Published online Jun 27, 2020. doi: 10.4240/wjgs.v12.i6.298
Peer-review started: February 6, 2020
First decision: March 29, 2020
Revised: April 6, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: June 27, 2020
Processing time: 133 Days and 19.5 Hours
A collecting duct carcinoma is a very rare, malignant renal epithelial tumor. Distant metastases are present in one third of cases at the time of diagnosis. It is known to have a poor prognosis.
A 42-year-old male was sent to our surgery clinic for removal of a 119.2 mm × 108.3 mm encapsulated cystic mass, which was localized in the 8th segment of the right liver lobe. The lesion was first identified on ultrasonography. A computed tomography scan confirmed the presence of a Bosniak type III cystic lesion, which affected the liver and convexity of the right kidney. Surgical intervention involved a right nephrectomy, with removal of the cystic mass. The patient was mobilized on the first postoperative day and was discharged after 7 d. The histological and immunohistochemical examination revealed a low-grade collecting duct renal carcinoma, which is a rare variant of papillary carcinoma, with low malignant potential. The patient did not receive chemotherapy and after 21 mo of follow-up, a radiological examination and laboratory analyses showed normal aspects. No relapse or other complications were reported.
To manage renal tumors properly, a correct histopathological diagnosis is crucial, as is early diagnosis and correct surgical treatment.
Core tip: We present a patient with a rare histological variant of renal carcinoma. There are at least three particularities related to this tumor. Firstly, during imaging investigations, renal carcinoma mimics an encapsulated cyst. Secondly, the differential diagnosis was difficult and was finally established by immunohistochemical staining. Thirdly, although collecting duct carcinoma is known to have an aggressive behavior, the histological assessment indicated a low-grade carcinoma. The patient is still alive after 21 mo of regular follow-up, without postoperative oncological therapy.
- Citation: Fulop ZZ, Gurzu S, Jung I, Simu P, Banias L, Fulop E, Dragus E, Bara TJ. Cystic low-grade collecting duct renal carcinoma with liver compression — A challenging diagnosis and therapy: A case report. World J Gastrointest Surg 2020; 12(6): 298-306
- URL: https://www.wjgnet.com/1948-9366/full/v12/i6/298.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i6.298
Collecting duct carcinoma (CDC), also known as carcinoma of the collecting ducts of Bellini, comprises fewer than 1% of malignant renal tumors[1,2]. It originates from the collecting duct epithelium, which is located in the renal medulla[3]. CDC has distinct clinical and pathological characteristics, such as hematuria, weight loss, back or flank pain and a local mass, but also fatigue and fever[2-6]. It mostly appears in middle-aged men (male-to-female ratio = 2:1), with a right-sided laterality predominance (> 2:1)[2,6,7,8]. At the time of diagnosis, approximately one third of patients present with metastases in supraclavicular or cervical lymph nodes or distant metastases in the lungs, bones, liver or adrenal glands[2,4,6,7,9].
The presumptive diagnosis is highlighted by computed tomography (CT) characteristics. CDC is characteristically described as having medullary localization, with renal sinus involvement, heterogeneous enhancement, infiltrative growth, and preserved renal curve[7].
Microscopically, it has a tubular or tubulopapillary architecture[1,4,10]. The World Health Organization established major criteria for diagnosing CDC, which is a diagnosis of exclusion. They include location in the medullary pyramid, irregular tubular architecture, without a component of urothelial carcinoma, with high nuclear grade, inflammatory desmoplastic stroma, which is accompanied by numerous neutrophils, together with immunohistochemical (IHC) reactivity to antibodies against high molecular weight cytokeratin and Ulex europaeus I (UEA1), respectively[4,7].
In this study, we present a patient with a large cystic low-grade CDC. After surgical excision, without postoperative oncological treatment but a strict postoperative follow-up, the patient is still alive, without complications, 21 mo after surgery.
A 42-year-old male patient, with a body mass index of 28.75, was transferred from a regional hospital, to our University Surgery Clinic, due to right hypochondria pain and suspicion of a hydatid cyst of the liver.
The patient confirmed a one-month history of right hypochondria pain, without any other significant symptoms.
The patient was known to have chronic cholecystitis and mild hepatic steatosis. He declared himself a social drinker.
No significant diseases or other information were confirmed.
During physical examination a large abdominal mass was palpated in the right hypochondria and the inferior edge of the liver, located 2.5 cm below the rib cage.
The serum lymphocyte level was 16.4%, with a neutrophil-to-lymphocyte ratio (NLR) of 4.42. The serum levels of tumor-specific markers such as α-fetoprotein, carcinoembryonic antigen, and human choriogonadotropin were not evaluated but their positivity was denied by IHC staining.
The primary imaging investigation included ultrasonography, which described a non-homogeneous round mass 119.2 mm × 108.3 mm in size, with increased echogenicity. The encapsulated mass was located in the 8th segment of the right liver. Based on the patient’s personal history, the presumptive diagnosis was a hepatic cyst, associated with hepatosplenomegaly and hepatic steatosis. A right renal malrotation, with the right kidney localized in the epigastrium, was also suspected. The ureteral jet from the right side of the bladder was not visible.
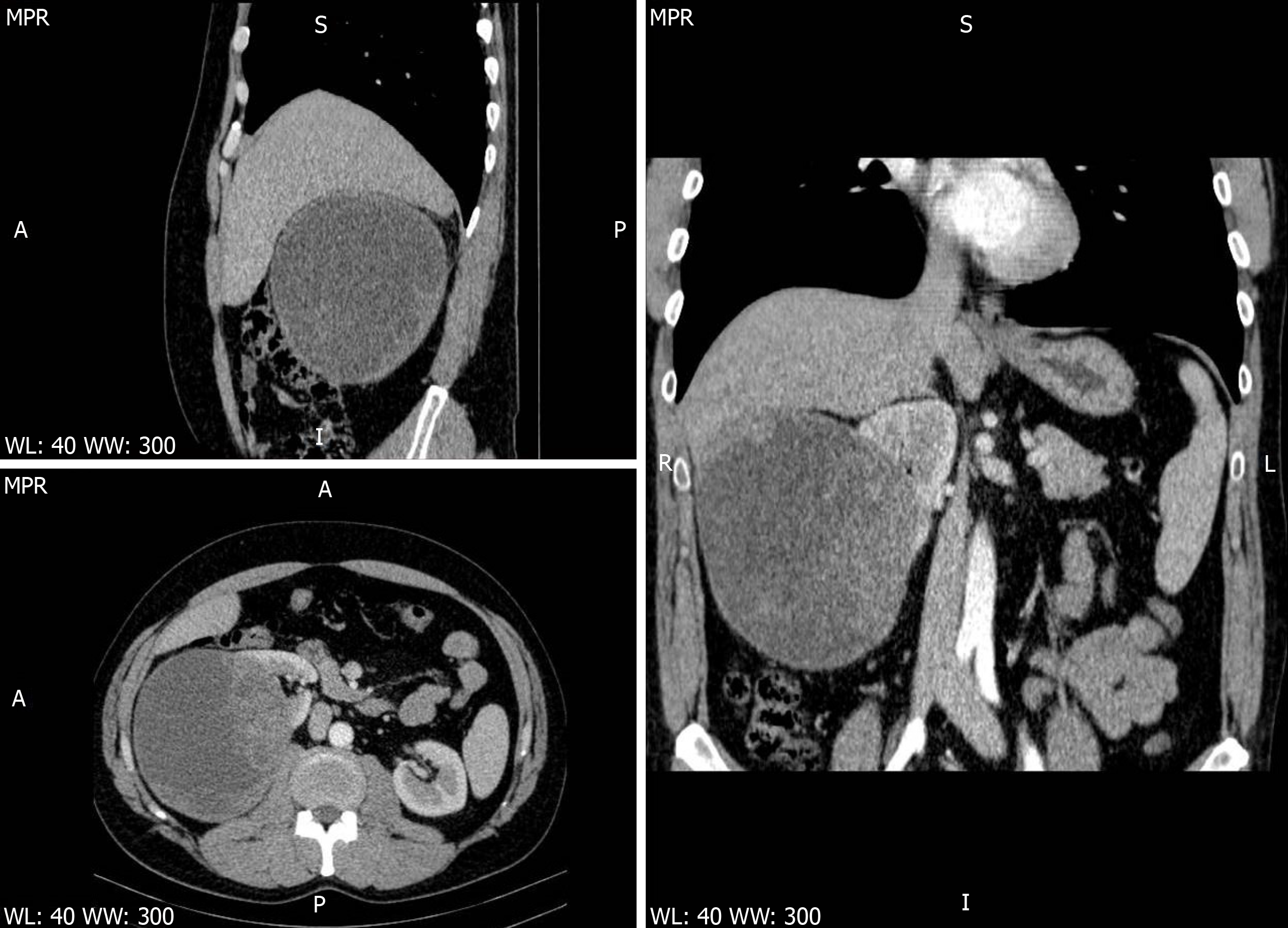
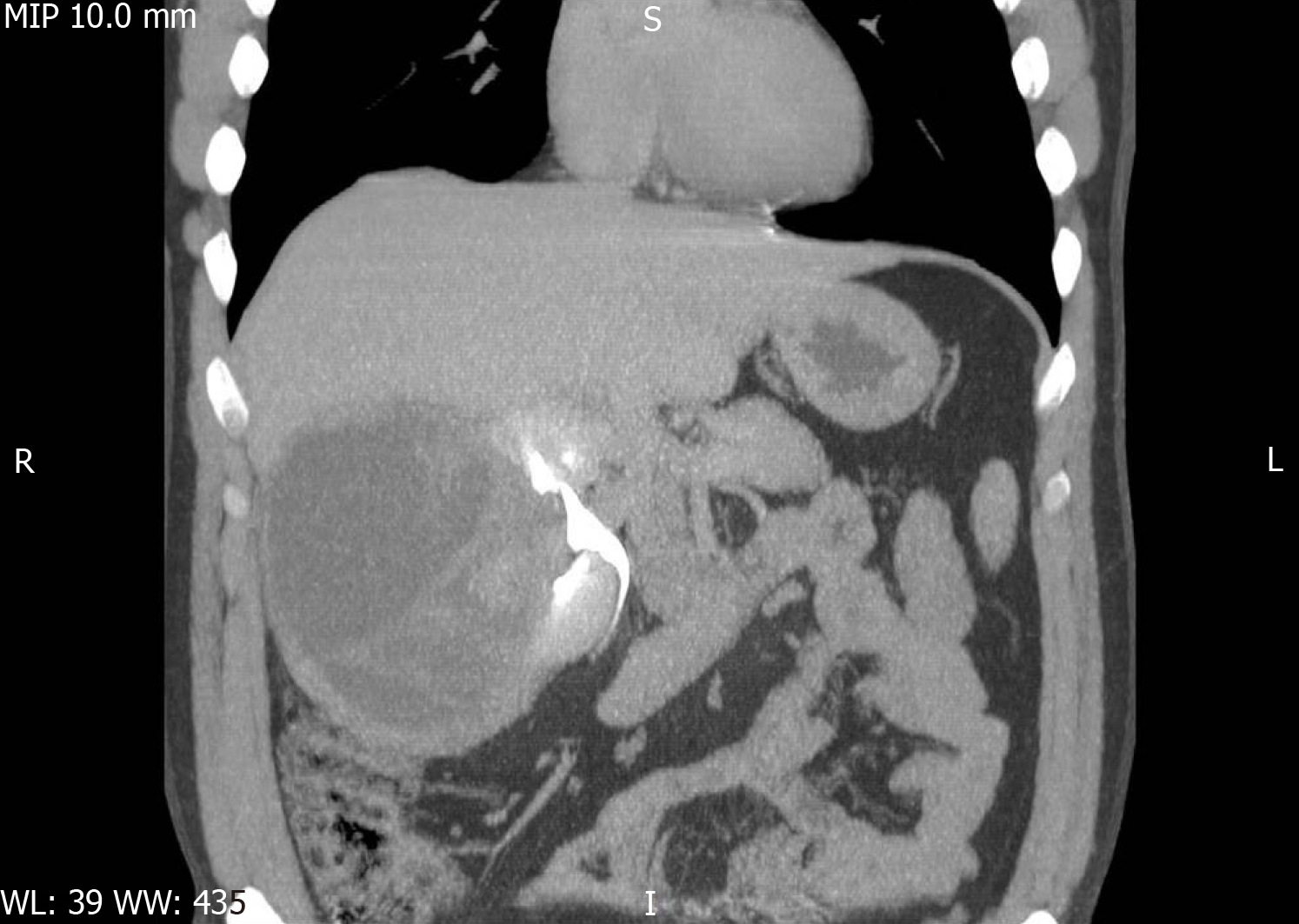
A CT scan was also carried out. The scan confirmed the hepatomegaly and presence of a well-defined 126 mm × 121 mm × 146 mm macronodular encapsulated cystic lesion. The wall thickness was estimated to be approximately 6 mm. The fluid content was estimated to be clear, with a density of 10 HU. The cystic mass was thought to involve the convex side of the right kidney, with the upper limit at the visceral face of the liver, the lateral limit at the abdominal wall and the inferior limit at the bifurcation of the abdominal aorta. It displaced the right kidney in the anterior and medial direction (Figures 1-3). Hydronephrosis or signs of disorders of secretion or excretion of the right kidney were not described, neither were modified lymph nodes. Free abdominal fluid was observed. As the cystic lesion was classified as Bosniak type III, with a 50% potential of malignancy, surgical excision was planned.
Macroscopic examination confirmed the presence of the encapsulated cystic mass, which involved the renal specimen. On the cut section, a blackish-brown appearance was evident.
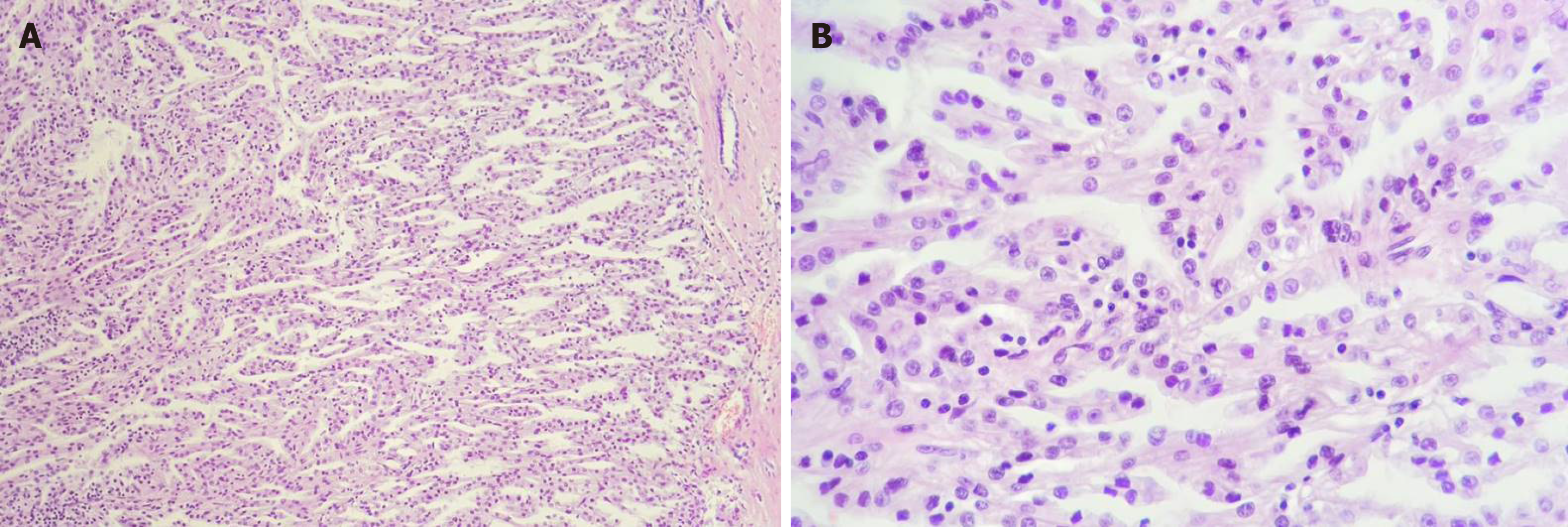
The microscopic findings revealed the presence of tubular and papillary-like structures. The nuclei predominantly showed Fuhrman grade 2; the stroma did not show obvious desmoplastic areas, although it was infiltrated with neutrophils (Figure 4). Based on histological findings, a papillary carcinoma was initially diagnosed. As foamy macrophages were seen within the fibrovascular core, a variant of CDC was taken into account, for differential diagnosis. The tumor extended into both the cortical and medullary area, exceeded the renal capsule and infiltrated the perirenal adipose tissue (pT3 stage). No lymph nodes were identified in the surgical specimen. No clear cells or sarcomatoid dedifferentiation was identified.
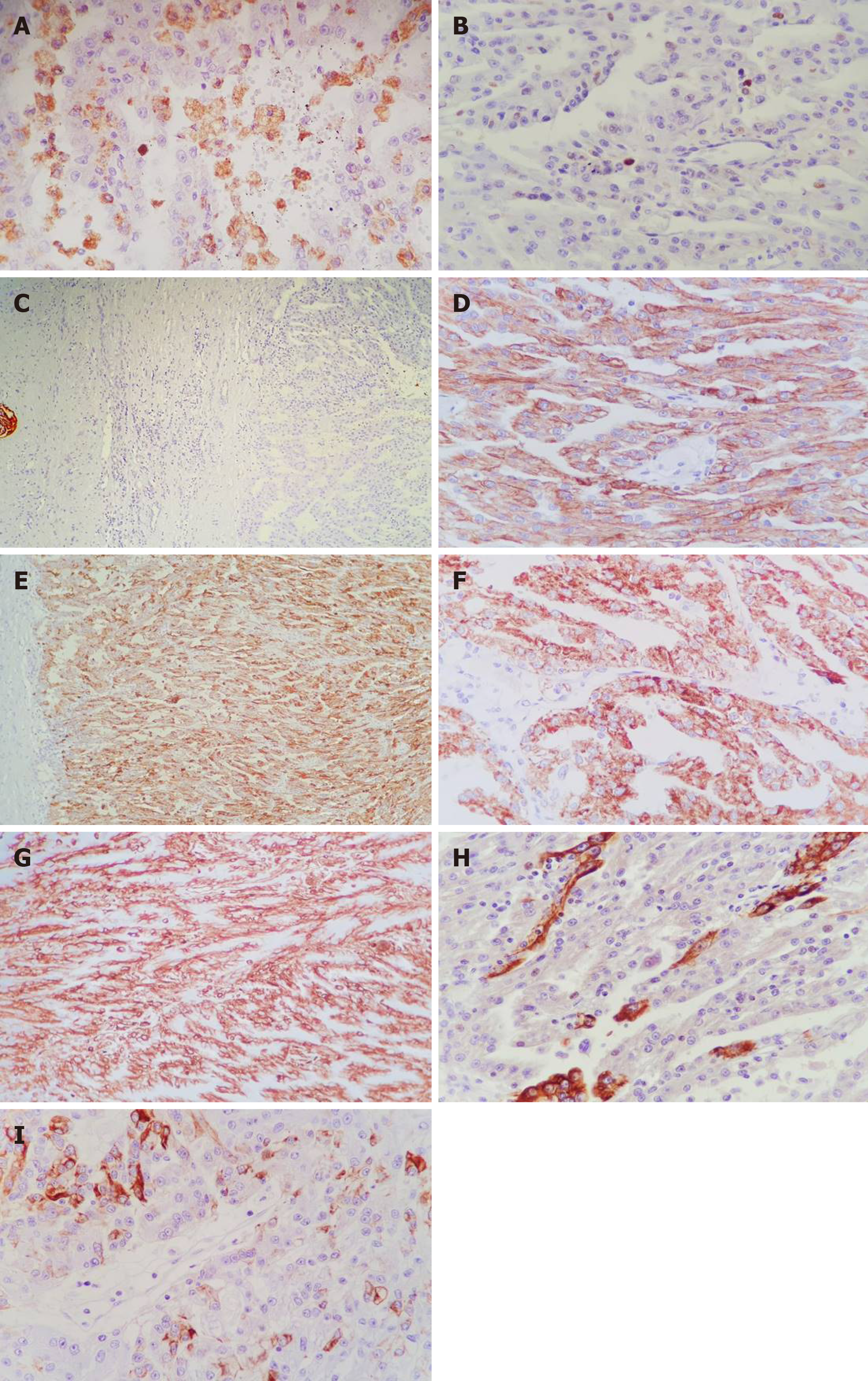
Tumor cells showed diffuse positivity for CD15 (clone: Carb-3, Dako) CTK AE1/AE3 (clone AE1/AE3, ImmunoLogic), alpha-methylacyl-CoA racemase (AMACR clone 13H4, Dako), and vimentin (clone V9, Leica Biosystems), focal positivity for CTK7 (clone OV-TL 12/30, A. Menarini Diagnostics) and CTK19 (clone RCK 108, Dako). No positivity was revealed for antibodies CD10 (clone Ab-2 56C6, Thermo Fisher Scientific), HER-2 (clone c-erbB-2, Dako), inhibin (clone Inhibin A, Leica), CD68 (clone KP1, ImmunoLogic; it marked the foamy macrophages within the fibrovascular core), Melan-A (clone A103, Cell Marque), Calretinin (clone Dak-Calret 1, Dako), human choriogonadotropin (polyclonal, Dako), carcinoembryonic antigen (polyclonal, Dako) or α-fetoprotein (polyclonal, Dako). The Ki67 (clone Mib-1, Dako) index was below 5% (Figure 5).
Based on the histological findings and immunoprofile of the tumor cells, the final diagnosis was established as pT3Nx-stage low-grade carcinoma of the collecting ducts of Bellini.
After antibiotic prophylaxis, with second-generation cephalosporins, and thromboembolic prophylaxis with low molecular weight heparin, surgical intervention was carried out. The POSSUM predicted morbidity rate was estimated to be 29.3%, with a POSSUM predicted mortality rate of 5.3%.
Exploratory laparoscopy was initiated, followed by conversion to open surgery. Intraoperatively, involvement of the right kidney was observed and a histological examination confirmed the malignant potential. Based on this fact, a right nephrectomy, with tumorectomy, was performed. Due to diligent hemostasis, the surgical intervention lasted 150 min.
The patient was mobilized on the first postoperative day. Presenting favorable evolution, he was discharged 7 d after surgery. Postoperatively the patient’s health conditions were assessed by phone at 3 mo, 6 mo, and 12 mo after discharge.
He did not receive postoperative oncological treatment, but in the first year following the surgical intervention, he attended oncological control examinations once every 3 mo. During the course of the first 12 mo, two ultrasonographies and one CT scan were performed, which described normal kidney function on the left side, without any suspicion of relapse on the right side. From the second postoperative year, control examinations were performed every 6 mo. After 18 mo, the laboratory investigations showed normal values and ultrasonography excluded any sign of relapse. The patient presents regularly at the nephrology clinic for evaluation of left-sided renal activity, which shows normal function. The patient states that he is in good general health, without any complaints 21 mo after surgery.
CDC is a rare but threatening genitourinary malignancy[9,10]. It usually presents as a locally advanced, high-grade carcinoma, with bone metastases[9]. In rare cases, these tumors can be large with a cystic appearance, such as in this case[3,11].
Due to its histological rarity, the optimal postoperative therapy requires elucidation[10]. It should be based on a correct estimation of malignancy grade. As CDC is considered to show highly malignant behavior, the identification of low-grade cases, as an exclusion diagnosis, might be crucial for a thorough follow-up. Recent studies proposed using inflammation-related parameters for estimation of prognosis[12,13]. An NLR ≥ 4 is considered an indicator of poor prognosis[12,14-16]. In this case, although the value of the NLR was 4.42, the patient had a favorable prognosis, 21 mo after diagnosis.
Current relevant literature is dominated mostly by case reports from small institutional cohort studies (Table 1)[3,11,17-27]. The reported survival rate is controversial, being mostly reported as a median survival of 13-22 mo after diagnosis[5,9]. The overall survival rate at 1 year and 3 years, for all stages, is reported to be 69% and 45%, respectively[2,5]. A survival benefit of 4-5 mo only, is predicted with chemo-radiotherapy[9]. To date, there is no standardized treatment for metastatic CDC[8]. In locally advanced cases, no chemotherapy is indicated after standard nephrectomy. It is recommended that the evolution of patients is followed, and if metastases appear, the histological examination and immunoprofile of the metastasis are necessary for individualized therapy[2]. As HER-2 may be overexpressed[10], HER-2/neu amplifications can be indicators of anti-HER-2 medications. In metastatic CDCs, tumor regression was reported after targeted therapy with sunitinib or sorafenib[2,8].
| Ref. | Publication year | Histological type | Mimicking | Tumor localization | Tumor diameter, (cm) | Tumor stage |
| Kumar et al[3] | 2018 | Collecting duct carcinoma | Hydatid cyst | Right kidney | 3.5 | Not specified |
| Uçar et al[11] | 2016 | Renal hydatid cyst | Renal tumor | Right kidney | 3.2 | Not specified |
| Dwivedi and Mourya[17] | 2018 | Renal cell carcinoma with disseminated intraperitoneal and retroperitoneal cystic metastases | Systemic hydatidosis | Right kidney | Not specified | Not specified |
| Kheir et al[18] | 2017 | Multicystic nephroma | Hydatid cyst | Right kidney | 13 | Not specified |
| Bhat et al[19] | 2015 | Renal hydatid cyst | Cystic renal cell carcinoma | Left kidney | 7 | Not specified |
| Nayyar et al[20] | 2011 | Cystic schwannoma | Simple renal cyst | Right kidney | 12 | Not specified |
| Straus and Osipov[21] | 2006 | Renal cell carcinoma, clear cell type | Epithelium lined cyst of the liver | Left kidney | 6.6 | Not specified |
| Mai et al[22] | 1996 | Papillary transitional cell carcinoma | Renal cyst | Right kidney | 8.5 | Not specified |
| Qadri et al[23] | 2015 | Renal cystic echinococcosis | Renal cell carcinoma | Right kidney | Not specified | Not specified |
| Sidani et al[24] | 2015 | Renal hydatid cyst | Renal cell carcinoma | Left kidney | 7.9 | Not specified |
| Anshu et al[25] | 2012 | Renal hydatid cyst | Renal cell carcinoma | Right kidney | 17 | Not specified |
| Liang et al[26] | 2019 | Recurrent renal cell carcinoma | Polycystic liver disease | Right kidney | Not specified | Not specified |
| Ahmet et al[27] | 2019 | Hydatid cyst | Kidney tumor | Left kidney | 13 | Not specified |
| Our study | 2020 | Collecting duct carcinoma | Hydatid cyst | Right kidney | 12.6 | T3 |
Although there is a tendency for a detailed imaging diagnosis, in this particular case, a hepatic cyst was suspected on ultrasonography. CDC usually presents as a central medullary renal mass, showing minimal contrast capture; in rare cases, cortically situated satellite nodes are also described[2]. Cystic renal masses are easily diagnosed on CT scans, if they belong to Bosniak type I or type IV. Bosniak type II and III cysts[3] may present a diagnostic challenge.
The diagnosis in our patient was difficult based on histological assessment, and supplementary IHC staining was carried out. As CDC is an exclusion diagnosis, the differential diagnosis should include papillary carcinoma, renal medullary carcinoma, and gland-forming urothelial carcinoma[3]. Suspicion of CDC should be confirmed by Hematoxylin-Eosin staining and positivity for UEA I and a large spectrum of keratins, such CKHMW, CK AE1/AE3, CK7, and CK19, associated with positivity for CD15. CDC is negative for CD10, CK20 and villin and might display vimentin and HER-2 expression[1,3,5,6]. HER-2 positivity, which was not identified in this case, is considered an indicator of poor prognosis[10]. CDC also presents loss of heterozygosity and deletion of 1q32.1-32.2 and monosomy of chromosomes 1q, 6p, 8p, 14, 18, and 22[1,2].
Multicenter studies and clinical trials, based on the genetic profile of the tumor cells, need to be conducted to establish an effective adjuvant therapy.
In patients with rare variants of renal carcinomas, such as CDC, an estimation of tumor grade is mandatory for an appropriate follow-up. The LNR cannot be used as a prognostic parameter. In metastatic cases, targeted therapy should focus on the genetic profile of tumor cells.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vagholkar K, Xia SJ S-Editor: Tang JZ L-Editor: Webster JR E-Editor: Qi LL
| 1. | Bose D, Das RN, Chatterjee U, Banerjee U. Collecting duct carcinoma: a rare malignancy. J Cancer Res Ther. 2013;9:94-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Procopio G, Testa I, Iacovelli R, Grassi P, Verzoni E, Garanzini E, Colecchia M, Torelli T, De Braud F. Treatment of collecting duct carcinoma: current status and future perspectives. Anticancer Res. 2014;34:1027-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Kumar V, Misra V, Chaurasiya D, Verma N. Collecting duct carcinoma kidney masquerading as hydatid cyst: A rare case report and review of literature. Indian J Pathol Microbiol. 2018;61:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Bansal P, Kumar S, Mittal N, Kundu AK. Collecting duct carcinoma: a rare renal tumor. Saudi J Kidney Dis Transpl. 2012;23:810-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Wang X, Hao J, Zhou R, Zhang X, Yan T, Ding D, Shan L, Liu Z. Collecting duct carcinoma of the kidney: a clinicopathological study of five cases. Diagn Pathol. 2013;8:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Tsoukalas N, Kostakis ID, Tolia M, Tryfonopoulos D, Lypas G, Panopoulos C, Barbounis V, Koumakis G, Efremidis A. A collecting duct carcinoma producing carcinoembryonic antigen and Ca-125 in a 29-year-old woman. Int Urol Nephrol. 2014;46:1313-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Dason S, Allard C, Sheridan-Jonah A, Gill J, Jamshaid H, Aziz T, Kajal B, Kapoor A. Management of renal collecting duct carcinoma: a systematic review and the McMaster experience. Curr Oncol. 2013;20:e223-e232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Zhao RN, Nie LH, Gong R, Wang JZ, Wazir R, Liu LR, Song TR, Wei Q. Active targeted therapy for metastatic collecting duct carcinoma of the kidney: a case report and review of the literature. Int Urol Nephrol. 2013;45:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Sui W, Matulay JT, Robins DJ, James MB, Onyeji IC, RoyChoudhury A, Wenske S, DeCastro GJ. Collecting duct carcinoma of the kidney: Disease characteristics and treatment outcomes from the National Cancer Database. Urol Oncol. 2017;35:540.e13-540.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Mennitto A, Verzoni E, Peverelli G, Alessi A, Procopio G. Management of Metastatic Collecting Duct Carcinoma: An Encouraging Result in a Patient Treated With Cabozantinib. Clin Genitourin Cancer. 2018;16:e521-e523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Uçar M, Karagözlü Akgül A, Çelik F, Kılıç N. Excisional treatment of renal hydatid cyst mimicking renal tumor with diode laser technique: A case report. J Pediatr Urol. 2016;12:264.e1-264.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Kucharz J, Dumnicka P, Giza A, Demkow U, Kusnierz-Cabala B, Demkow T, Wiechno P. Radiological Response and Neutrophil-to-Lymphocyte Ratio as Predictive Factors for Progression-Free and Overall Survival in Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib. Adv Exp Med Biol. 2019;1153:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Widz D, Mitura P, Buraczynski P, Plaza P, Bar M, Cabanek M, Nowak G, Ostrowska A, Bar K. Preoperative Neutrophil-lymphocyte Ratio as a Predictor of Overall Survival in Patients with Localized Renal Cell Carcinoma. Urol J. 2020;17:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Nunno VD, Mollica V, Gatto L, Santoni M, Cosmai L, Porta C, Massari F. Prognostic impact of neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. Immunotherapy. 2019;11:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Huszno J, Kolosza Z, Mrochem-Kwarciak J, Rutkowski T, Skladowski K. The Role of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Platelets in the Prognosis of Metastatic Renal Cell Carcinoma. Oncology. 2019;97:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Taguchi S, Fukuhara H, Miyakawa J, Morikawa T, Naito A, Kawai T, Fujimura T, Kume H. Prognostic significance of neutrophil-to-lymphocyte ratio in collecting duct carcinoma. Jpn J Clin Oncol. 2018;48:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Dwivedi AND, Mourya C. Disseminated cystic nodal metastasis in renal cell carcinoma mimicking systemic hydatidosis on imaging. J Cancer Res Ther. 2018;14:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Kheir AE, Elnaeema AM, Gafer SM, Mohammed SA, Bahar ME. Multicystic nephroma masquerading as hydatid cyst: a diagnostic challenge. BMC Urol. 2017;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Bhat GS, Burude VA, Hegde SD, Tembadamani VS. Isolated renal hydatid cyst masquerading as cystic renal cell carcinoma: a case report. J Clin Diagn Res. 2015;9:PD07-PD08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Nayyar R, Khattar N, Sood R, Bhardwaj M. Cystic retroperitoneal renal hilar ancient schwannoma: Report of a rare case with atypical presentation masquerading as simple cyst. Indian J Urol. 2011;27:404-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Straus T, Osipov V. Ciliated hepatic foregut cyst in a patient with renal cell carcinoma. BMC Cancer. 2006;6:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mai KT, Gerridzen RG, Millward SF. Papillary transitional cell carcinoma arising in a calyceal cyst and masquerading as a renal cyst. Arch Pathol Lab Med. 1996;120:879-882. [PubMed] |
| 23. | Qadri S, Sherwani RK, Ahmed M. Isolated cystic echinococcosis of kidney burlesquing as renal cell carcinoma: a diagnostic pitfall. Ann Parasitol. 2015;61:57-60. [PubMed] |
| 24. | Sidani MN, Graziano CE, Soni SD, Smith TG. Renal Hydatid Cyst Mimicking Renal Cell Carcinoma. Austin J Urol. 2015;2:1028. |
| 25. | Anshu G, Shivani K, Manish KS, Onis S, Viplesh K. Isolated Renal Hydatid Cyst Mimicking Renal Cell Carcinoma: A Diagnostic Dilemma. J Clin Diagn Res. 2012;6:890-892. |
| 26. | Liang C, Takahashi K, Kurata M, Sakashita S, Oda T, Ohkohchi N. Recurrent renal cell carcinoma leading to a misdiagnosis of polycystic liver disease: A case report. World J Gastroenterol. 2019;25:2264-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Ahmet C, Hüseyin Ç, Ahmet Y, Ramazan A, Cemal T. A Case of Hydatid Cyst Mimicking Kidney Tumor. Cyprus J Med Sci. 2019;4:70-72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |