Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1293
Peer-review started: May 18, 2021
First decision: June 5, 2021
Revised: June 24, 2021
Accepted: September 29, 2021
Article in press: September 29, 2021
Published online: November 27, 2021
Processing time: 192 Days and 4.3 Hours
Being one of the most common causes of the acute abdomen, acute appendicitis (AA) forms the bread and butter of any general surgeon’s practice. With the recent advancements in AA’s management, much controversy in diagnostic algorithms, possible differential diagnoses, and weighing the management options has been generated, with no absolute consensus in the literature. Since Alvarado described his eponymous clinical scoring system in 1986 to stratify AA risk, there has been a burgeoning of additional scores for guiding downstream management and mortality assessment. Furthermore, advancing literature on the role of antibiotics, variations in appendicectomy, and its adjuncts have expanded the surgeon’s repertoire of management options. Owing to the varied presentation, diagnostic tools, and management of AA have also been proposed in special groups such as pregnant patients, the elderly, and the immunocompromised. This article seeks to raise the critical debates about what is currently known about the above aspects of AA and explore the latest controversies in the field. Considering the ever-evolving coronavirus disease 2019 situation worldwide, we also discuss the pandemic’s repercussions on patients and how surgeons’ practices have evolved in the context of AA.
Core Tip: Many controversies exist for the management of acute appendicitis (AA). Imaging modalities complement the clinical examination in AA diagnosis. Various imaging features of different imaging modalities should be considered to reduce diagnostic inaccuracies. Various diagnostic scoring systems augment clinical judgment, but uncertainty exists about the best score. Non-operative management of both uncomplicated and complicated AA is possible and reasonable, especially during the coronavirus disease 2019 pandemic. Intra-operative techniques of securing the base of the appendix stump via suture, clips, or stapling devices are all debated for superiority. Adjuncts and novel treatment ideas using endoscopic retrograde appendicitis therapy are emerging.
- Citation: Teng TZJ, Thong XR, Lau KY, Balasubramaniam S, Shelat VG. Acute appendicitis–advances and controversies. World J Gastrointest Surg 2021; 13(11): 1293-1314
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1293.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1293
Acute appendicitis (AA) is a commonly encountered surgical emergency at all levels of seniority and across different specialties. First described by Fitz[1] in 1886, it is characterized by inflammation of the vermiform appendix. Treves[2] is credited as the first to treat AA in 1902. AA occurs when there is obstruction of the appendiceal orifice (such as lymphoid hyperplasia or fecaliths), resulting in inflammation. This causes progressive distension of the appendix, eventually leading to vascular compromise, allowing the growth of pathogenic microorganisms[3]. Left untreated, this culminates in the perforation of the appendix with a localized abscess or generalized peritonitis.
The diagnosis and management of AA have not changed radically over the years despite advances in imaging and technology and an improved understanding of sepsis. To consolidate these advancements, we set out to review current literature to reassess relevant issues in the diagnosis and management of AA, including the impact of the coronavirus disease 2019 (COVID-19) pandemic on previously accepted proto
A search of relevant articles on PubMed, OVID/MEDLINE, and Web of Science was conducted on 6 May 2021 for literature published in English by Teng TZJ, Thong XR, and Lau KY. Disagreements were resolved by mutual discussions and consensus with senior authors Balasubramaniam S and Shelat VG. The following terms were used, and relevant articles were considered: [“appendicitis” (MeSH Terms)/etiology, surgery, therapy, “appendectomy” (MeSH Terms), “diagnosis” or “differential”, “Guidelines”]. Results were screened by title, and relevant articles were obtained in full text for review. We present our findings as a narrative review covering current practices and advancements in appendicitis diagnosis, followed by management, differentials, histological variations, and finally, surgical variations in AA management
The diagnosis of AA has historically been based on clinical judgment, though imaging is increasingly common where resources permit. The classic sequence of periumbilical pain radiating to the right iliac fossa (RIF), nausea or vomiting, and fever is Murphy's syndrome. Many clinical signs, such as Blumberg's sign (rebound tenderness), Rovsing's sign (RIF pain on left iliac fossa palpation), or the Psoas sign (pain upon right hip flexion suggesting retrocecal appendicitis) are described to augment diagnosis of AA. Clinical diagnosis is not absolute. Therefore, scoring systems combining clinical signs with serum markers of inflammation are widely advocated.
Scoring systems combine information from multiple sources to increase accuracy. Each score has its own merits and demerits discussed in Table 1[4-20]. The mere existence of so many systems endorses that none is perfect. The Alvarado score is widely cited and adopted in routine clinical care. However, the Alvarado score lacks specificity and is not widely validated in Asian populations. The Raja Isteri Pengiran Anak Saleha (RIPASA) scoring system was developed explicitly for the Asian population. Frountzas et al[17]'s meta-analysis comparing RIPASA and Alvarado reported higher accuracy for RIPASA score [area under the curve (AUC) 0.9431 vs 0.7944][17]. According to a retrospective study, including pregnant and non-pregnant female patients by Mantoglu et al[21], the RIPASA score was most helpful in pregnant patients (highest AUC at 0.806)[21]. In a trial involving 3878 patients, Andersson et al[5] noted the Appendicitis Inflammatory Response (AIR) score to have high sensitivity for complicated appendicitis[5]. As the AIR score more correctly identifies those with a high likelihood of appendicitis in whom supplemental imaging is unlikely to change management, AIR helps guide patient triaging for imaging. Sammalkorpi et al[6] compared the Adult Appendicitis Score (AAS) performance to the Alvarado and AIR scores in a prospective study of 829 patients. They reported that AAS had the highest AUC (0.882, 95%CI: 0.858-0.906)[6]. AAS is not widely validated. There are many other scoring systems, such as the Ohmann score, the Lintula score, the Tsanakis score, and the Fenyo-Lindburg score[22-27]. Validation studies for each scoring system are few. Ohmann's score differentiated innocent appendices from phlegmonous ones and phlegmonous from gangrenous appendices[22]. The Lintula score was developed for use in the pediatric population[28]. The Lintula score is advantageous in resource-limited settings, as no laboratory parameters are required. Tzanakis score includes the ultrasound scan (US), which can is validated in pregnant patients. Due to inter-observer variability for US scans, the scoring is not objective[29].
| Scoring system | Patient features | Clinical features | Laboratory/imaging features | Sensitivity | Specificity | Risk strata/recommended action | |
| Alvarado | - | RIF tenderness (2); Elevated temperature (1); Rebound tenderness (1); Migration of pain to RIF (1); Anorexia (1); Nausea or vomiting (1) | Leucocytosis (2); Leukocyte left shift (1) | 94.1% | 90.4% | 1-4: Discharge; 5-6: Admit and observe; 7-10: Surgery | |
| AIR | - | Elevated temperature (1); Rebound tenderness: Light (1), medium (2), strong (3); RIF pain (1); Vomiting (1) | Leucocytosis, × 109/L: 10-14.9 (1); ≥ 15 (2); Polymorphonuclear leucocytosis, %: 70-84 (1); ≥ 85 (2); CRP level, mg/L: 10-49 (1); ≥ 50 (2) | 97% | 0-4: Outpatient follow-up; 5-8: Admit and observe; 9-12: Surgery | ||
| AAS | - | RIF tenderness: Women 16-49 yr (1); all other patients (3); Migration of pain (2); RIF pain (2); Guarding: Mild (2); moderate or severe (4) | Leucocytosis, × 109/L: ≥ 7.2 and < 10.9 (1); ≥ 10.9 and < 14.0 (2); ≥ 14.0 (3). Neutrophilia, %: ≥ 62 and < 75 (2); ≥ 75 and < 83 (3); ≥ 83 (4). CRP level, mg/L and symptoms < 24 h: ≥ 4 and < 11 (2); ≥ 11 and < 25 (3); ≥ 25 and < 83 (5); ≥ 83 (1). CRP level, mg/L and symptoms > 24 h: ≥ 12 and < 53 (2); ≥ 53 and < 152 (2); ≥ 152 (1) | 1-10: Discharge without imaging; 11-15: Imaging; ≥ 16: Surgery | |||
| RIPASA | Age: < 40 (1); Age > 40 (0.5). Gender: Male (1); female (0.5). Foreign nationality registration identity card (1) | RIF tenderness (1); Elevated temperature (1); Rebound tenderness (1); Migration of pain to RIF (0.5); Anorexia (1); Nausea or vomiting (1); RIF pain (0.5); Duration of symptoms: < 48 h (1); > 48 h (0.5); Guarding (2); Rovsing sign (2) | Leucocytosis (1); Negative urine analysis (1) | 91.67% | 93.18% | - | |
| Ohmann | Age < 50 (1.5) | RIF tenderness (4.5); Rebound tenderness (2.5); Migration of pain (1); No micturition difficulties (2.0); Steady pain (2); Rigidity (1) | Leucocytosis (1.5) | 98.1% at cut-off score 9; 82.9% at cut-off score 13 | 94% at cut-off score 12 | < 6: Low risk; 6-11.5: Monitoring; ≥ 12: Surgery | |
| Lintula | Gender: Male (2); female (0) | Elevated temperature (3); Rebound tenderness (7); Migration of pain (4); Vomiting (2); RIF pain (4); Guarding (4); Pain intensity: severe (2); mild or moderate (0); Bowel sounds absent, tinkling or high-pitched (4) | - | 79.0% at cut-off score 21 | 58.3% at cut-off score 21 | ≤ 15: Discharge; 16-20: Monitoring; ≥ 21: Surgery | |
| Tzanakis | - | RIF tenderness (4); Rebound tenderness (3) | Leucocytosis (2); US imaging showing appendiceal inflammation (6) | 0-4: Discharge; 5-7: Monitoring; 8-15: Surgery | |||
| Fenyo-Lindberg | Gender: Male (8); female (-8) | Rebound tenderness: Yes (5); no (-10); migration of pain to RIF: Yes (7); no (-9); Vomiting: Yes (7); no (-5); Duration of pain: < 24 h (3); > 48 h (-12); Progression of pain: Yes (3); no (-4); Aggravation with cough: Yes (4); no (-11); Rigidity: Yes (15); no (-4); Pain outside RIF: Yes (-6); no (4) | Leucocytosis, × 109/L: < 8.9 (-15); 9-13.9 (2); > 14 (10) | In a cross-sectional study including 100 patients with RIF pain, Sahu reported a sensitivity of 72% and specificity of 71% | ≤ -17: Non-specific abdominal pain; ≥ -2: AA likely | ||
| Modified Alvarado Score | - | RIF tenderness (2); Elevated temperature (1); Rebound tenderness (1); Migration of pain to RIF (1); Anorexia (1); Nausea or vomiting (1) | Leucocytosis (2) | < 5: Surgery not required; 5-6: Monitor; 7-9: Surgery indicated | |||
| Christian | - | RIF tenderness (1); Elevated temperature (1); Vomiting (1); Abdominal pain (1) | Polymorphonuclear leucocytosis (1) | < 4: Monitoring; ≥ 4: Surgery | |||
| van den Broek et al[14] | Gender: Male (2) | Elevated temperature (1); Rebound tenderness (2); Duration of symptoms ≤ 48 h (1) | Leucocytosis (3) | 0-3: Observe; 4-6: Diagnostic laparoscopy | |||
| Simplified Appendicitis Score | - | RIF tenderness (1); Elevated temperature (1); Rebound tenderness (1); Migration of pain to RIF (1) | Leucocytosis (1) | < 4: AA excluded with 90.1% sensitivity; ≥ 6: AA included with 91.7% specificity | |||
As most scoring systems are generated from retrospective data, a scoring system derived from prospective medical records may be more accurate, especially if it is derived from multiple hospitals. The World Society of Emergency Surgery (WSES) made such an attempt. Complicated intra-abdominal infections (cIAIs) are defined as abdominal infections that extend beyond organs, causing localized or diffused peritonitis. The WSES Sepsis Severity Score predicts mortality in patients with cIAIs, including AA. In a prospective multi-center validation study including 4533 patients from 132 hospitals, Sartelli et al[25] reported that the WSES sepsis severity score cut-off of 5.5 helps differentiate survivors from non-survivors (sensitivity 89.2%, specificity 83.5%)[25]. WSES sepsis severity score needs validation in AA patients. The systemic inflammatory response syndrome (SIRS) criteria are validated in the management of AA. In a single-center prospective study including 268 patients, Beltrán et al[26] reported that a longer interval between symptom onset and surgery was significantly correlated with a higher SIRS score and an increased rate of perforated appendicitis[26]. The perforation rate for patients rose from 7% for those operated on within 24 h to as high as 85% among those operated on after 73 h. This reinforces the fact that untreated AA worsens with time and supports the utility of SIRS in determining the urgency of surgical intervention. The SIRS score can be used as an adjunct in deciding between surgical and conservative antibiotic management. In a retrospective study including 125 patients, Nozoe et al[27] reported that the SIRS score was lower in patients who were recommended non-operative management (NOM)[27]. Similarly, diverticular disease of the appendix (DDA) is associated with a higher perforation rate, and Chia et al[30] have shown that a high SIRS score is useful in clinical decision making for surgery in DDA[30]. The total white blood cell count is a non-specific biochemical marker, and novel markers may improve the performance of scoring systems. The relationship between biochemical markers [C-reactive protein (CRP), leukocyte count, procalcitonin, bilirubin] and AA has been extensively studied, either as part of clinical scores mentioned above or as standalone diagnostic predictors. The appendistat™ scoring system uses biochemical parameters to differentiate uncomplicated from complicated AA. In a validation study, Birben et al[31] reported that CRP was adequate to differentiate uncomplicated from complicated AA[31]. In a prospective study involving 544 patients, Körner et al[32] noted that perforation was more likely when CRP concentration > 50 U/L (OR 4.6, 95%CI: 2.44-8.75)[32]. Birben et al[31] also reported that a high total bilirubin level at 0.75 mg/dL could diagnose AA even if leukocyte levels were normal[31]. In a meta-analysis of seven studies and 1011 patients by Yu et al[33], CRP had the best discriminative capability in diagnosing AA[33]. Although not helpful in diagnosing AA, procalcitonin has a high positive likelihood ratio in identifying complicated AA. Thus, different scoring systems serve to aid diagnostic accuracy, triage for imaging, differentiate complicated from uncomplicated AA, determine the timing of surgical intervention, and predict morbidity outcomes. No one-size-fits-all, so prudence is required if a scoring system is used to guide bed-side decisions.
The European Association of Endoscopic Surgery (EAES) recommended a diagnostic algorithm in 2016. It risk stratifies patients into three main groups based on clinical scoring. Low-risk patients can be discharged following work-up for other possible causes. Moderate risk patients first undergo US, with computed tomography (CT) being recommended as a second-level diagnostic study only for those with incon
In 2018, the American Academy of Family Physicians published their clinical recommendations on the efficient diagnosis and management of AA[35]. Some key recommendations for AA diagnosis include the use of Alvarado, Pediatric Appendicitis Score or AIR, and US as a front-line diagnostic sieve to reduce CT use. Unlike in EAES’ guidelines, CT with IV or oral contrast or magnetic resonance imaging (MRI) is recommended for patients with negative (in addition to intermediate) US findings and high clinical suspicion to account for US’ lower sensitivity.
The 2020 WSES algorithm recommends using either the Alvarado, AIR, or AAS systems to classify low, moderate, and high-risk AA patients. This algorithm differs from EAES by using the original < 5 cut-off for low-risk AA based on the Alvarado score. WSES applies a graded imaging strategy with US as the first-line imaging choice like the above two guidelines. Low-risk patients can be discharged as appropriate or worked up for other causes of abdominal pain[36]. Moderate-risk patients are recommended to undergo an US, proceeding to CT or MRI only if the US is equivocal or negative, but the patient fails to respond to treatment. Whether CT or US should be used as second-line imaging after the US for pediatric patients is mainly dependent on local resources[36]. High-risk patients may proceed for surgery without further imaging.
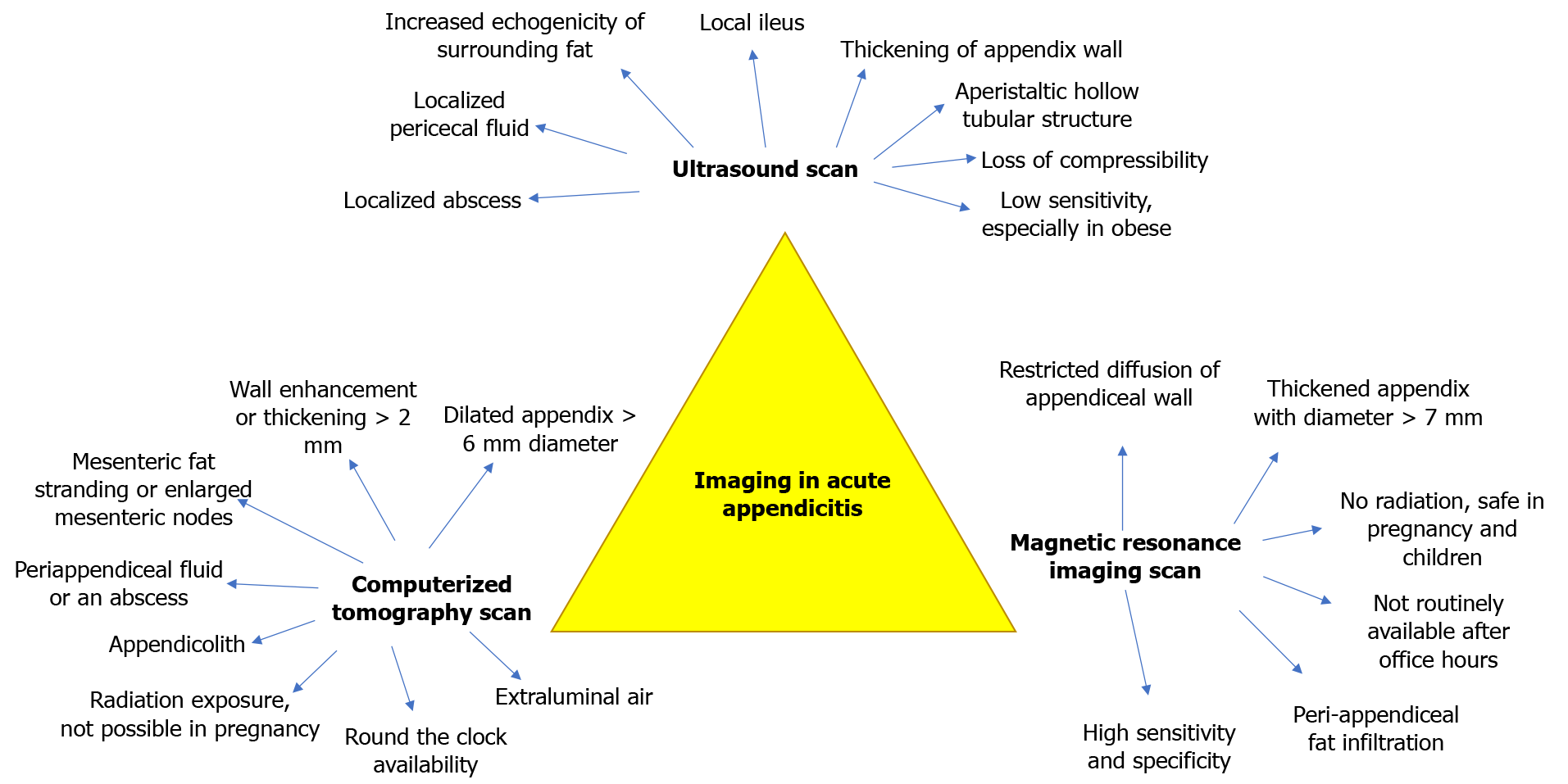
Imaging is widely accessible and has become integral to AA's management–as an adjunct to confirm the diagnosis, rule out differential diagnoses, or assist surgical planning. Free air under the diaphragm on erect chest radiograph is rare in patients with perforated AA[37]. The plain abdominal radiograph showing an appendicolith, right lower quadrant soft tissue mass or extraluminal air, and psoas margin concealment is of historical interest[38]. As such, radiographs have a minimal role in AA diagnosis. Figure 1 illustrates the key imaging features of the US scan, CT scan, and MRI scan[39-44].
Although CT scans having higher sensitivity in diagnosis[45], WSES and EAES guidelines recommend the US scan as the first line and reserve CT scan in patients with inconclusive US findings. Such a strategy increases cost-effectiveness and reduces radiation exposure. CT scan may be a more appropriate first-line investigation in overweight or elderly patients. In a prospective cohort study of 106 patients with suspected AA, Keller et al[46] reported that the US scan was five times more likely to be non-diagnostic in overweight patients[46]. Similarly, Sauvain et al[39] reported that the US scan was seven times more likely to be inconclusive in patients with a body mass index (BMI) > 25 kg/m2[39]. In a retrospective study including 105 patients, Pelin et al[40] reported that CT scan was more accurate in patients with high BMI [26.7 ± 4.3 (mean ± SD) kg/m2] and increased age [31 ± 14 (mean ± SD) years], possibly because of higher rates of complicated appendicitis[40]. This is consistent with the American College of Radiology Appropriateness Criteria's call for a lower threshold for CT imaging in elderly patients with RIF pain[41].
Intravenous contrasted CT scan enhances appendiceal wall thickening and aids AA diagnosis[38]. Per-rectal contrast does not increase diagnostic accuracy and is unnecessary[42]. Recently, there has been interest in low-dose CT scans that reduce radiation exposure without compromising diagnostic accuracy or impact on normal appendectomy rates (NARs). Randomized controlled studies and meta-analyses have shown that the low-dose protocol's diagnostic accuracy was non–inferior[43].
The role of CT scans in the evaluation of the complications of AA is well established. In particular, CT accurately detects periappendiceal abscess, peritonitis, and gangrenous changes[44]. CT scan findings of appendix mass, asymmetric wall abnormality, and diameter > 15 mm can also accurately detect concomitant appendiceal neoplasm[47]. Appendiceal mucocele, defined as a dilated mucin-filled appendix, can also be diagnosed via CT scan, with a luminal diameter > 1.3 cm having 88.2% accuracy in diagnosing a mucocele[48]. CT scan also aids in diagnosing complications such as portal vein thrombosis[49], pyogenic liver abscesss[50], and pyle
MRI is a reasonable alternative to CT in diagnosing AA and confers the advantage of avoiding ionizing radiation and intravenous contrast in the investigation of pregnant and pediatric patients. Unfortunately, the cost and logistics involved in MRI mean it is usually not used as a first-line modality except in children[52] and pregnant women[53]. A meta-analysis of 11 studies has reported that an MRI scan improves diagnostic accuracy, reduces time to appendectomy, NAR, and aids in alternative diagnosis. Other considerations for children include an incomplete MRI due to fear from claustrophobia, staying still, and noise emitted from MRI. These concerns can be addressed with child and parental counseling or sedation[54].
In addition to diagnosis, imaging also assists in the severity grading of AA. With the increasing adoption of NOM of AA, it is essential to distinguish between complicated and uncomplicated AA. In a retrospective study of 223 patients, Rybkin and Thoeni[55] reported that retroperitoneal inflammatory changes predicted complicated AA (pars plana vitrectomy 0.64-0.92 for patients above 16-years)[55]. Imaging has also been shown to play a role in scoring systems, as previously mentioned, such as the Tzanakis scoring system[10]. A positive US finding of AA such as periappendiceal fluid, localized abscess, appendicolith, wall thickness, and other findings[56] yielded 6 out of the 15 points in the score, where a score of 8 and above is suggestive of AA.
Imaging improves diagnostic accuracy at a financial cost. Patients from lower-income countries may not have accessibility and affordability to CT scans and MRI scans. Management of AA in different countries revealed that CT scans were done more liberally in accordance with the countries’ income level[36]. Within a country itself, there are discrepancies on which modality of imaging to consider first as well. This may be due to the proportion of special populations in the country (obese, children, pregnant women), the logistical constraints of the hospital (primary vs tertiary hospital) as well as the availability of radiologists’ opinion (working hours, overnight shifts, public holidays)[57]. While prudence needs to be exercised to request imaging to aid AA diagnosis, a refusal or rejection of imaging request on the pretext of “appendicitis is a clinical diagnosis, and please do appendicectomy if clinically you feel so” or “do a serial examination and it will reveal itself over next few days” etc. from radiology colleagues is unacceptable. In our experience, liberal imaging policy is associated with low NAR. In a local audit of 2603 appendectomy patients, NAR was 3.34% (n = 87)[58]. The unmet need remains the lack of uniform standardized criteria that define imaging diagnosis of AA. In particular, the imaging features of the prominent or dilated appendix can be subjective and international collaboration is needed to define thresholds for AA imaging diagnosis.
A decision tree (DT) analysis model is a tree-shaped graphical representation derived from empirical data to chart out a statistical probability outcome. In the setting of ambiguous CT scan findings, Kang et al[59] compared the diagnostic accuracy of various clinical scoring systems with DT analysis. DT analysis based on rebound tenderness severity, pain migration, urinalysis, symptom duration, leukocytosis, neutrophil levels, and CRP was more accurate (receiver operating characteristic and AUC 0.85) as compared to the Alvarado score (AUC 0.695), the Eskelinen score (AUC 0.715), and the AAS (AUC 0.749)[59]. In a study by Akmese et al[60] involving 595 clinical records, a boosted tree algorithm based on demographic data and serum biochemistry had predicted surgery necessity with 95.3% accuracy[60]. However, due to the retrospective nature, the subjective clinical judgment of the surgeon could influence the results. Evidence is emerging, and machine learning algorithms will have an increasing role in decision-making in AA management.
No report on AA is complete without mentioning the common diagnostic pitfalls and possible differential diagnoses. Imaging is integral not only to establish a diagnosis but also to rule out another diagnosis. These include right colonic diverticulitis[30,61-63], Yersinia enterocolitis[64,65], right-sided renal disease[66], mesenteric lymphadenitis[67] and Meckel’s Diverticulitis[68,69]. The meta-analysis investigating the role of MRI scan in pediatric AA reported that alternative diagnosis was present in about 20% of patients, most common being adnexal cyst and enteritis/colitis[61]. Various scoring systems, serum, and imaging biomarkers have improved diagnostic accuracy, and diagnostic dilemmas are uncommon. With the advent of minimal access surgery, the adage of “when in doubt, open and see” is replaced with “when in doubt, do a scan” or “when in doubt, look (diagnostic laparoscopy) and see.”
It is essential to distinguish between complicated and uncomplicated AA as it impacts management. Complicated AA typically includes perforation with peritonitis, phlegmon, or abscess formation, making up 2%-10% of all AA cases[70]. A phlegmon is described as an inflammatory mass including the inflamed adjacent viscera and greater omentum, while an abscess is described as a pus-containing appendiceal mass[71]. Appendicitis in the absence of these is defined as uncomplicated. Appendectomy (open or laparoscopic) is the standard of care for AA. However, recent evidence suggests that antibiotics alone may be adequate in selected patients–NOM. The classic description of NOM principles by Ochsner-Sherren relates to complicated AA-a patient with RIF mass. Currently, NOM is described both in uncomplicated and complicated AA[72].
In a meta-analysis including five studies and 1116 patients, Sallinen et al[73] reported lower rates of complications with NOM. However, the authors reported an increased incidence of recurrence of AA at one year and longer hospital stay[73]. Surgical intervention has higher treatment efficacy and a shorter length of stay than antibiotic treatment[74]. However, heterogeneity in antibiotic choice, dose and duration, inclusion and exclusion criteria, and other confounding variables could impact the results. In a retrospective cohort study of 81 uncomplicated AA patients, Loftus et al[75] reported NOM was more successful if patients had a longer duration of symptoms before admission, a lower temperature within 6 h of admission, lower modified Alvarado score, and a smaller appendiceal diameter[75]. Studies with long-term follow-up data are reported. In a 7-year prospective observational study involving 423 patients, Sippola et al[76] reported a 39.3% recurrence rate when uncomplicated AA patients were managed by NOM[76]. Patient satisfaction between the appendectomy and NOM group was similar (95%CI: 0.86-1.0; P = 0.96). Podda et al[77] reported that patients managed by NOM had a higher visual analog scale at 30-d follow-up (0.3 ± 0.6 vs 2.1 ± 1.7)[77]. O'Leary et al[78] reported that patients managed by surgery had a better quality of life (94.3 vs 91.0, P < 0.001)[78]. Thus, the decision for NOM vs surgery has multiple domains to consider, and each patient should be assessed and counseled on his own merits. Ideally, a patient-centric healthcare decision ought to be made, but a survey by Reinisch et al[79] involving 1300 surgeons revealed that decisions are made by surgeon preferences. Authors reported that only 14% of surgeons treat uncomplicated AA by NOM, 38.1% in selected cases, and 48.8% rejected NOM[79]. Thus, the inherent bias of the surgical community against NOM should be considered while critically appraising the evidence. More prospective multi-center collaborative studies, with long-term follow-up comparing NOM with appendectomy, including total cost of care, quality of life domains as outcome measures, are necessary before meaningful conclusions and valid recommendations can be made. In our opinion, NOM imposes a long-term recurrence risk and adds the burden of missing incidental tumours. In a systematic review of 455 patients, Peltrini et al[80] reported a 11% incidence of appendiceal neoplasms after interval appendectomies for complicated appendicitis[80]. It is possible that with such information, young patients may not participate in a randomized study due to fear of being allocated to the NOM group. Lastly, many authors have reported using carbapenems for NOM, which could contribute to antimicrobial resistance. Percutaneous drainage is integral to the NOM concept. Percutaneous drainage in perforated AA lowers the risk of hemorrhage, fistula formation, wound infection, prolonged ileus, and adhesions compared to immediate appendectomy[81].
Antibiotics are the bare minimum in AA management, regardless of NOM or appendectomy. A 2005 Cochrane review included 45 studies with 9576 patients and reported that antibiotics were superior to placebo in preventing wound infection and intra-abdominal abscess[72]. Beyond 24-h postoperative antibiotics are generally prescribed in patients with complicated AA[82]. Three days of antibiotics are as effective as a five-day course in reducing infectious complications[83,84]. The com
Choice and selection of antibiotics are equally crucial as duration. Local antibiotic stewardship initiatives and individual surgeons must ensure that antibiotics are rationally used to reduce the emergence of multi-drug resistance organisms. Our unit uses amoxicillin-clavulanate with a stat dose of gentamicin or ceftriaxone and metronidazole in AA patients. Studies reporting NOM tend to use more broad-spectrum antibiotics to increase treatment success. A meta-analysis by Wang et al[88] involving nine randomized controlled trials with 4551 patients reported that carbapenems were associated with fewer treatment-related complications than an appendectomy in uncomplicated AA[88]. Additionally, carbapenems were noted to be the only antibiotic with one-year treatment success rates greater than appendectomy. However, we caution to generalize these results, as each institution should remain guided to select antibiotics based on local antibiogram.
Timing of antibiotic administration is essential in managing patients with sepsis, as delay can increase mortality. An early administration of antibiotics is recommended[74]. In a systemic review involving 34 studies and 2944 uncomplicated AA patients, Talan et al[86] reported that most patients showed treatment response within 1-2 d[86]. On the other hand, complicated AA patients had a mean response time of approximately three days. This suggests that prolonged course antibiotics may be necessary for complicated AA patients[12]. An electronic clinical decision support tool allows for the rational use of antibiotics[89].
Appendectomy or NOM both remain valid options in selected patients with both uncomplicated and complicated AA. There is enough data that NOM is safe, feasible, cost-effective, and restores quality of life. In a retrospective study including 231,678 patients, McCutcheon et al[90] reported no differences in mortality and cost between appendectomy and NOM[90]. We remain cautious about recurrent AA risk, missing tumors, and antimicrobial resistance. In patients selected for appendectomy, timing (interval vs index appendectomy) and approach (laparoscopic vs open appendectomy) need discussion. In addition, with the laparoscopic approach, single incision vs conventional three-port incision and stump closure methods need discussion.
The timing of an appendectomy depends on the patient's clinical stability, available resources, and patient preference. Emergency appendectomy is warranted in patients who manifest signs of sepsis with hemodynamic instability[91]. If the patient is deemed to have high risk due to medical co-morbidity or organ failure, then percutaneous drainage of an abscess may be considered. If the patient with perforated AA is clinically stable, an appendectomy can be performed at the next available opportunity. Various studies have demonstrated both superior and inferior outcomes with early appendectomy when compared to NOM. Young et al[92] reported that early appendectomy resulted in reduced bowel resection incidence[92]. Others have reported higher morbidity, including the need for hemicolectomy in patients with complicated AA[93]. This is consistent with Gavriilidis et al[83]'s recent meta-analysis, where the overall complications, abdominal/pelvic abscess, wound infections and unplanned procedure performance were significantly lower in conservative treatment cohorts[83]. In our experience, surgeon experience and skill are essential to avoid a limited right hemicolectomy. In patients treated conservatively, Snyder et al[35] reported a 12% risk of recurrence[35]. Thus, a patient must be counseled adequately for possible increased morbidity from imminent surgery or interval appendectomy after a trial of conservative management.
Interval appendectomy can be done routinely following conservative management or selectively in patients with recurrent AA after NOM. We distinguish NOM from conservative management with relation to intent. NOM intends to avoid surgery, while conservative management intends to delay surgery later, accounting for safety. NOM can be repeated in patients with recurrent AA. In a systematic review by Darwazeh et al[84] involving 1943 patients and 21 studies, there was no morbidity difference between patients managed via interval appendectomy or repeat NOM (10.4% vs 13.3%)[84]. The study by Hall et al[94] involving 106 children who had a recurrence of AA recommended a conservative "wait-and-see" approach over interval appendectomy given the low incidence of complications[94]. A routine interval appendectomy may be beneficial in patients of advanced age to check for a possible malignancy. However, this could be circumvented by offering follow-up imaging and colonoscopy[95]. Due to the short follow-up duration of studies that recommend NOM, the authors practice recommending a routine interval appendectomy to all patients, especially in the presence of a fecolith at the appendix base.
Laparoscopic appendectomy is as safe as open appendectomy. Smaller wounds translate to less pain, a faster return to normal activities, and a shorter length of stay[23,96,97]. A surgical scar is a determinant of adhesive small bowel obstruction[98]. It is debatable if minimal access approach results in lower rates of postoperative adhesions and small bowel obstruction in patients with AA. In a retrospective analysis of 619 children managed with appendectomy, Håkanson et al[99] concluded that the risk for small bowel obstruction after appendectomy was significantly related to perforation or postoperative intra-abdominal abscess and not to the surgical approach[99]. Buia et al[96] revealed in a systematic review of 185 articles that laparoscopic appendectomy provides lower short-term bowel obstruction rates in pediatric and perforated AA populations while having lower long-term bowel obstruction rates in all patients[96]. There is a paucity of data regarding postoperative incisional hernia incidence. In a systematic review of 37 studies on appendectomy with sample size > 500 patients each and follow-up > 30 d, Rasmussen et al[100] reported a pooled estimate of 0.7% for incisional hernia at follow-up of 6.5 (range 1.9-10) years[100]. In our opinion, minimal access surgery probably reduces the rates of postoperative adhesions and incisional hernia.
Surgical site infection and intra-abdominal infection are crucial key performance indicators of appendectomy. Surgical site infection results in prolonged hospital stay, extended recovery time, increased total cost of care, and drain on healthcare resources[101]. In an umbrella review including ten meta-analyses, Poprom et al[102] concluded that surgical site infection rate was 48% to 70% lower in laparoscopic appendectomy than an open appendectomy, and intra-abdominal abscess rate was 1.34 to 2.20 higher in laparoscopic appendectomy than open appendectomy[102]. A higher rate of intra-abdominal abscess could be mitigated by judicious peritoneal lavage and a standard policy to aspirate peritoneal cavity dry before closure.
Laparoscopic appendectomy is associated with reduced 30-d readmission. In a meta-analysis including 45 studies and 836921 appendectomies, Bailey et al[103] has reported a 4.3% (range 0.0-14.4%) 30-d readmission rate. Diabetes mellitus, complicated appendicitis, and open appendectomy predicted 30-d readmission[103], and thus laparoscopic appendectomy may be superior if available and accessible. Laparoscopic appendectomy is also notably more cost-effective compared to not only open surgery but NOM as well. In an umbrella study by Sugiura et al[104], it is noted that three meta-analyses revealed NOM costs $235 more than operative management, making it less cost-effective than laparoscopic management[104].
Laparoscopic appendectomy can be performed by a single port or conventional three-port technique. A study involving 101 patients by Kim et al[105] reported that Single-incision laparoscopic appendectomy (SILA) reduced the length of hospitalization (1.2 ± 0.8 d vs 1.6 ± 0.8 d, P = 0.037) vs three-port appendectomy[105]. Systematic reviews and meta-analyses report that SILA is associated with a shorter length of hospital stay but longer operation duration and increased risk of open conversion[106,107]. SILA requires special training and may be associated with an increased risk of incisional hernia.
Laparoscopic appendectomy is safe and reduces postoperative morbidity in patients with morbid obesity[96]. In a systematic review and meta-analysis including 12 studies with 126237 elderly patients in the laparoscopy group and 213201 elderly patients in the open group, Wang et al[108] reported that laparoscopic appendectomy was associated with lower postoperative mortality, wound infection, and shorter length of hospital stay[108]. Thus, laparoscopic appendectomy is safe in obese and elderly patients. While there is the benefit of percutaneous drainage to manage a postoperative intra-abdominal abscess > 4 cm in size[109,110], routine abdominal drainage following an appendectomy for complicated appendicitis has no clinical benefit[111].
Appendiceal stump closure techniques, e.g., surgical stapler or conventional sutures such as Endoloop, are debated. In a study of 333 patients, Rakić et al[112] reported that Endoloop was preferred over the stapler given the cost benefits and lack of difference in perioperative morbidity[112]. In a retrospective study of 708 patients, Escolino et al[113] reported that the use of Endoloop was associated with a higher incidence of an intra-abdominal abscess, postoperative ileus, and re-operations/readmissions compared to the use of a stapler[113]. Sohn et al[114] made a simplified reco
Two categories of incidental findings need discussion. Firstly, situations where intra-operative AA is established, but a separate incidental pathology is detected[118]. In such instances, it is our opinion that a surgeon should proceed with an appendectomy and document the operative findings. The incidental pathology can be investigated and managed later. Secondly, situations where the appendix appears normal to visualization. Laparoscopy has an advantage in such situations; a surgeon can thoroughly explore the peritoneal cavity. If a definitive pathology is detected and the patient appropriately consented, the surgeon can proceed accordingly. It is debatable and controversial if a normal appendix must be removed, especially if another pathology is established. Our practice is to remove a “normal-appearing” appendix in the absence of other established diagnoses[119]. We do this for two reasons. Firstly, a “normal-appearing” appendix may be an early AA. Secondly, removal of the appendix eliminates future diagnostic dilemmas for RIF symptoms.
Other techniques such as endoscopic retrograde appendicitis therapy have also been proposed to treat uncomplicated AA[120]. In another retrospective study by Ding et al[121] involving 210 patients, there was a 100% success rate with a recurrence rate of 2.86% during the first 6 mo of postoperative follow-up[121]. Given the relatively low-powered studies currently, more evidence is necessary.
There are reports of AA associated with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Many authors have also made suggestions for NOM and avoid surgery in selected AA patients. In patients managed by surgery, the use of personal protective equipment, strategies to reduce surgical aerosols, and the role of peritoneal fluid in viral isolation is proposed.
In a case series by Prichard et al[122], including 6047 patients, it was noted that AA was more likely in patients with COVID-19 positive results compared to those without (10.8% vs 1.3%, P < 0.001)[122]. Meyer et al[123] reported a higher prevalence of SARS-CoV-2 infection in children. Ahmad et al[124] reported a case where SARS-CoV-2 isolates were found in tissue samples of mesenteric lymph nodes despite having a SARS-CoV-2 negative swab[124]. The converse was also reported, where a patient reported by Ngaserin et al[125] was COVID-19 positive but did not detect SARS-CoV-2 in the peritoneal fluid[125]. However, a small sample size limits the generalizability of such observations. We have not observed a similar trend in Singapore (unpublished data). More evidence is required, including histology analysis, before any meaningful conclusions can be drawn.
To reduce risk exposure to healthcare personnel and intra-operative airborne or droplet transmission of the virus, various societies have made recommendations including but not limited to: (1) Strict donning of personal protective equipment; (2) Goggles; (3) N-95 mask; (4) Gradual decompression of pneumoperitoneum; and (5) Reducing the operating personnel to bare necessary, etc. and so on. One of the primary considerations is the possibility of avoiding surgery to reduce COVID-19 risk, i.e., NOM[126]. This is supported by the high risk of perioperative COVID-19-associated mortality[127]. In a large, randomized trial comparing outcomes of drugs and appendectomy involving 1552 patients, 29% of patients in the NOM group required surgical intervention[128]. Mai et al[127] did not detect perioperative COVID-19 infections and advocate that surgical treatment should be first-line unless COVID-19 infections have been proven or suspected[127]. However, the management should not only be dictated by the COVID-19 pandemic. Collard et al[126] propose using the Saint-Antoine scale, including BMI < 28 kg/m2, leucocyte count < 15000/uL, CRP < 3 mg/dL, and no radiological signs of perforation and diameter of appendix ≤ 10 mm each for 1 point. A score of ≥ 4 is more likely to respond to antibiotic treatment only[126]. More evidence is required if such criteria could guide NOM during a pandemic.
The effect of COVID-19 on the severity of AA has to be considered in two ways–the fear of the virus delaying COVID-19 negative patients from seeking treatment and the effect of the virus itself in worsening AA.
The combination of government restrictions to leaving the house and fear of exposure in high-risk environments such as hospitals may cause a delay in seeking treatment. In a prospective study by Mowbray et al[129], only 64 patients presented with AA in April 2019 (before lockdown) compared to those previously (190 patients in April 2020 during lockdown)[129]. Patients were also noted to have increased their threshold for seeking treatment, presenting to the hospital one day later (2 d vs 3 d, P = 0.03). Consequently, some authors noted that the delay in seeking AA treatment might have resulted in more complex AA presentations. Mowbray et al[129] noted a higher American Society of Anesthesiology (ASA) score (P = 0.049)[129]. Finkelstein et al[130]'s retrospective analysis of 107 patients revealed a similar increase in AA perforations (33% vs 17% P = 0.04) than pre-COVID-19[130]. Interestingly, Finkelstein et al[130] did not notice a delay in presentation to the hospital (2 ± 3 d in both 2019 and 2020, P = 0.50) but noted that complicated AA seemed to present with a longer duration of symptoms (2 d vs 1 d, P = 0.03). The idea that more complicated AA presented in the COVID-19 era is also supported by Yang et al[131] in a study of 235 patients, where there was a significantly longer interval from onset of symptoms to seeking treatment (37.92 h vs 24.57 h comparing registration time of onset of symptoms to registration, P = 0.028) and higher incidence of complex AA (35.8% vs 19.4%, P = 0.005)[131]. However, the converse has also been reported where no differences in complications or severity in AA presentation were seen in other regions. In a retrospective study by Griffith et al[132] comparing 2020 and 2019 AA admissions, there was an increased admission rate (40.8% vs 34.1%, P = 0.036)[132]. Kohler et al[133] revealed in a population-based study in Germany that there was no difference in the number of perforated AA diagnosed during the pandemic or pre-pandemic[133]. Additionally, Bajomo et al[134] noted, in a study involving 78 patients, higher inflammatory markers (CRP 103 mg/L vs 53 mg/L, P = 0.03) and more severe disease on the histological examination pre-pandemic[134].
Liberal use of imaging may improve diagnostic accuracy and reduce NAR. In a study by Somers et al[135] comparing AA management in 2020 and 2019, there was increased use of imaging (89.3% vs 69.3%, P = 0.007) and an accompanying decrease in NAR (0% vs 24.6%)[135]. Other additional measures enforced for surgeons' safety during the pandemic can also be considered in future circumstances where aerosol-driven pathogens are suspected. Examples include the presence of a negative-pressure operating room, enhanced personal protective equipment, and avoiding the use of electrocautery and other aerosol-generating instruments[136].
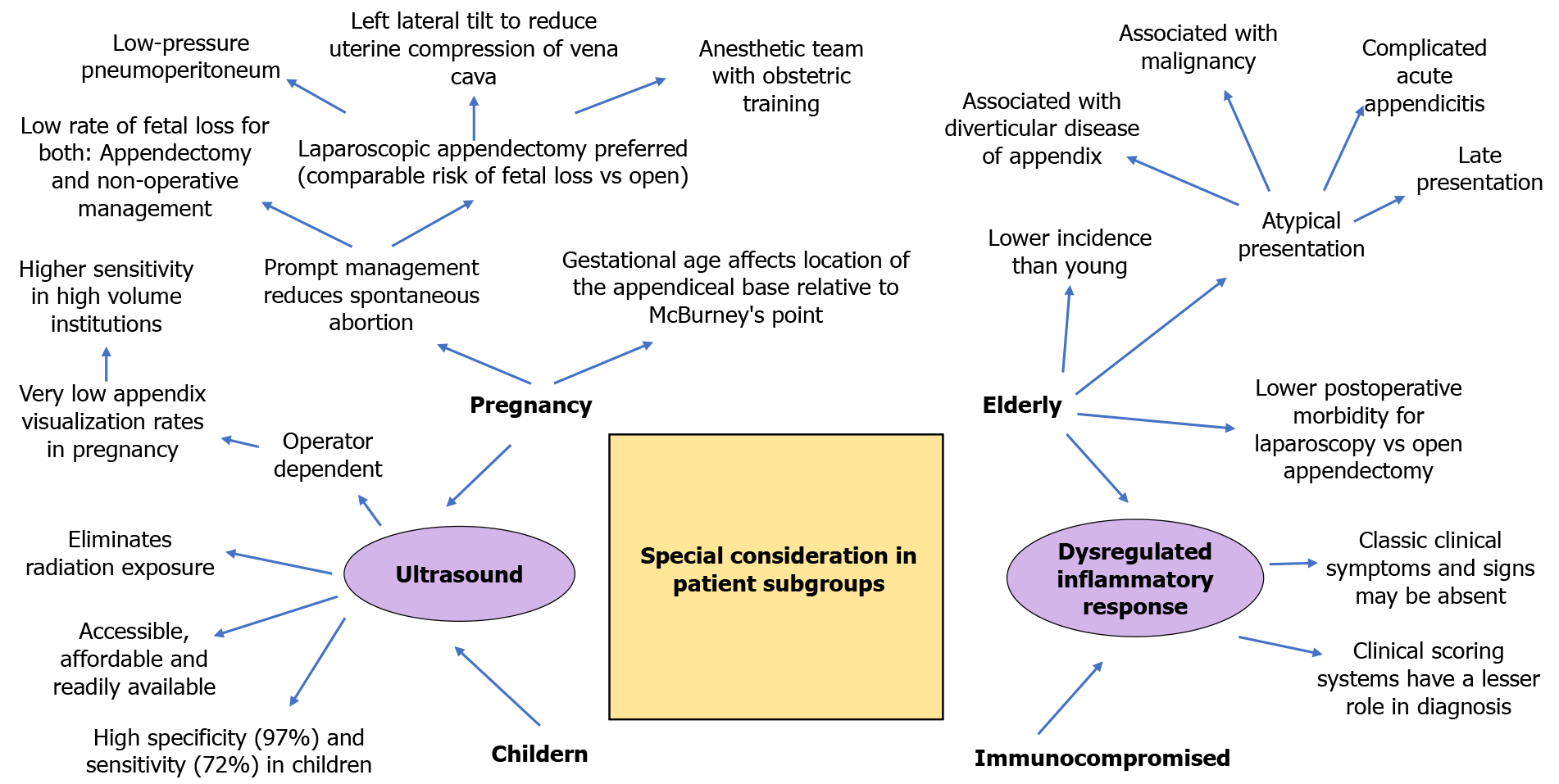
Children, pregnant women, the elderly, and immunocompromised status pose unique diagnostic and management challenges for AA. We briefly discuss pertinent issues in Figure 2. The US scan is simple, cheap, readily available, and an accurate diagnostic modality. It also avoids radiation exposure. Mittal et al[137] conducted a 10-center prospective observational study on 2625 pediatric patients with suspected AA and reported that the US scan had an overall sensitivity of 72% and specificity of 97% in diagnosing AA[137]. The US is operator-dependent, and US sensitivity is higher at sites using it more frequently. US scan is also recommended in pregnant patients as it eliminates fetus radiation exposure[56]. However, Wi et al[138] reported very low appendix visualization rates and proposed using MRI scan as first-line imaging in pregnant patients with abdominal pain suspicious for AA[138]. We suggest that hospitals conduct regular audits and implement quality improvement practices to track US performance. Prompt management can reduce spontaneous abortion. In a study by Nakashima et al[139] involving 169 pregnant women, the incidence of fetal loss was low in NOM compared to appendectomy (4% vs 5%)[139]. Surgeons must be aware that gestational age leads to a significant change in the location of the appendiceal base relative to McBurney's point[140]. We routinely offer laparoscopic appendectomy in pregnant patients. Low-pressure pneumoperitoneum, left lateral tilt to reduce uterine compression of vena cava, and an anesthetic team with obstetric training are essential to good outcomes. In a systematic review and meta-analysis of 22 comparative cohort studies, including 4694 pregnant women (905 Laparoscopic appendectomies and 3789 open appendectomies), Lee et al[141] reported that fetal loss was significantly higher for laparoscopic appendectomy patients (pooled OR was 1.72, 95%CI: 1.22-2.42). However, the results were skewed due to one study. On excluding the outlying study, there was no significant difference between laparoscopic and open appendectomy concerning the risk of fetal loss (OR 1.163, 95%CI: 0.68-1.99; P = 0.581)[141]. As such, while caution should be taken, patients should not be unduly refused appendicectomy while pregnant. We recommend that appendectomy be done in a facility with resources available to deal with obstetric urgencies.
No age is immune to AA. AA in the elderly is uncommon and atypical. Late presentation, association with malignancy, association with DDA, and complicated AA are common. In a meta-analysis involving 12 studies and 126,237 patients, Wang et al[108] report that postoperative mortality was lower in elderly patients treated with laparoscopy vs open appendectomy (OR, 0.33; 95%CI: 0.28-0.39)[108]. Elderly and immunocompromised patients have limited inflammatory responses. In such patients, clinical scoring systems have a lesser role in diagnosis. Anshul et al[142] reported a patient with a silent abdomen but AA diagnosed on CT scan[142]. Perioperative care must be customized with a low threshold to suspect complications.
Routine histopathological examination after appendectomy is the prevalent standard practice globally. In a meta-analysis of twenty-five studies and 57357 patients, Bastiaenen et al[143] reported 2.5% unexpected findings. They also observed that surgeons could rarely (3%) detect unexpected findings during surgery. Though granulomatous diseases such as Crohn's could be macroscopically detected almost half of the time (47.1%), endometriosis and parasitic infections could only be diagnosed following histopathology.
Neoplasms account for 1% of appendectomy histology specimens[144]. Patients above 50 years of age, with family history of colon cancer or inflammatory bowel disease, or with unexplained anemia are at risk of appendiceal neoplasm[47]. The most common appendiceal neoplasm is neuroendocrine tumours[145]. Appendiceal carcinoid tumors are seen in 1% of appendectomy specimens and same managed with the same caution as adenocarcinomas[139,140,146]. The presence of adenocarcinoma in the appendectomy specimen requires a right hemicolectomy. In a meta-analysis of six studies including 261 patients who had an appendiceal carcinoid tumor, Ricci et al[147] found a significant recurrence rate in tumors larger than 2 cm in size compared to those smaller than 2 cm, with a higher risk of lymph node metastases in the former group[147]. A right hemicolectomy is warranted for carcinoid tumors > 2 cm size, located at the base of the appendix, or involved lymph nodes. Locally, the multidisciplinary oncology board makes management recommendations in such instances. In perforated AA, the goblet cell subtype of appendiceal carcinoid is associated with a greater risk of peritoneal metastasis than the classical subtype. In a systematic review involving 121 cases of appendiceal carcinoid tumors with perforation, Madani et al[148] noted that perforation accelerates the metastatic process[148]. A surgeon should avoid a spill of luminal contents. Metastasis to the appendix is a rare occurrence. The gastrointestinal tract is the most likely site of breast tumor metastases. Ng et al[149] reported 15 patients with breast cancer and appendix metastasis[149]. Each patient's treatment should be determined by multidisciplinary oncology teams considering disease stage, the extent of metastases, patient performance status, physician expertise, and patient choices. Appendiceal endometriosis (AE) may be associated with low-grade appendiceal mucinous neoplasms and small bowel obstruction secondary to an endometrial ileal stricture[150]. Prophylactic appendectomy in patients with AE may reduce intestinal obstruction risk, and further data is needed.
Multiple aspects of approach to management of AA remain well debated in the literature. The role of clinical scoring systems and imaging in the early and accurate diagnosis of AA can reduce NARs. NOM and appendectomy both remain valid options with their own merits and demerits. Laparoscopic appendectomy is widely accepted as safe with the benefits of early recovery and reduced wound infection compared to open appendectomy. Fear-related behavior is proven during the COVID-19 pandemic, as evidenced by a delay in presentation. Histologic evaluation of appendix specimens has value in detecting incidental malignancies. As the management of AA evolves with technological strides and a more refined under
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: MacCormick AP, Sun C S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Fitz RH. Perforating inflammation of the vermiform appendix: with special reference to its early diagnosis and treatment. Am J Med Sci. 188;321-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Treves F. The CAVENDISH LECTURE on SOME PHASES of INFLAMMATION of the APPENDIX: Delivered before the West London Medico-Chirurgical Society on June 20th, 1902. Br Med J. 1902;1:1589-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Jeon HG, Ju HU, Kim GY, Jeong J, Kim MH, Jun JB. Bacteriology and changes in antibiotic susceptibility in adults with community-acquired perforated appendicitis. PLoS One. 2014;9:e111144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? Ann Emerg Med. 2014;64:365-372.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) Score. World J Surg. 2021;45:2081-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Sammalkorpi HE, Mentula P, Leppäniemi A. A new adult appendicitis score improves diagnostic accuracy of acute appendicitis--a prospective study. BMC Gastroenterol. 2014;14:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Chong CF, Adi MI, Thien A, Suyoi A, Mackie AJ, Tin AS, Tripathi S, Jaman NH, Tan KK, Kok KY, Mathew VV, Paw O, Chua HB, Yapp SK. Development of the RIPASA score: a new appendicitis scoring system for the diagnosis of acute appendicitis. Singapore Med J. 2010;51:220-225. [PubMed] |
| 8. | Ohmann C, Franke C, Yang Q. Clinical benefit of a diagnostic score for appendicitis: results of a prospective interventional study. German Study Group of Acute Abdominal Pain. Arch Surg. 1999;134:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lintula H, Pesonen E, Kokki H, Vanamo K, Eskelinen M. A diagnostic score for children with suspected appendicitis. Langenbecks Arch Surg. 2005;390:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Tzanakis NE, Efstathiou SP, Danulidis K, Rallis GE, Tsioulos DI, Chatzivasiliou A, Peros G, Nikiteas NI. A new approach to accurate diagnosis of acute appendicitis. World J Surg. 2005;29:1151-1156, discussion 1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Fenyö G, Lindberg G, Blind P, Enochsson L, Oberg A. Diagnostic decision support in suspected acute appendicitis: validation of a simplified scoring system. Eur J Surg. 1997;163:831-838. [PubMed] |
| 12. | Luo CC, Cheng KF, Huang CS, Lo HC, Wu SM, Huang HC, Chien WK, Chen RJ. Therapeutic effectiveness of percutaneous drainage and factors for performing an interval appendectomy in pediatric appendiceal abscess. BMC Surg. 2016;16:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Christian F, Christian GP. A simple scoring system to reduce the negative appendicectomy rate. Ann R Coll Surg Engl. 1992;74:281-285. [PubMed] |
| 14. | van den Broek WT, Bijnen BB, Rijbroek B, Gouma DJ. Scoring and diagnostic laparoscopy for suspected appendicitis. Eur J Surg. 2002;168:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Goh PL. A Simplified Appendicitis Score in the Diagnosis of Acute Appendicitis. Hong Kong J Emerg Med. 2010;17:230-235. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Ohle R, O'Reilly F, O'Brien KK, Fahey T, Dimitrov BD. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Frountzas M, Stergios K, Kopsini D, Schizas D, Kontzoglou K, Toutouzas K. Alvarado or RIPASA score for diagnosis of acute appendicitis? Int J Surg. 2018;56:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Karami MY, Niakan H, Zadebagheri N, Mardani P, Shayan Z, Deilami I. Which One is Better? Ann Coloproctol. 2017;33:227-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Karpagavel C, Velayudhan N. Comparison of predictive validity of Alvarado score and Lintula score in acute appendicitis in adults. Inter J Surg, Trauma Orthop. 2017;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Omar AS, Kadhim SJ. Accuracy of lintula score vs alvarado score in diagnosis of acute appendicitis in Al-yarmouk teaching hospital. Int J Surg. 2020;4:18-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Mantoglu B, Gonullu E, Akdeniz Y, Yigit M, Firat N, Akin E, Altintoprak F, Erkorkmaz U. Which appendicitis scoring system is most suitable for pregnant patients? World J Emerg Surg. 2020;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Rastović P, Trninić Z, Galić G, Brekalo Z, Lesko J, Pavlović M. Accuracy of Modified Alvarado Score, Eskelinen Score and Ohmann Score in Diagnosing Acute Appendicitis. Psychiatr Danub. 2017;29:134-141. [PubMed] |
| 23. | Güler Y, Karabulut Z, Çaliş H, Şengül S. Comparison of laparoscopic and open appendectomy on wound infection and healing in complicated appendicitis. Int Wound J. 2020;17:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Sharma P, Jain A, Shankar G, Jinkala S, Kumbhar US, Shamanna SG. Diagnostic accuracy of Alvarado, RIPASA and Tzanakis scoring system in acute appendicitis: A prospective observational study. Trop Doct. 2021;494755211030165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Sartelli M, Abu-Zidan FM, Catena F, Griffiths EA, Di Saverio S, Coimbra R, Ordoñez CA, Leppaniemi A, Fraga GP, Coccolini F, Agresta F, Abbas A, Abdel Kader S, Agboola J, Amhed A, Ajibade A, Akkucuk S, Alharthi B, Anyfantakis D, Augustin G, Baiocchi G, Bala M, Baraket O, Bayrak S, Bellanova G, Beltràn MA, Bini R, Boal M, Borodach AV, Bouliaris K, Branger F, Brunelli D, Catani M, Che Jusoh A, Chichom-Mefire A, Cocorullo G, Colak E, Costa D, Costa S, Cui Y, Curca GL, Curry T, Das K, Delibegovic S, Demetrashvili Z, Di Carlo I, Drozdova N, El Zalabany T, Enani MA, Faro M, Gachabayov M, Giménez Maurel T, Gkiokas G, Gomes CA, Gonsaga RA, Guercioni G, Guner A, Gupta S, Gutierrez S, Hutan M, Ioannidis O, Isik A, Izawa Y, Jain SA, Jokubauskas M, Karamarkovic A, Kauhanen S, Kaushik R, Kenig J, Khokha V, Kim JI, Kong V, Koshy R, Krasniqi A, Kshirsagar A, Kuliesius Z, Lasithiotakis K, Leão P, Lee JG, Leon M, Lizarazu Pérez A, Lohsiriwat V, López-Tomassetti Fernandez E, Lostoridis E, Mn R, Major P, Marinis A, Marrelli D, Martinez-Perez A, Marwah S, McFarlane M, Melo RB, Mesina C, Michalopoulos N, Moldovanu R, Mouaqit O, Munyika A, Negoi I, Nikolopoulos I, Nita GE, Olaoye I, Omari A, Ossa PR, Ozkan Z, Padmakumar R, Pata F, Pereira Junior GA, Pereira J, Pintar T, Pouggouras K, Prabhu V, Rausei S, Rems M, Rios-Cruz D, Sakakushev B, Sánchez de Molina ML, Seretis C, Shelat V, Simões RL, Sinibaldi G, Skrovina M, Smirnov D, Spyropoulos C, Tepp J, Tezcaner T, Tolonen M, Torba M, Ulrych J, Uzunoglu MY, van Dellen D, van Ramshorst GH, Vasquez G, Venara A, Vereczkei A, Vettoretto N, Vlad N, Yadav SK, Yilmaz TU, Yuan KC, Zachariah SK, Zida M, Zilinskas J, Ansaloni L. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study). World J Emerg Surg. 2015;10:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Beltrán MA. The Systemic Inflammatory Response in Patients with Appendicitis: a Progressive Phenomenon. Indian J Surg. 2015;77:1050-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Nozoe T, Matsumata T, Sugimachi K. Significance of SIRS score in therapeutic strategy for acute appendicitis. Hepatogastroenterology. 2002;49:444-446. [PubMed] |
| 28. | Ojuka D, Sangoro M. Alvarado vs Lintula Scoring Systems in Acute Appendicitis. Ann Afric Surg. 2018;14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Lakshminarasimhaiah AKS, Nagaraja A, Srinivasaiah M. Evaluation of Tzanakis scoring system in acute appendicitis: a prospective study. Int Surg J. 2017;4:3338-3343. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Chia ML, Chan SWY, Shelat VG. Diverticular Disease of the Appendix Is Associated with Complicated Appendicitis. GE Port J Gastroenterol. 2021;28:236-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 31. | Birben B, Sönmez BM, Er S, Özden S, Kösa MT, Tez M. External validation of the AppendistatTM score and comparison with CRP levels for the prediction of complicated appendicitis. Ulus Travma Acil Cerrahi Derg. 2021;27:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Körner H, Söndenaa K, Söreide JA. Perforated and non-perforated acute appendicitis--one disease or two entities? Eur J Surg. 2001;167:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Yu CW, Juan LI, Wu MH, Shen CJ, Wu JY, Lee CC. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg. 2013;100:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | Gorter RR, Eker HH, Gorter-Stam MA, Abis GS, Acharya A, Ankersmit M, Antoniou SA, Arolfo S, Babic B, Boni L, Bruntink M, van Dam DA, Defoort B, Deijen CL, DeLacy FB, Go PM, Harmsen AM, van den Helder RS, Iordache F, Ket JC, Muysoms FE, Ozmen MM, Papoulas M, Rhodes M, Straatman J, Tenhagen M, Turrado V, Vereczkei A, Vilallonga R, Deelder JD, Bonjer J. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 262] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 35. | Snyder MJ, Guthrie M, Cagle S. Acute Appendicitis: Efficient Diagnosis and Management. Am Fam Physician. 2018;98:25-33. [PubMed] |
| 36. | Di Saverio S, Podda M, De Simone B, Ceresoli M, Augustin G, Gori A, Boermeester M, Sartelli M, Coccolini F, Tarasconi A, De' Angelis N, Weber DG, Tolonen M, Birindelli A, Biffl W, Moore EE, Kelly M, Soreide K, Kashuk J, Ten Broek R, Gomes CA, Sugrue M, Davies RJ, Damaskos D, Leppäniemi A, Kirkpatrick A, Peitzman AB, Fraga GP, Maier RV, Coimbra R, Chiarugi M, Sganga G, Pisanu A, De' Angelis GL, Tan E, Van Goor H, Pata F, Di Carlo I, Chiara O, Litvin A, Campanile FC, Sakakushev B, Tomadze G, Demetrashvili Z, Latifi R, Abu-Zidan F, Romeo O, Segovia-Lohse H, Baiocchi G, Costa D, Rizoli S, Balogh ZJ, Bendinelli C, Scalea T, Ivatury R, Velmahos G, Andersson R, Kluger Y, Ansaloni L, Catena F. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 594] [Article Influence: 118.8] [Reference Citation Analysis (109)] |
| 37. | Matar ZS. Acute abdomen with pneumoperitoneum. J Family Community Med. 2004;11:71-72. [PubMed] |
| 38. | Karul M, Berliner C, Keller S, Tsui TY, Yamamura J. Imaging of appendicitis in adults. Rofo. 2014;186:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Sauvain MO, Tschirky S, Patak MA, Clavien PA, Hahnloser D, Muller MK. Acute appendicitis in overweight patients: the role of preoperative imaging. Patient Saf Surg. 2016;10:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Pelin M, Paquette B, Revel L, Landecy M, Bouveresse S, Delabrousse E. Acute appendicitis: Factors associated with inconclusive ultrasound study and the need for additional computed tomography. Diagn Interv Imaging. 2018;99:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Expert Panel on Gastrointestinal Imaging, Garcia EM, Camacho MA, Karolyi DR, Kim DH, Cash BD, Chang KJ, Feig BW, Fowler KJ, Kambadakone AR, Lambert DL, Levy AD, Marin D, Moreno C, Peterson CM, Scheirey CD, Siegel A, Smith MP, Weinstein S, Carucci LR. ACR Appropriateness Criteria® Right Lower Quadrant Pain-Suspected Appendicitis. J Am Coll Radiol. 2018;15:S373-S387. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 42. | Kepner AM, Bacasnot JV, Stahlman BA. Intravenous contrast alone vs intravenous and oral contrast computed tomography for the diagnosis of appendicitis in adult ED patients. Am J Emerg Med. 2012;30:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Sippola S, Virtanen J, Tammilehto V, Grönroos J, Hurme S, Niiniviita H, Lietzen E, Salminen P. The Accuracy of Low-dose Computed Tomography Protocol in Patients With Suspected Acute Appendicitis: The OPTICAP Study. Ann Surg. 2020;271:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Pinto Leite N, Pereira JM, Cunha R, Pinto P, Sirlin C. CT evaluation of appendicitis and its complications: imaging techniques and key diagnostic findings. AJR Am J Roentgenol. 2005;185:406-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Bhangu A; RIFT Study Group on behalf of the West Midlands Research Collaborative. Evaluation of appendicitis risk prediction models in adults with suspected appendicitis. Br J Surg. 2020;107:73-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 46. | Keller C, Wang NE, Imler DL, Vasanawala SS, Bruzoni M, Quinn JV. Predictors of Nondiagnostic Ultrasound for Appendicitis. J Emerg Med. 2017;52:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Hatch QM, Gilbert EW. Appendiceal Neoplasms. Clin Colon Rectal Surg. 2018;31:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Louis TH, Felter DF. Mucocele of the appendix. Proc (Bayl Univ Med Cent). 2014;27:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Wichmann D, Königsrainer A, Schweizer U, Archid R, Nadalin S, Manncke S. Pyogenic Liver Abscesses Caused by Acute Appendicitis: Frequency and Diagnostic and Therapeutic Recommendations. Surg Infect (Larchmt). 2021;22:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Ayers BC, Weinberg GA, Caserta M, Kauffman A, Wakeman D. Pyogenic liver abscess following perforated appendicitis. J Pediatr Surg Case Rep. 2019;44:101196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Castro R, Fernandes T, Oliveira MI, Castro M. Acute appendicitis complicated by pylephlebitis: a case report. Case Rep Radiol. 2013;2013:627521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Moore MM, Kulaylat AN, Hollenbeak CS, Engbrecht BW, Dillman JR, Methratta ST. Magnetic resonance imaging in pediatric appendicitis: a systematic review. Pediatr Radiol. 2016;46:928-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Dewhurst C, Beddy P, Pedrosa I. MRI evaluation of acute appendicitis in pregnancy. J Magn Reson Imaging. 2013;37:566-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Covelli JD, Madireddi SP, May LA, Costello JE, Lisanti CJ, Carlson CL. MRI for Pediatric Appendicitis in an Adult-Focused General Hospital: A Clinical Effectiveness Study-Challenges and Lessons Learned. AJR Am J Roentgenol. 2019;212:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Rybkin AV, Thoeni RF. Current concepts in imaging of appendicitis. Radiol Clin North Am. 2007;45:411-422, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Mostbeck G, Adam EJ, Nielsen MB, Claudon M, Clevert D, Nicolau C, Nyhsen C, Owens CM. How to diagnose acute appendicitis: ultrasound first. Insights Imaging. 2016;7:255-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 57. | Debnath J, George RA, Ravikumar R. Imaging in acute appendicitis: What, when, and why? Med J Armed Forces India. 2017;73:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Chia ML, Justin K, Hong HTC, Vishal GS. Computerized tomography scan in acute appendicitis with eventual negative appendectomy. J Clin Transl Res. 2021;7:326-332. [PubMed] |
| 59. | Kang HJ, Kang H, Kim B, Chae MS, Ha YR, Oh SB, Ahn JH. Evaluation of the diagnostic performance of a decision tree model in suspected acute appendicitis with equivocal preoperative computed tomography findings compared with Alvarado, Eskelinen, and adult appendicitis scores: A STARD compliant article. Medicine (Baltimore). 2019;98:e17368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Akmese OF, Dogan G, Kor H, Erbay H, Demir E. The Use of Machine Learning Approaches for the Diagnosis of Acute Appendicitis. Emerg Med Int. 2020;2020:7306435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Sasaki Y, Komatsu F, Kashima N, Sato T, Takemoto I, Kijima S, Maeda T, Ishii T, Miyazaki T, Honda Y, Shimada N, Urita Y. Clinical differentiation of acute appendicitis and right colonic diverticulitis: A case-control study. World J Clin Cases. 2019;7:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Shin JH, Son BH, Kim H. Clinically distinguishing between appendicitis and right-sided colonic diverticulitis at initial presentation. Yonsei Med J. 2007;48:511-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Sibileau E, Boulay-Coletta I, Jullès MC, Benadjaoud S, Oberlin O, Zins M. Appendicitis and diverticulitis of the colon: misleading forms. Diagn Interv Imaging. 2013;94:771-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Adamkiewicz TV, Berkovitch M, Krishnan C, Polsinelli C, Kermack D, Olivieri NF. Infection due to Yersinia enterocolitica in a series of patients with beta-thalassemia: incidence and predisposing factors. Clin Infect Dis. 1998;27:1362-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Zińczuk J, Wojskowicz P, Kiśluk J, Fil D, Kemona A, Dadan J. Mesenteric lymphadenitis caused by Yersinia enterocolitica. Prz Gastroenterol. 2015;10:118-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Cheng YZ, Lin HJ, Wu CM. Acute Pyelonephritis of an Ectopic Kidney Mimicking Acute Appendicitis: Two Unusual Cases in an Emergency Department. Tzu Chi Med J. 2009;21:70-72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Gross I, Siedner-Weintraub Y, Stibbe S, Rekhtman D, Weiss D, Simanovsky N, Arbell D, Hashavya S. Characteristics of mesenteric lymphadenitis in comparison with those of acute appendicitis in children. Eur J Pediatr. 2017;176:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Chohan T, Tabook S, Elmukashfi E, Sakroon S. Acute Appendicitis or…. is it Meckel’s Diverticulitis? Oman Med J. 2010;25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Mittal BR, Kashyap R, Bhattacharya A, Singh B, Radotra BD, Narasimha Rao KL. Meckel's diverticulum in infants and children; technetium-99m pertechnetate scintigraphy and clinical findings. Hell J Nucl Med. 2008;11:26-29. [PubMed] |
| 70. | Elkbuli A, Diaz B, Polcz V, Hai S, McKenney M, Boneva D. Operative vs non-operative therapy for acute phlegmon of the appendix: Is it safer? Int J Surg Case Rep. 2018;50:75-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Tannoury J, Abboud B. Treatment options of inflammatory appendiceal masses in adults. World J Gastroenterol. 2013;19:3942-3950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Andersen BR, Kallehave FL, Andersen HK. Antibiotics vs placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst Rev. 2005;CD001439. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 73. | Sallinen V, Akl EA, You JJ, Agarwal A, Shoucair S, Vandvik PO, Agoritsas T, Heels-Ansdell D, Guyatt GH, Tikkinen KA. Meta-analysis of antibiotics vs appendicectomy for non-perforated acute appendicitis. Br J Surg. 2016;103:656-667. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 74. | Podda M, Cillara N, Di Saverio S, Lai A, Feroci F, Luridiana G, Agresta F, Vettoretto N; ACOI (Italian Society of Hospital Surgeons) Study Group on Acute Appendicitis. Antibiotics-first strategy for uncomplicated acute appendicitis in adults is associated with increased rates of peritonitis at surgery. A systematic review with meta-analysis of randomized controlled trials comparing appendectomy and non-operative management with antibiotics. Surgeon. 2017;15:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 75. | Loftus TJ, Brakenridge SC, Croft CA, Stephen Smith R, Efron PA, Moore FA, Mohr AM, Jordan JR. Successful nonoperative management of uncomplicated appendicitis: predictors and outcomes. J Surg Res. 2018;222:212-218.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Sippola S, Haijanen J, Viinikainen L, Grönroos J, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T, Hurme S, Mecklin JP, Sand J, Jartti A, Salminen P. Quality of Life and Patient Satisfaction at 7-Year Follow-up of Antibiotic Therapy vs Appendectomy for Uncomplicated Acute Appendicitis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2020;155:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 77. | Podda M, Poillucci G, Pacella D, Mortola L, Canfora A, Aresu S, Pisano M, Erdas E, Pisanu A, Cillara N; ACTUAA Study Collaborative Working Group. Appendectomy versus conservative treatment with antibiotics for patients with uncomplicated acute appendicitis: a propensity score-matched analysis of patient-centered outcomes (the ACTUAA prospective multicenter trial). Int J Colorectal Dis. 2021;36:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | O'Leary DP, Walsh SM, Bolger J, Baban C, Humphreys H, O'Grady S, Hegarty A, Lee AM, Sheehan M, Alderson J, Dunne R, Morrin MM, Lee MJ, Power C, McNamara D, McCawley N, Robb W, Burke J, Sorensen J, Hill AD. A Randomised Clinical Trial Evaluating the Efficacy and Quality of Life of Antibiotic Only Treatment of Acute Uncomplicated Appendicitis: Results of the COMMA trial. Ann Surg. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 79. | Reinisch A, Reichert M, Hecker A, Padberg W, Ulrich F, Liese J. Nonoperative Antibiotic Treatment of Appendicitis in Adults: A Survey among Clinically Active Surgeons. Visc Med. 2020;36:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Peltrini R, Cantoni V, Green R, Lionetti R, D'Ambra M, Bartolini C, De Luca M, Bracale U, Cuocolo A, Corcione F. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: A systematic review and meta-analysis. Surgeon. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Marin D, Ho LM, Barnhart H, Neville AM, White RR, Paulson EK. Percutaneous abscess drainage in patients with perforated acute appendicitis: effectiveness, safety, and prediction of outcome. AJR Am J Roentgenol. 2010;194:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Ramson DM, Gao H, Penny-Dimri JC, Liu Z, Khong JN, Caruana CB, Campbell R, Jackson S, Perry LA. Duration of post-operative antibiotic treatment in acute complicated appendicitis: systematic review and meta-analysis. ANZ J Surg. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Gavriilidis P, de'Angelis N, Katsanos K, Di Saverio S. Acute Appendicectomy or Conservative Treatment for Complicated Appendicitis (Phlegmon or Abscess)? J Clin Med Res. 2019;11:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Darwazeh G, Cunningham SC, Kowdley GC. A Systematic Review of Perforated Appendicitis and Phlegmon: Interval Appendectomy or Wait-and-See? Am Surg. 2016;82:11-15. [PubMed] |
| 85. | Varadhan KK, Neal KR, Lobo DN. Safety and efficacy of antibiotics compared with appendicectomy for treatment of uncomplicated acute appendicitis: meta-analysis of randomised controlled trials. BMJ. 2012;344:e2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 86. | Talan DA, Saltzman DJ, DeUgarte DA, Moran GJ. Methods of conservative antibiotic treatment of acute uncomplicated appendicitis: A systematic review. J Trauma Acute Care Surg. 2019;86:722-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Abounozha S, Ibrahim R, Alshehri FM, Nawara H, Alawad A. The role of postoperative antibiotics in preventing surgical site infections in uncomplicated appendicitis. Ann Med Surg (Lond). 2021;62:203-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Wang CH, Yang CC, Hsu WT, Qian F, Ding J, Wu HP, Tsai JJ, Yang CJ, Su MY, Chen SC, Lee CC. Optimal initial antibiotic regimen for the treatment of acute appendicitis: a systematic review and network meta-analysis with surgical intervention as the common comparator. J Antimicrob Chemother. 2021;76:1666-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Marulanda K, Willis Z, Wilson W, Koonce RD, Lamm A, McLean SE, Hayes-Jordan A, Phillips MR. Implementation of Electronic Clinical Decision Support Tools for Antibiotic Stewardship in Pediatric Appendicitis. Am Surg. 2021;3134821989035. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | McCutcheon BA, Chang DC, Marcus LP, Inui T, Noorbakhsh A, Schallhorn C, Parina R, Salazar FR, Talamini MA. Long-term outcomes of patients with nonsurgically managed uncomplicated appendicitis. J Am Coll Surg. 2014;218:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Becker P, Fichtner-Feigl S, Schilling D. Clinical Management of Appendicitis. Visc Med. 2018;34:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Young KA, Neuhaus NM, Fluck M, Blansfield JA, Hunsinger MA, Shabahang MM, Torres DM, Widom KA, Wild JL. Outcomes of complicated appendicitis: Is conservative management as smooth as it seems? Am J Surg. 2018;215:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 93. | Simillis C, Symeonides P, Shorthouse AJ, Tekkis PP. A meta-analysis comparing conservative treatment vs acute appendectomy for complicated appendicitis (abscess or phlegmon). Surgery. 2010;147:818-829. [RCA] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 94. | Hall NJ, Eaton S, Stanton MP, Pierro A, Burge DM; CHINA study collaborators and the Paediatric Surgery Trainees Research Network. Active observation versus interval appendicectomy after successful non-operative treatment of an appendix mass in children (CHINA study): an open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Mohamed I, Chan S, Bhangu A, Karandikar S. Appendicitis as a manifestation of colon cancer: should we image the colon after appendicectomy in patients over the age of 40 years? Int J Colorectal Dis. 2019;34:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Buia A, Stockhausen F, Hanisch E. Laparoscopic surgery: A qualified systematic review. World J Methodol. 2015;5:238-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (4)] |
| 97. | Sakpal SV, Bindra SS, Chamberlain RS. Laparoscopic appendectomy conversion rates two decades later: an analysis of surgeon and patient-specific factors resulting in open conversion. J Surg Res. 2012;176:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Tong JWV, Lingam P, Shelat VG. Adhesive small bowel obstruction - an update. Acute Med Surg. 2020;7:e587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 99. | Håkanson CA, Fredriksson F, Lilja HE. Adhesive small bowel obstruction after appendectomy in children - Laparoscopic vs open approach. J Pediatr Surg. 2020;55:2419-2424. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Rasmussen T, Fonnes S, Rosenberg J. Long-Term Complications of Appendectomy: A Systematic Review. Scand J Surg. 2018;107:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Chia CL, Shelat VG, Low W, George S, Rao J. The use of Collatamp G, local gentamicin-collagen sponge, in reducing wound infection. Int Surg. 2014;99:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Poprom N, Wilasrusmee C, Attia J, McEvoy M, Thakkinstian A, Rattanasiri S. Comparison of postoperative complications between open and laparoscopic appendectomy: An umbrella review of systematic reviews and meta-analyses. J Trauma Acute Care Surg. 2020;89:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 103. | Bailey K, Choynowski M, Kabir SMU, Lawler J, Badrin A, Sugrue M. Meta-analysis of unplanned readmission to hospital post-appendectomy: an opportunity for a new benchmark. ANZ J Surg. 2019;89:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Sugiura K, Suzuki K, Umeyama T, Omagari K, Hashimoto T, Tamura A. Cost-effectiveness analysis of initial nonoperative management vs emergency laparoscopic appendectomy for acute complicated appendicitis. BMC Health Serv Res. 2020;20:1019. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Kim WJ, Jin HY, Lee H, Bae JH, Koh W, Mun JY, Kim HJ, Lee IK, Lee YS, Lee CS. Comparing the postoperative outcomes of single incision laparoscopic appendectomy and three port appendectomy with enhanced recovery after surgery protocol for acute appendicitis: A propensity score matching analysis. Ann Coloproctol. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 106. | Zaman S, Mohamedahmed AYY, Stonelake S, Srinivasan A, Sillah AK, Hajibandeh S. Single-port laparoscopic appendicectomy vs conventional three-port approach for acute appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2021;37:119-127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Deng L, Xiong J, Xia Q. Single-incision vs conventional three-incision laparoscopic appendectomy: A meta-analysis of randomized controlled trials. J Evid Based Med. 2017;10:196-206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Wang D, Dong T, Shao Y, Gu T, Xu Y, Jiang Y. Laparoscopy vs open appendectomy for elderly patients, a meta-analysis and systematic review. BMC Surg. 2019;19:54. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Fugazzola P, Coccolini F, Tomasoni M, Stella M, Ansaloni L. Early appendectomy vs. conservative management in complicated acute appendicitis in children: A meta-analysis. J Pediatr Surg. 2019;54:2234-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Shelat VG. Appendicectomy, intra-abdominal abscess, percutaneous drainage and non-operative management. ANZ J Surg. 2020;90:2145-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Li Z, Zhao L, Cheng Y, Cheng N, Deng Y. Abdominal drainage to prevent intra-peritoneal abscess after open appendectomy for complicated appendicitis. Cochrane Database Syst Rev. 2018;5:CD010168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 112. | Rakić M, Jukić M, Pogorelić Z, Mrklić I, Kliček R, Družijanić N, Perko Z, Patrlj L. Analysis of endoloops and endostaples for closing the appendiceal stump during laparoscopic appendectomy. Surg Today. 2014;44:1716-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Escolino M, Becmeur F, Saxena A, Till H, Holcomb GW 3rd, Esposito C. Endoloop vs endostapler: what is the best option for appendiceal stump closure in children with complicated appendicitis? Surg Endosc. 2018;32:3570-3575. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Sohn M, Hoffmann M, Pohlen U, Lauscher JC, Zurbuchen U, Holmer C, Buhr HJ, Lehmann KS. [Stump closure in laparoscopic appendectomy. Influence of endoloop or linear stapler on patient outcome]. Chirurg. 2014;85:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 115. | Ates M, Dirican A, Ince V, Ara C, Isik B, Yilmaz S. Comparison of intracorporeal knot-tying suture (polyglactin) and titanium endoclips in laparoscopic appendiceal stump closure: a prospective randomized study. Surg Laparosc Endosc Percutan Tech. 2012;22:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Wilson M, Maniam P, Ibrahim A, Makaram N, Knight SR, Patil P. Polymeric clips are a quicker and cheaper alternative to endoscopic ligatures for securing the appendiceal stump during laparoscopic appendicectomy. Ann R Coll Surg Engl. 2018;100:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Petersen LF, Nally MC, Agos A, Petty K. Internal hernia and small bowel obstruction caused by a linear cutter staple at appendiceal stump following laparoscopic appendectomy. J Surg Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Akbulut S, Koc C, Kocaaslan H, Gonultas F, Samdanci E, Yologlu S, Yilmaz S. Comparison of clinical and histopathological features of patients who underwent incidental or emergency appendectomy. World J Gastrointest Surg. 2019;11:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Phillips AW, Jones AE, Sargen K. Should the macroscopically normal appendix be removed during laparoscopy for acute right iliac fossa pain when no other explanatory pathology is found? Surg Laparosc Endosc Percutan Tech. 2009;19:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Song MY, Ullah S, Yang HY, Ahmed MR, Saleh AA, Liu BR. Long-term effects of appendectomy in humans: is it the optimal management of appendicitis? Expert Rev Gastroenterol Hepatol. 2021;15:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 121. | Ding W, Du Z, Zhou X. Endoscopic retrograde appendicitis therapy for management of acute appendicitis. Surg Endosc. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Prichard C, Canning M, McWilliam-Ross K, Birbari J, Parker W, Wasson L, Hollingsworth JW. Case series of acute appendicitis association with SARS-CoV-2 infection. BMC Infect Dis. 2021;21:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Meyer JS, Robinson G, Moonah S, Levin D, McGahren E, Herring K, Poulter M, Waggoner-Fountain L, Shirley DA. Acute appendicitis in four children with SARS-CoV-2 infection. J Pediatr Surg Case Rep. 2021;64:101734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 124. | Ahmad S, Ahmed RN, Jani P, Ullah M, Aboulgheit H. SARS-CoV-2 isolation from an appendix. J Surg Case Rep. 2020;2020:rjaa245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 125. | Ngaserin SH, Koh FH, Ong BC, Chew MH. COVID-19 not detected in peritoneal fluid: a case of laparoscopic appendicectomy for acute appendicitis in a COVID-19-infected patient. Langenbecks Arch Surg. 2020;405:353-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 126. | Collard M, Lakkis Z, Loriau J, Mege D, Sabbagh C, Lefevre JH, Maggiori L. Antibiotics alone as an alternative to appendectomy for uncomplicated acute appendicitis in adults: Changes in treatment modalities related to the COVID-19 health crisis. J Visc Surg. 2020;157:S33-S42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 127. | Mai DVC, Sagar A, Menon NS, Claydon O, Park JY, Down B, Keeler BD. A local experience of non-operative management for an appendicitis cohort during COVID-19. Ann Med Surg (Lond). 2021;63:102160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | CODA Collaborative, Flum DR, Davidson GH, Monsell SE, Shapiro NI, Odom SR, Sanchez SE, Drake FT, Fischkoff K, Johnson J, Patton JH, Evans H, Cuschieri J, Sabbatini AK, Faine BA, Skeete DA, Liang MK, Sohn V, McGrane K, Kutcher ME, Chung B, Carter DW, Ayoung-Chee P, Chiang W, Rushing A, Steinberg S, Foster CS, Schaetzel SM, Price TP, Mandell KA, Ferrigno L, Salzberg M, DeUgarte DA, Kaji AH, Moran GJ, Saltzman D, Alam HB, Park PK, Kao LS, Thompson CM, Self WH, Yu JT, Wiebusch A, Winchell RJ, Clark S, Krishnadasan A, Fannon E, Lavallee DC, Comstock BA, Bizzell B, Heagerty PJ, Kessler LG, Talan DA DA. A Randomized Trial Comparing Antibiotics with Appendectomy for Appendicitis. N Engl J Med. 2020;383:1907-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 129. | Mowbray NG, Hurt L, Powell-Chandler A, Reeves N, Chandler S, Walters E, Cornish J. Where have all the appendicectomies gone? Ann R Coll Surg Engl. 2021;103:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Finkelstein P, Picado O, Muddasani K, Wodnicki H, Mesko T, Unger S, Bao P, Jorge I, Narayanan S, Ben-David K. A Retrospective Analysis of the Trends in Acute Appendicitis During the COVID-19 Pandemic. J Laparoendosc Adv Surg Tech A. 2021;31:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 131. | Yang Y, Li Y, Du X. Acute complex appendicitis during the COVID-19 epidemic: A single-institution retrospective analysis based on real-world data. Am J Emerg Med. 2021;46:74-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Griffith AM, Ockerse P, Shaaban A, Kelly C. Effect of the COVID-19 pandemic on CT scans ordered from the emergency department for abdominal complaints. Emerg Radiol. 2021;28:485-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | Köhler F, Acar L, van den Berg A, Flemming S, Kastner C, Müller S, Diers J, Germer CT, Lock JF, L'hoest H, Marschall U, Wiegering A. Impact of the COVID-19 pandemic on appendicitis treatment in Germany-a population-based analysis. Langenbecks Arch Surg. 2021;406:377-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 134. | Bajomo O, Hampal R, Sykes P, Miah A. Managing appendicitis during the COVID-19 era: A single centre experience & implications for future practice. Ann Med Surg (Lond). 2021;63:102168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Somers K, Abd Elwahab S, Raza MZ, O'Grady S, DeMarchi J, Butt A, Burke J, Robb W, Power C, McCawley N, McNamara D, Kearney D, Hill ADK. Impact of the COVID-19 pandemic on management and outcomes in acute appendicitis: Should these new practices be the norm? Surgeon. 2021;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Oh D, Kang YM, Choi JY, Lee WJ. What surgeons should know about emergency operation for COVID-19 confirmed patients: A case report. Int J Surg Case Rep. 2020;77:503-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | Mittal MK, Dayan PS, Macias CG, Bachur RG, Bennett J, Dudley NC, Bajaj L, Sinclair K, Stevenson MD, Kharbanda AB; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Performance of ultrasound in the diagnosis of appendicitis in children in a multicenter cohort. Acad Emerg Med. 2013;20:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 138. | Wi SA, Kim DJ, Cho ES, Kim KA. Diagnostic performance of MRI for pregnant patients with clinically suspected appendicitis. Abdom Radiol (NY). 2018;43:3456-3461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 139. | Nakashima M, Takeuchi M, Kawakami K. Clinical Outcomes of Acute Appendicitis During Pregnancy: Conservative Management and Appendectomy. World J Surg. 2021;45:1717-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | de Moya MA, Sideris AC, Choy G, Chang Y, Landman WB, Cropano CM, Cohn SM. Appendectomy and pregnancy: gestational age does not affect the position of the incision. Am Surg. 2015;81:282-288. [PubMed] |
| 141. | Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy vs open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 142. | Anshul F, Naids JM, Thakur K. Silent Appendicitis in an Immunocompromised Patient: A Diagnostic Dilemma: 2416. ACG. 2017;112:S1317-S1318. [DOI] [Full Text] |
| 143. | Bastiaenen VP, Allema WM, Klaver CEL, van Dieren S, Koens L, Tanis PJ, Bemelman WA. Routine histopathologic examination of the appendix after appendectomy for presumed appendicitis: Is it really necessary? Surgery. 2020;168:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 144. | Shaib WL, Assi R, Shamseddine A, Alese OB, Staley C 3rd, Memis B, Adsay V, Bekaii-Saab T, El-Rayes BF. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist. 2017;22:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (3)] |
| 145. | Stinner B, Rothmund M. Neuroendocrine tumours (carcinoids) of the appendix. Best Pract Res Clin Gastroenterol. 2005;19:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | Fornaro R, Frascio M, Sticchi C, De Salvo L, Stabilini C, Mandolfino F, Ricci B, Gianetta E. Appendectomy or right hemicolectomy in the treatment of appendiceal carcinoid tumors? Tumori. 2007;93:587-590. [PubMed] |
| 147. | Ricci C, Ingaldi C, Alberici L, Brighi N, Santini D, Mosconi C, Ambrosini V, Campana D, Minni F, Casadei R. Histopathological diagnosis of appendiceal neuroendocrine neoplasms: when to perform a right hemicolectomy? Endocrine. 2019;66:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 148. | Madani A, van der Bilt JD, Consten EC, Vriens MR, Borel Rinkes IH. Perforation in appendiceal well-differentiated carcinoid and goblet cell tumors: impact on prognosis? Ann Surg Oncol. 2015;22:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 149. | Ng CYD, Nandini CL, Chuah KL, Shelat VG. Right hemicolectomy for acute appendicitis secondary to breast cancer metastases. Singapore Med J. 2018;59:284-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 150. | Sali PA, Yadav KS, Desai GS, Bhole BP, George A, Parikh SS, Mehta HS. Small bowel obstruction due to an endometriotic ileal stricture with associated appendiceal endometriosis: A case report and systematic review of the literature. Int J Surg Case Rep. 2016;23:163-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |