaitkevicius V. Foamy gland pattern of pancreatic ductal adenocarcinoma: a deceptively benign-appearing variant. Am J Surg Pathol. 2000;24:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Kelly PJ, Shinagare S, Sainani N, Hong X, Ferrone C, Yilmaz O, Fernández-del Castillo C, Lauwers GY, Deshpande V. Cystic papillary pattern in pancreatic ductal adenocarcinoma: a heretofore undescribed morphologic pattern that mimics intraductal papillary mucinous carcinoma. Am J Surg Pathol. 2012;36:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Trikudanathan G, Dasanu CA. Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J. 2010;103:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Voong KR, Davison J, Pawlik TM, Uy MO, Hsu CC, Winter J, Hruban RH, Laheru D, Rudra S, Swartz MJ, Nathan H, Edil BH, Schulick R, Cameron JL, Wolfgang CL, Herman JM. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Kovi J. Adenosquamous carcinoma of the pancreas: a light and electron microscopic study. Ultrastruct Pathol. 1982;3:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Moslim MA, Lefton MD, Ross EA, Mackrides N, Reddy SS. Clinical and Histological Basis of Adenosquamous Carcinoma of the Pancreas: A 30-year Experience. J Surg Res. 2021;259:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Brody JR, Costantino CL, Potoczek M, Cozzitorto J, McCue P, Yeo CJ, Hruban RH, Witkiewicz AK. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Fang Y, Su Z, Xie J, Xue R, Ma Q, Li Y, Zhao Y, Song Z, Lu X, Li H, Peng C, Bai F, Shen B. Genomic signatures of pancreatic adenosquamous carcinoma (PASC). J Pathol. 2017;243:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Borazanci E, Millis SZ, Korn R, Han H, Whatcott CJ, Gatalica Z, Barrett MT, Cridebring D, Von Hoff DD. Adenosquamous carcinoma of the pancreas: Molecular characterization of 23 patients along with a literature review. World J Gastrointest Oncol. 2015;7:132-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Mostafa ME, Erbarut-Seven I, Pehlivanoglu B, Adsay V. Pathologic classification of "pancreatic cancers": current concepts and challenges. Chin Clin Oncol. 2017;6:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, Brennan MF, Klimstra DS. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 54. | Scarpa A, Real FX, Luchini C. Genetic unrelatedness of co-occurring pancreatic adenocarcinomas and IPMNs challenges current views of clinical management. Gut. 2018;67:1561-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Felsenstein M, Noë M, Masica DL, Hosoda W, Chianchiano P, Fischer CG, Lionheart G, Brosens LAA, Pea A, Yu J, Gemenetzis G, Groot VP, Makary MA, He J, Weiss MJ, Cameron JL, Wolfgang CL, Hruban RH, Roberts NJ, Karchin R, Goggins MG, Wood LD. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018;67:1652-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Yamada M, Sekine S, Ogawa R, Taniguchi H, Kushima R, Tsuda H, Kanai Y. Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol. 2012;228:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 58. | Hutchings D, Jiang Z, Skaro M, Weiss MJ, Wolfgang CL, Makary MA, He J, Cameron JL, Zheng L, Klimstra DS, Brand RE, Singhi AD, Goggins M, Klein AP, Roberts NJ, Hruban RH. Histomorphology of pancreatic cancer in patients with inherited ATM serine/threonine kinase pathogenic variants. Mod Pathol. 2019;32:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Choi M, Kipps T, Kurzrock R. ATM Mutations in Cancer: Therapeutic Implications. Mol Cancer Ther. 2016;15:1781-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 351] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 60. | Schmitt A, Feldmann G, Zander T, Reinhardt HC. Targeting Defects in the Cellular DNA Damage Response for the Treatment of Pancreatic Ductal Adenocarcinoma. Oncol Res Treat. 2018;41:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | El Hussein S, Khader SN. Primary signet ring cell carcinoma of the pancreas: Cytopathology review of a rare entity. Diagn Cytopathol. 2019;47:1314-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Radojkovic M, Ilic D, Ilic I. Primary signet ring cell carcinoma of the pancreas with a good response to chemotherapy: case report and literature review. Tumori. 2017;103:e50-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, Kadkol SS, Yeo CJ, Choti M, Zahurak M, Johnson K, Tascilar M, Offerhaus GJ, Hruban RH, Kern SE. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am J Pathol. 2000;156:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 64. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7247] [Article Influence: 724.7] [Reference Citation Analysis (0)] |
| 65. | Kryklyva V, Ter Linden E, Kroeze LI, de Voer RM, van der Kolk BM, Stommel MWJ, Hermans JJ, Luchini C, Wood LD, Hruban RH, Nagtegaal ID, Ligtenberg MJL, Brosens LAA. Medullary Pancreatic Carcinoma Due to Somatic POLE Mutation: A Distinctive Pancreatic Carcinoma With Marked Long-Term Survival. Pancreas. 2020;49:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Vanoli A, Argenti F, Vinci A, La Rosa S, Viglio A, Riboni R, Necchi V, Pugliese L, Sessa F, Pietrabissa A, Paulli M. Hepatoid carcinoma of the pancreas with lymphoid stroma: first description of the clinical, morphological, immunohistochemical, and molecular characteristics of an unusual pancreatic carcinoma. Virchows Arch. 2015;467:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Tomino T, Ninomiya M, Matono R, Narutomi F, Oshiro Y, Watanabe K, Taniguchi D, Nishimura S, Zaitsu Y, Kajiwara Y, Yokota T, Minami K, Nishizaki T. Pure pancreatic hepatoid carcinoma: a surgical case report and literature review. Surg Case Rep. 2019;5:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Chang JM, Katariya NN, Lam-Himlin DM, Haakinson DJ, Ramanathan RK, Halfdanarson TR, Borad MJ, Pannala R, Faigel D, Moss AA, Mathur AK. Hepatoid Carcinoma of the Pancreas: Case Report, Next-Generation Tumor Profiling, and Literature Review. Case Rep Gastroenterol. 2016;10:605-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |