Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1696
Peer-review started: July 22, 2021
First decision: August 19, 2021
Revised: August 30, 2021
Accepted: November 1, 2021
Article in press: November 1, 2021
Published online: December 27, 2021
Processing time: 154 Days and 20.2 Hours
Liver resection and radiofrequency ablation are considered curative options for hepatocellular carcinoma. The choice between these techniques is still controversial especially in cases of hepatocellular carcinoma affecting posterosuperior segments in elderly patients.
To compare post-operative outcomes between liver resection and radiofrequency ablation in elderly with single hepatocellular carcinoma located in posterosuperior segments.
A retrospective multicentric study was performed enrolling 77 patients age ≥ 70-years-old with single hepatocellular carcinoma (≤ 30 mm), located in posterosuperior segments (4a, 7, 8). Patients were divided into liver resection and radiofrequency ablation groups and preoperative, peri-operative and long-term outcomes were retrospectively analyzed and compared using a 1:1 propensity score matching.
After propensity score matching, twenty-six patients were included in each group. Operative time and overall postoperative complications were higher in the resection group compared to the ablation group (165 min vs 20 min, P < 0.01; 54% vs 19% P = 0.02 respectively). A median hospital stay was significantly longer in the resection group than in the ablation group (7.5 d vs 3 d, P < 0.01). Ninety-day mortality was comparable between the two groups. There were no significant differences between resection and ablation group in terms of overall survival and disease free survival at 1, 3, and 5 years.
Radiofrequency ablation in posterosuperior segments in elderly is safe and feasible and ensures a short hospital stay, better quality of life and does not modify the overall and disease-free survival.
Core Tip: A retrospective multicentric study was performed enrolling 77 patients with ≥ 70 years of age and a single hepatocellular carcinoma (≤ 30 mm), located in the posterosuperior segments (4a, 7, 8). Patients were divided into two groups: liver resection and radiofrequency ablation. Peri-operative and long-term outcomes were analyzed and compared using a 1:1 propensity score matching. The study results show that radiofrequency ablation in posterosuperior segments in elderly patients is safe and feasible and ensures a short hospital stay, reduces overall postoperative complications, increases the quality of life and does not modify the overall and disease-free survival.
- Citation: Delvecchio A, Inchingolo R, Laforgia R, Ratti F, Gelli M, Anelli MF, Laurent A, Vitali G, Magistri P, Assirati G, Felli E, Wakabayashi T, Pessaux P, Piardi T, di Benedetto F, de'Angelis N, Briceño J, Rampoldi A, Adam R, Cherqui D, Aldrighetti LA, Memeo R. Liver resection vs radiofrequency ablation in single hepatocellular carcinoma of posterosuperior segments in elderly patients. World J Gastrointest Surg 2021; 13(12): 1696-1707
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1696.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1696
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and the fifth most common cancer[1]. According to Barcelona Clinic Liver Cancer (BCLC) staging system, ablation, resection and liver transplantation (LT) are considered the best treatment for patients affected by HCC very early and early stage[2]. Considering the increasing number of elderly patients in our population, LT could not be considered as a valid therapeutic option in these patients, due to the limit of age that is contraindicated in many liver transplantation centers[3]. Nevertheless, for elderly patients, liver resection (LR) and radiofrequency ablation (RFA) remains a valid alternative. LR guarantees a complete removal of the tumor with a wide margin either in anatomical and non-anatomical resection. Even if in recent periods the use of minimally invasive approaches, laparoscopic and robotic, has been increasing, they still remain invasive procedures performed under general anesthesia[4]. On the contrary, RFA has very low invasiveness and morbidity but literature is still unclear in terms of disease free and overall survival compared to liver resection[5,6].
The choice between LR and RFA is still controversial, especially in cases of HCC affecting posterosuperior segments (PSS).
PSS are more difficult to access than the anterolateral ones for the anatomical position and are technically complex for the bleeding control and poor liver field visualization. Open liver resection (OLR) is widely considered as preferred procedure for HCC located in PSS[7], instead, laparoscopic liver resection (LLR) in PSS is challenging and needs to be approached by experienced surgeons in major centers. LLR presents important benefits with less invasiveness, less postoperative pain, early discharge and similar mortality and morbidity compared to OLR according to 2017 Southampton Consensus Guidelines[8].
In the literature there are few studies with focus on surgical treatments in elderly patients with HCC especially in PSS[9]. The aim of our study is to compare short and long-term outcomes between LR and RFA in elderly patients with single HCC located in PSS.
A multicentric retrospective study was performed enrolling 77 patients with ≥ 70 years of age, from January 2009 to January 2019 in the following European hospital centers: IRCCS San Raffaele Hospital, Milan, Italy; Paul Brousse University Hospital, Villejuif, France; University Hospital Reina Sofía, Córdoba, Spain; Henri Mondor University Hospital, Créteil, France; University Hospital Policlinico of Modena, Modena, Italy; Miulli Hospital, Bari, Italy; Hospital Niguarda, Milan, Italy; Strasbourg University Hospital, IRCAD, Strasbourg, France; Robert Debré University Hospital, Reims, France; University Hospital Geneva, Switzerland.
Inclusion criteria were elderly patients (age ≥ 70) with single HCC ≤ 30 mm, located in PSS, treated with RFA or LR. Exclusion criteria are multiple HCC or single > 30mm, patients younger than 70 years and American Society of Anesthesiologists (ASA) score > IV.
Patients were divided into two groups according to the treatment, LR or RFA. LR group included open liver resection and laparoscopic liver resection.
The choice of treatment was generally based on the tumor location, the history of previous upper abdominal surgery and each center experience.
Preoperative, peri-operative data and long term outcomes were retrospectively analyzed and compared in both groups before propensity score matching (b-PSM) and after propensity score matching (a-PSM).
Patient demographic data and preoperative variables were collected: blood tests, i.e. serum α-fetoprotein (AFP), platelets, bilirubin and coagulation; American Society of Anesthesiologists (ASA) score; comorbidities; cause of cirrhosis; Child-Pugh and the model for end-stage liver disease (MELD) scores.
All patients were staged preoperatively following computer tomography of the chest-abdomen-pelvis and/or abdominal magnetic resonance.
Tumor involving segments 4a, 7, 8 or between them were defined as located in PSS. HCC location and size were recorded and the type of treatment was discussed in multidisciplinary teams including surgeons, hepatologists, oncologists, interventional radiologists and pathologists.
Diagnosis was based on non-invasive criteria according to European Association for the Study of the Liver (EASL) and biopsy was used in case of inconclusive diagnosis[10].
The procedure was performed by expert surgeons and interventional radiologists with a minimum consolidated experience of 10 years.
An intraoperative Doppler Ultrasound was systematically achieved to confirm the procedure to be performed.
Percutaneous RFA was performed using a single internally cooled electrode under a continuous sonographic guidance with local anesthesia and intravenous sedation. Post-RFA ultrasound was performed to control that there were no immediate complications such as hemorrhage or hematoma. On the 1st post-op day, an ultrasound was performed to assess the quality of the ablation in terms of necrotic area.
The Couinaud classification was used to define liver segmentation and the Brisbane 2000 terminology was used to define liver resections[11,12]. During surgical resection, attempts were made to maintain an adequate parenchymal margin of at least 1 cm.
The Pringle maneuver was routinely prepared for surgical resection and used according to the experience of each center. Perioperative variables included operative time, rate of blood transfusion, complications and length of hospital stay which were recorded. Clavien-Dindo grading system was used to classify postoperative complications.
Ninety-day mortality was defined as any deaths occurring 90 d from surgery or RFA.
Patients undergoing RFA were given a CT scan 1 mo after ablation, in order to evaluate the results of the treatment according to mRECIST (modified Response Evaluation Criteria in Solid Tumors) criteria[13].
A standardized follow-up was adopted, every 2 mo for the first 2 years and then every 4 mo. During such follow-up, the patients were subjected to blood testing including alpha-fetoprotein, liver function and imaging, such as abdominal ultrasonography, CT, or MRI. Recurrence treatment included repeat resection or RFA, trans-arterial chemo-embolization, chemotherapy or supportive care according to the EASL clinical practice guidelines[10].
All HCC-related deaths and recurrences were estimated and used to calculate the overall survival (OS) and disease-free survival (DFS) in both groups.
A propensity score-based analysis was performed to minimize selection bias and limit confusion in the retrospective study. The propensity score was estimated using a 1:1 Logistical regression regarding the following variables: ASA score, MELD score and the tumors size.
Continuous variables, expressed as median with range, were compared using Mann-Whitney U test. Instead, categorical variables, expressed as numbers with percentages, were compared using chi-square test.
Overall survival and disease-free survival were estimated using the Kaplan-Meier method and compared using a log-rank test. A P value of < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS software version 20.
The preoperative characteristics, before and after propensity score matching of RFA and LR groups are presented in Table 1.
| Before PSM (n: 77) | After PSM (n: 52) | |||||||||
| RFA (n: 40) | Surgery (n: 37) | P value | RFA (n: 26) | Surgery (n: 26) | P value | |||||
| Male, n (%) | 28 (70) | 27 (73) | 0.80 | 17 (65) | 16 (62) | 1.0 | ||||
| Age (yr) median (range) | 74.5 (70-87) | 74.98 (70-83) | 0.80 | 75 (70-81) | 74.26 (70-81) | 0.43 | ||||
| BMI (kg/cm²) median (range) | 26.7 (19-51) | 26.7 (22-36) | 0.90 | 26.7 (19-51) | 26.7 (22-36) | 0.48 | ||||
| Comorbidity ≥ 2, n (%) | 23 (57) | 14 (38) | 0.10 | 12 (46) | 10 (40) | 0.78 | ||||
| Cause of cirrhosis, n (%) | 0.30 | 0.71 | ||||||||
| Hepatitis C virus, n (%) | 21 (53) | 19 (50) | 16 (61) | 16 (61) | ||||||
| Hepatitis B virus, n (%) | 5 (12) | 10 (27) | 4 (15) | 6 (23) | ||||||
| Alcohol, n (%) | 6 (15) | 4 (12) | 3 (12) | 1 (4) | ||||||
| Others, n (%) | 8 (20) | 4 (11) | 3 (12) | 3 (12) | ||||||
| F4 cirrhosis, n (%) | 33 (82) | 19 (51) | 0.01 | 22 (85) | 12 (46) | 0.01 | ||||
| ASA score, n (%) | 0.05 | 0.35 | ||||||||
| I/II, n (%) | 11 (28) | 19 (51) | 10 (40) | 11 (42) | ||||||
| III/IV, n (%) | 29 (72) | 18 (49) | 16 (61) | 15 (58) | ||||||
| Preoperative blood tests median (range) | ||||||||||
| Bilirubin (µmol/L) median (range) | 1 (1-1.1) | 1 (1-2) | 0.55 | 1 (1-1.1) | 1 (1-1) | 0.90 | ||||
| Platelet count × 109/L median (range) | 118 (52-380) | 173 (55-387) | 0.01 | 137 (69-380) | 183 (55-340) | 0.06 | ||||
| INR median (range) | 1 (1-2) | 1 (1-2) | 0.40 | 1 (1-1.2) | 1 (1-2) | 0.06 | ||||
| AFP (mg/mL) median (range) | 5 (1-1988) | 12.5 (2-3900) | 0.14 | 6.5 (1-1988) | 7 (2-3900) | 0.64 | ||||
| Child-Pugh, n (%) | 0.20 | 0.06 | ||||||||
| A | 37 (93) | 30 (81) | 26 (100) | 21 (81) | ||||||
| B | 3 (7) | 7 (19) | 0 (0) | 5 (19) | ||||||
| MELD median (range) | 8 (6-15) | 6 (6-16) | 0.01 | 8 (6-15) | 7 (6-16) | 0.23 | ||||
| Tumors size (mm) median (range) | 23 (10-30) | 29 (12-30) | 0.02 | 20.5 (10-30) | 23 (15-30) | 0.08 | ||||
| Tumor locations, n (%) | 0.10 | 0.45 | ||||||||
| 4a | 3 (7) | 3 (8) | 2 (8) | 2 (8) | ||||||
| 7 | 8 (20) | 17 (46) | 4 (15) | 9 (34) | ||||||
| 8 | 23 (58) | 14 (38) | 17 (65) | 13 (50) | ||||||
| 7-8 | 6 (15) | 3 (8) | 3 (12) | 2 (8) | ||||||
| Histological proven, n (%) | 7 (17) | 8 (22) | 0.80 | 6 (23) | 8 (31) | 0.75 | ||||
During the study period, 77 patients were enrolled and divided into two groups according to the procedure performed: 40 patients in the RFA group and 37 patients in the LR group. After a 1:1 PSM, 52 patients were enrolled: 26 patients for each group.
The rate of F4 cirrhosis was lower in LR group than in the RFA group both before (51% vs 82%, P = 0.01) and after PSM (46% vs 85%, P = 0.01). ASA scores and MELD scores were lower in liver resection group b-PSM than in the RFA group (P = 0.05, P = 0.01, respectively) and equal between the two groups a-PSM (P = 0.35, P = 0.23, respectively). Tumor size was higher in the LR group than in the RFA group b-PSM (median, 29 mm vs 23 mm, P = 0.02) and comparable between two groups a-PSM (median, 23 mm vs 20.5 mm, P = 0.08).
The perioperative characteristics, before and after propensity score matching of the RFA and LR groups are presented in Table 2.
| Before PSM (n: 77) | After PSM (n: 52) | ||||||
| RFA (n: 40) | Surgery (n: 37) | P value | RFA (n: 26) | Surgery (n: 26) | P value | ||
| Operative time (min) median (range) | 23.5 (5-55) | 260 (120-600) | < 0.01 | 20 (5-26) | 165 (120-383) | < 0.01 | |
| Blood transfusion, n (%) | 3 (7) | 7 (19) | 0.20 | 3 (12) | 5 (19) | 0.70 | |
| Postoperative complications, n (%) | 9 (22) | 16 (43) | 0.09 | 5 (19) | 14 (54) | 0.02 | |
| Dindo-Clavien classification, n (%) | |||||||
| I-II | 9 (22) | 11 (30) | 0.60 | 5 (19) | 10 (40) | 0.20 | |
| III-IV | 0 (0) | 5 (13) | 0.02 | 0 (0) | 2 (8) | 0.50 | |
| Type of complications, n (%) | |||||||
| Liver failure | 0 (0) | 2 (5) | 0.22 | 0 (0) | 2 (8) | 0.50 | |
| Ascites | 0 (0) | 4 (11) | 0.05 | 0 (0) | 4 (15) | 0.11 | |
| Biliary leakage | 0 (0) | 1 (3) | 0.50 | 0 (0) | 1 (4) | 1 | |
| Hemorrhage | 1 (2) | 2 (5) | 0.60 | 1 (4) | 1 (4) | 1.0 | |
| Systemic infection | 0 (0) | 4 (11) | 0.05 | 0 (0) | 4 (15) | 0.11 | |
| Intra-abdominal abscess | 0 (0) | 2 (5) | 0.23 | 0 (0) | 1 (4) | 1.0 | |
| Wound infection | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Portal thrombosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Pulmonary | 3 (7) | 3 (8) | 1 | 1 (4) | 3 (12) | 0.61 | |
| Cardiac | 1 (2) | 2 (5) | 0.60 | 0 (0) | 2 (8) | 0.50 | |
| Renal | 1 (2) | 3 (8) | 0.35 | 1 (4) | 2 (8) | 1.0 | |
| Reoperation, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Postoperative treatment, n (%) | 0 (0) | 2 (5) | 0.23 | 0 (0) | 1 (4) | 1.0 | |
| Length of hospital stay (d) median (range) | 2 (1-15) | 6 (2-203) | < 0.01 | 3 (1-9) | 7.5 (2-203) | < 0.01 | |
| 90 d mortality, n (%) | 2 (5) | 2 (5) | 1.0 | 2 (8) | 2 (8) | 1.0 | |
| Recurrence, n (%) | 21 (52) | 15 (40) | 0.40 | 12 (46) | 11 (42) | 1.0 | |
Operative time was higher in the LR group than in the RFA group b-PSM (260 min vs 23.5 min, P < 0.01) and this was confirmed also after restricting the analysis to propensity score matching (165 min vs 20 min, P < 0.01). Intraoperative blood transfusion was comparable between the LR group and the RFA group both before (19% vs 7%, P = 0.20) and after PSM (19% vs 12%, P = 0.70). There were no differences in overall postoperative complications between the LR group and the RFA group b-PSM (43% vs 22%, P = 0.09), conversely, for a-PSM were significantly higher in the LR group than in the RFA group (54% vs 19%, P = 0.02). A median hospital stay was significantly longer in the LR group than in the RFA group both before and after PSM (6 d vs 2 d, P < 0.01; 7.5 d vs 3 d, P < 0.01, respectively). There was no difference in the 90-d mortality between the LR and the RFA groups both before (5% vs 5%, P = 1.0) and after PSM (8% vs 8%, P = 1.0).
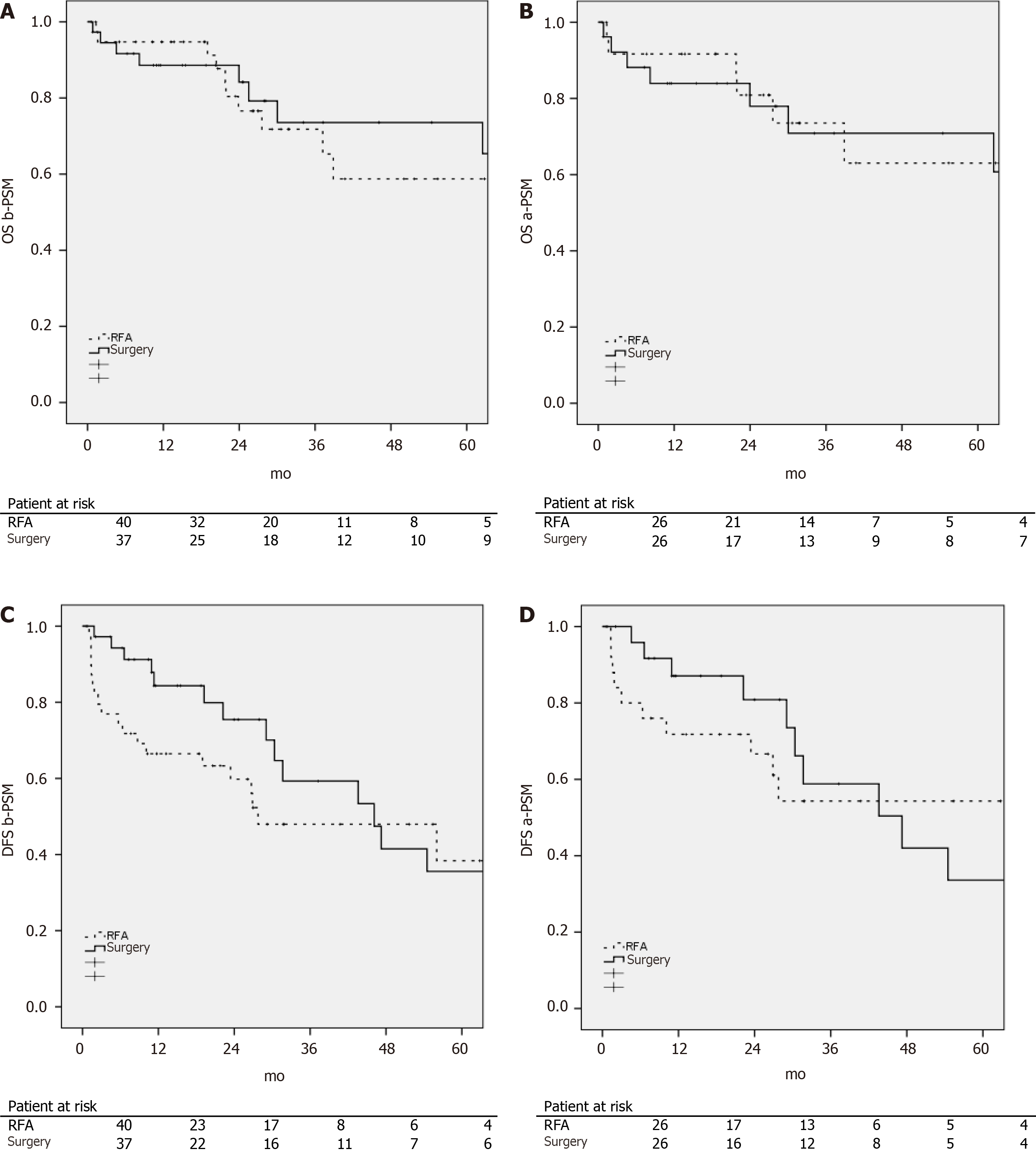
OS and DFS were calculated before and after the propensity score matching according to the procedure performed and are presented in Figure 1.
There were no statistically significant differences between each group in terms of OS (b-PSM P = 0.50; a-PSM P = 0.91) and DFS (b-PSM P = 0.17; a-PSM P = 0.70).
The estimated 1-, 3-, and 5-year OS rates b-PSM were 9%, 72%, and 59% for the RFA group and 88, 74, and 74% for the LR group respectively.
The estimated 1-, 3- and 5-year OS rates a-PSM were 92%, 73%, and 63% for the RFA group and 84, 71, and 71% in the LR group respectively.
DFS b-PSM at 1-, 3- and 5-years was 66%, 48%, and 38% in the RFA group as compared to 84, 59, and 35% in the LR group respectively.
DFS a-PSM at 1-, 3- and 5-years was 72%, 54%, and 54% in the RFA group as compared to 87, 59 and 34% in the LR group respectively.
To our knowledge, our retrospective multicentric study is one of the few series reported in literature comparing short and long-term outcomes between RFA and LR in elderly patients and it is the first considering the HCC located in PSS.
The number of elderly patients is constantly growing thanks to improved medical care and an increase in life expectancy; therefore, the cut-off age to define the elderly has moved from > 65 years to 70 years[14].
Elderly patients should be treated with RFA or LR for a curative intent because they are unsuitable for LT due to advanced age [3]. Elderly are considered fragile as a result of the accumulation of chronic diseases, the gradual loss of reserve capacity and the increase in the tumor’s rate including HCC[15].
HCC located in PSS still represent a surgical challenge and the best therapeutic option is still controversial. PSS segments are difficult to access, located in the posterior part of the abdominal cavity where exposure is not ideal[16,17].
The resections of lesions located in PSS are technically complex and should be performed by experienced surgeons in open and laparoscopic surgery and in a high-volume centers, as recommended by Southampton Guidelines[18]. Experience is essential to ensure success without compromise to oncological outcomes and surgical safety. Laparoscopic approach was considered difficult for these kind of lesions and also the anatomical landmarks are not clear as in anterior segments of the liver[8,19]. In complex cases including major hepatectomy, biliary reconstruction and difficult segmentectomy of the PSS, robotic surgery improved intra-operative and short-term postoperative outcomes[20].
In recent years RFA has been increasingly used for the treatment of small HCC as first line curative treatment when patients are not candidates for LR or LT and also as bridging treatment for patients on the waiting list for liver transplantation[21].
Technological improvements have increased effectiveness of RFA characterized by less invasiveness and morbidity and better tolerability compared to LR; on the other hand, liver resection guarantees removal of the tumor-bearing portal and hepatic veins territory affected by micro metastases and microscopic vascular tumor invasions[5,22-24].
The choice between percutaneous RFA and LR is still controversial because many randomized prospective studies and meta-analyses were not conclusive[6,25-27]. Several aspects must be considered for choosing the best procedure including patient’s age, HCC characteristics, oncological outcome, periprocedural risks, length of hospitalization and costs[22].
According to Asian Pacific Association for the Study of the Liver(APASL) HCC guidelines[28], RFA is recommended as first line treatment for HCC ≤ 2 cm because it showed similar results in terms of OS compared to LR. Instead, American Association for the Study of Liver Diseases (AASLD) highlight that surgical resection remains the first therapeutic option in small size HCC, leaving RFA for patients not eligible for surgery[29]. In cases of a single HCC > 2 cm, all guidelines recommend LR as the first approach when feasible. RFA has the advantage of cost effectiveness, feasibility, minimal invasiveness, short hospital stay, excellent efficacy and is particularly suitable for older patients and tumors located in deep positions in the liver, also in PSS.
Our retrospective multicentric study showed better short-term outcomes and similar long-term outcomes for RFA compared to LR in elderly patients with HCC ≤ 30 mm located in PSS.
In our work, operative time and hospital stay were shorter in the RFA group compared to the LR group. This highlights the less invasive nature of the ablative treatment and is corroborated by randomized controlled trials[6,25,27].
According to the literature data, overall postoperative complications were significantly lower in the RFA group than in the LR group. These data emphasize the minimally invasiveness and improved post-operative quality of life of percutaneous treatment, necessary features especially for elderly patients[30,31].
In our study, OS and DFS had no significant difference between the RFA and LR group and this is confirmed by Chen et al[25] Conversely, many articles reported a decreased recurrence risk and improvement in OS of LR compared to the RFA group[32-35], but we would underline that there was no specificity regarding patient’s age.
LR has been associated with less HCC recurrences due to complete eradication of the tumor and venous tumor thrombi, and could therefore result in better long-term survival compared to RFA[36]. In addition, RFA may be associated with an increased risk of neoplastic dissemination after treatment due to repeated puncture and temperature-related intratumoral explosion[37].
Compared to LR, it is clear from numerous reports that percutaneous RFA treating liver tumors ≥ 40-50 mm in diameter or located in difficult sites of the liver (subcapsular, adjacent gallbladder or diaphragm) is associated with an increased rate of incomplete treatment, which is usually reported erroneously as a local recurrence[37,38].
Liver resection should be considered for patients with better liver function and longer life expectation in order to balance the postoperative risk of treatment with the benefits in long-term survival.
It is evident that most of the studies and guidelines comparing LR with RFA do not consider the patient’s age and the tumors locations, hence the need for additional prospective randomized studies focusing on elderly patients with HCC located in PSS.
RFA in PSS segments in elderly patients is safe and feasible, ensures a short hospital stay, increases the quality of life and does not modify the overall success rate. This technique should be recommended mainly in elderly patients because it allows a reduction of postoperative complications and a fast discharge to home.
Liver resection and radiofrequency ablation are considered curative options for hepatocellular carcinoma, but the choice among them is still controversial, especially in cases of hepatocellular carcinoma affecting posterosuperior segments in elderly.
In literature there are few studies which focus on surgical treatments in elderly patients with hepatocellular carcinoma especially in posterosuperior segments.
To compare short and long-term outcomes between liver resection and radiofrequency ablation in elderly patients with single hepatocellular carcinoma located in posterosuperior segments.
We performed a multicentric retrospective study enrolling 77 patients with ≥ 70 years of age, from January 2009 to January 2019 in 10 European hospital centers. Patients were divided into two groups according to the treatment, liver resection or radiofrequency ablation. Preoperative, peri-operative data and long term outcomes were retrospectively analyzed and compared in both groups before propensity score matching and after propensity score matching.
After propensity score matching, 26 patients were included in each group. Operative time and overall postoperative complications were higher in the resection group compared to the ablation group. A median hospital stay was significantly longer in the resection group than in the ablation group. There was no significant differences between resection and ablation groups in terms of overall survival and disease free survival at 1, 3 and 5 years.
Radiofrequency ablation in posterosuperior segments in elderly is safe and feasible and ensures a short hospital stay, better quality of life and does not modify the overall and disease-free survival.
Radiofrequency ablation can be considered a gold standard for the treatment of single hepatocellular carcinoma located in posterosuperior segments in elderly. These results must be a starting point for future research and to ensure a higher level of evidence in clinical practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim JH S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4265] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 2. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 3. | Wu FH, Shen CH, Luo SC, Hwang JI, Chao WS, Yeh HZ, Jan YG, Yen Y, Cheng SB, Wu CC, Lin YL, P'eng FK. Liver resection for hepatocellular carcinoma in oldest old patients. World J Surg Oncol. 2019;17:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Ciria R, Berardi G, Alconchel F, Briceño J, Choi GH, Wu YM, Sugioka A, Troisi RI, Salloum C, Soubrane O, Pratschke J, Martinie J, Tsung A, Araujo R, Sucandy I, Tang CN, Wakabayashi G. The impact of robotics in liver surgery: A worldwide systematic review and short-term outcomes meta-analysis on 2,728 cases. J Hepatobiliary Pancreat Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 6. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 598] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG. Laparoscopic vs open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc. 2015;29:2994-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Abu Hilal M, Tschuor C, Kuemmerli C, López-Ben S, Lesurtel M, Rotellar F. Laparoscopic posterior segmental resections: How I do it: Tips and pitfalls. Int J Surg. 2020;82S:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Delvecchio A, Conticchio M, Ratti F, Gelli M, Anelli FM, Laurent A, Vitali GC, Magistri P, Assirati G, Felli E, Wakabayashi T, Pessaux P, Piardi T, Di Benedetto F, de'Angelis N, Briceño-Delgado J, Adam R, Cherqui D, Aldrighetti L, Memeo R. Laparoscopic major hepatectomy for hepatocellular carcinoma in elderly patients: a multicentric propensity scorebased analysis. Surg Endosc. 2021;35:3642-3652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 11. | Couinaud C. Definition of hepatic anatomical regions and their value during hepatectomy (author's transl). Chirurgie. 1980;106:103-108. [PubMed] |
| 12. | Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 14. | Orimo H. [Reviewing the definition of elderly]. Nihon Ronen Igakkai Zasshi. 2006;43:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Badawy A, Seo S, Toda R, Fuji H, Fukumitsu K, Ishii T, Taura K, Kaido T, Uemoto S. A Propensity Score-Based Analysis of Laparoscopic Liver Resection for Liver Malignancies in Elderly Patients. J Invest Surg. 2019;32:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Coelho FF, Kruger JA, Fonseca GM, Araújo RL, Jeismann VB, Perini MV, Lupinacci RM, Cecconello I, Herman P. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg. 2016;8:5-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 17. | Mostaedi R, Milosevic Z, Han HS, Khatri VP. Laparoscopic liver resection: Current role and limitations. World J Gastrointest Oncol. 2012;4:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 19. | Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery. 2008;144:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Becker F, Morgül H, Katou S, Juratli M, Hölzen JP, Pascher A, Struecker B. Robotic Liver Surgery - Current Standards and Future Perspectives. Z Gastroenterol. 2021;59:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 22. | Viganò L, Laurenzi A, Solbiati L, Procopio F, Cherqui D, Torzilli G. Open Liver Resection, Laparoscopic Liver Resection, and Percutaneous Thermal Ablation for Patients with Solitary Small Hepatocellular Carcinoma (≤ 30 mm): Review of the Literature and Proposal for a Therapeutic Strategy. Dig Surg. 2018;35:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Wood BJ, Kruecker J, Abi-Jaoudeh N, Locklin JK, Levy E, Xu S, Solbiati L, Kapoor A, Amalou H, Venkatesan AM. Navigation systems for ablation. J Vasc Interv Radiol. 2010;21:S257-S263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Leung U, Kuk D, D'Angelica MI, Kingham TP, Allen PJ, DeMatteo RP, Jarnagin WR, Fong Y. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation vs hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9:e84484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, Yuen J, Poon RTP, Fan ST, Lo CM. Randomized clinical trial of hepatic resection vs radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 28. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 29. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3241] [Article Influence: 463.0] [Reference Citation Analysis (1)] |
| 30. | Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, Wang K, Pan H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Polanczyk CA, Marcantonio E, Goldman L, Rohde LE, Orav J, Mangione CM, Lee TH. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 360] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Chu HH, Kim JH, Kim PN, Kim SY, Lim YS, Park SH, Ko HK, Lee SG. Surgical resection vs radiofrequency ablation very early-stage HCC (≤ 2 cm Single HCC): A propensity score analysis. Liver Int. 2019;39:2397-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Kaibori M, Yoshii K, Hasegawa K, Ogawa A, Kubo S, Tateishi R, Izumi N, Kadoya M, Kudo M, Kumada T, Sakamoto M, Nakashima O, Matsuyama Y, Takayama T, Kokudo N; Liver Cancer Study Group of Japan. Treatment Optimization for Hepatocellular Carcinoma in Elderly Patients in a Japanese Nationwide Cohort. Ann Surg. 2019;270:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann Surg. 2016;263:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation vs hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 37. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, Solé M, Rodés J, Bruix J; Barcelona Clínic Liver Cancer (BCLC) Group. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 38. | Conticchio M, Inchingolo R, Delvecchio A, Laera L, Ratti F, Gelli M, Anelli F, Laurent A, Vitali G, Magistri P, Assirati G, Felli E, Wakabayashi T, Pessaux P, Piardi T, di Benedetto F, de'Angelis N, Briceño J, Rampoldi A, Adam R, Cherqui D, Aldrighetti LA, Memeo R. Radiofrequency ablation vs surgical resection in elderly patients with hepatocellular carcinoma in Milan criteria. World J Gastroenterol. 2021;27:2205-2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |