Published online Jun 27, 2020. doi: 10.4240/wjgs.v12.i6.259
Peer-review started: April 23, 2020
First decision: April 29, 2020
Revised: May 1, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 27, 2020
Processing time: 56 Days and 15.2 Hours
One-hundred years after the 1918-19 H1N1 flu pandemic and 10 years after the 2009 H1N1 flu pandemic, another respiratory virus has now inserted itself into the human population. Severe acute respiratory syndrome coronavirus has become a critical challenge to global health with immense economic and social disruption. In this article we review salient aspects of the coronavirus disease 2019 (COVID-19) outbreak that are relevant to surgical practice. The emphasis is on considerations during the pre-operative and post-operative periods as well as the utility and limitations of COVID-19 testing. The focus of the media during this pandemic is centered on predictive epidemiologic curves and models. While epidemiologists and infectious disease physicians are at the forefront in the fight against COVID-19, this pandemic is also a “stress test” to evaluate the capacity and resilience of our surgical community in dealing with the challenges imposed to our health system and society. As recently pointed out by Dr. Anthony Fauci, the virus decides the timelines in the models. However, the models can also change based on our decisions and behavior. It is our role as surgeons, to make every effort to bend the curves against the virus’ will.
Core tip: Severe acute respiratory syndrome coronavirus has become a critical challenge to global health with immense economic and social disruption. Coronavirus disease 2019 (COVID-19) pandemic has become a “stress test” to evaluate the capacity and resilience of our surgical community in dealing with the challenges imposed to our health system and society. Some aspects of the COVID-19 outbreak are relevant to surgical practice and modifying practice can reduce risk to patients and staff alike. We discuss considerations during the pre-operative and post-operative periods as well as the utility and limitations of COVID-19 testing. Computed tomography scan is currently the most sensitive modality for COVID-19, and while reverse transcription polymerase chain reaction is highly specific, sensitivity is considerably viable based on multiple factors.
- Citation: Grubic AD, Ayazi S, Zebarjadi J, Tahmasbi H, Ayazi K, Jobe BA. COVID-19 outbreak and surgical practice: The rationale for suspending non-urgent surgeries and role of testing modalities. World J Gastrointest Surg 2020; 12(6): 259-268
- URL: https://www.wjgnet.com/1948-9366/full/v12/i6/259.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i6.259
One-hundred years after the 1918-19 H1N1 flu pandemic and 10 years after the 2009 H1N1 flu pandemic, another respiratory virus has now insinuated itself into the human population. This new virus is not as virulent as the causative pathogen of 1918 flu that infected a quarter of the world's population and resulted in 50 million deaths. However, this modern virus has become a critical challenge to global health with immense economic and social disruption. The 200-nanometer virus has tested the capacity of almost every continent, country and the facility of health care systems in dealing with a large scale, multifaceted problem.
With the arrival of coronavirus disease 2019 (COVID-19) in the United States[1], numerous governmental agencies as well as professional societies have issued guidelines to healthcare systems and medical providers. Of these recommendations, temporarily postponing elective surgeries is particularly relevant to surgeons. The measure was first proposed by the Centers of Disease Control on February 29th 2020[2]. Similar guidance was mirrored by the American College of Surgeons on March 13th, 2020 with additional recommendations for triaging elective cases[3]. On the same day, the United States Surgeon General echoed the recommendation from the American College of Surgeons and urged hospitals and healthcare systems to consider suspending elective surgical procedures during the outbreak of COVID-19. Shortly thereafter, Centers for Medicare and Medicaid Services released formal guidelines for surgical triage during the COVID-19 pandemic[4]. Clear priority was given to emergent/urgent cases, with deferring semi-urgent cases to lower-risk settings, and completely postponing non-urgent, elective procedures. Based on this, most hospital systems underwent large-scale rescheduling as a component of COVID-19 action plans. As of March 20th, 2020 only three United States health systems were still performing elective procedures[5].
These efforts are all part of a broader concept commonly referred to as “flattening the curve”. By limiting interposal contact, especially high-risk close contact, the population can assert some control over disease incidence. Although continued transmission may be inevitable, reducing the rate by which the virus spreads prevents catastrophic burden on the healthcare system. If adequate personnel and resources are available, patients can be properly cared for, thus reducing morbidity and mortality. Ethical dilemmas regarding triage and rationing are also abated. Additionally, reducing transmission provides more time for the development of potential therapies and vaccines.
In this article we present a surgical case that highlights the challenges inherent in COVID-19 diagnosis and then review salient aspects of the COVID-19 outbreak that are relevant to surgical practice. The emphasis is on the considerations during the pre-operative and post-operative periods and the utility and limitations of COVID-19 testing.
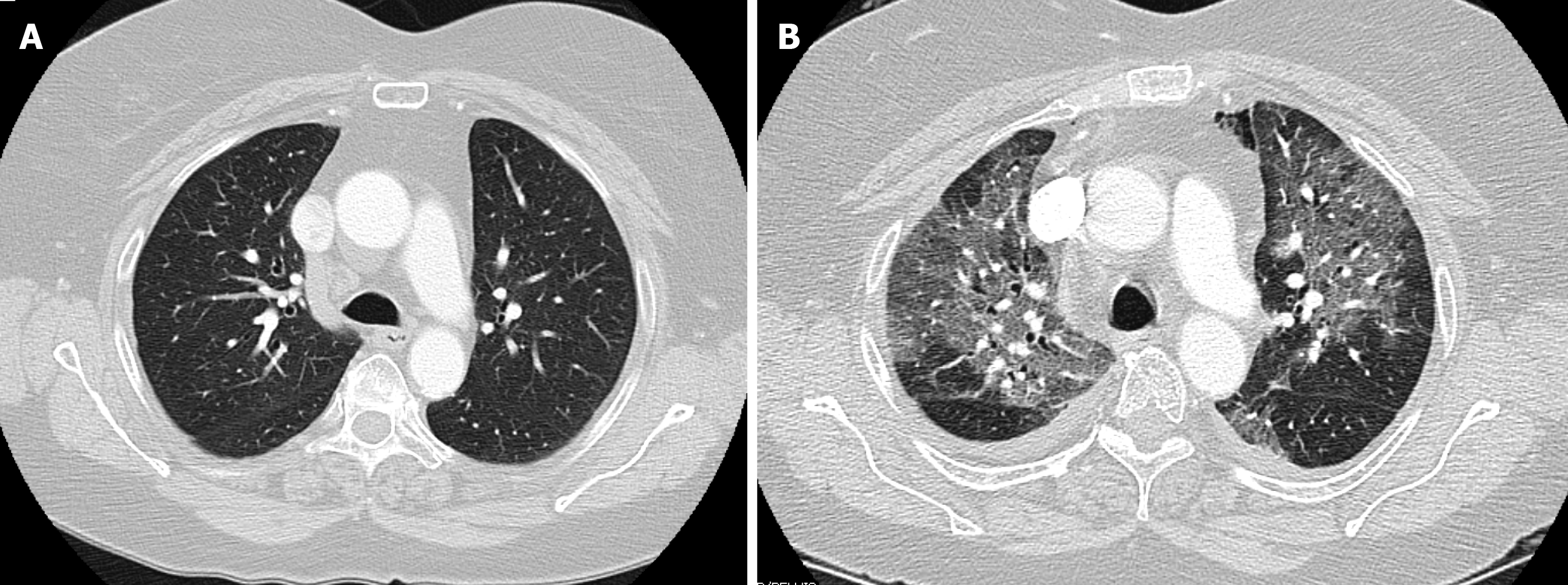
The patient is a 66-year old female with history of prior hiatal hernia repair with Nissen fundoplication, who presented to our practice with recurrent heartburn, dysphagia, and chest pain. She reported a history of mild chronic obstructive pulmonary disease however pre-operative computed tomography (CT)-scan showed no emphysematous or interstitial changes (Figure 1A), and pulmonary function testing was normal. Her surgical workup revealed a recurrent paraesophageal hernia (intact fundoplication), moderately delayed gastric emptying, and mildly impaired esophageal motility. In the time leading up to surgery, the patient reported feeling generally well and did not endorse any sick contacts, or recent travel. On March 12th, 2020 she underwent elective laparoscopic revision paraesophageal hernia repair with Nissen fundoplication, as well as pyloroplasty. On post-operative day 1, an upper-gastrointestinal contrast study was negative for leak or obstruction, her diet was advanced to liquids, and she was subsequently discharged per routine protocol.
On March 16th, 2020 (post-operative day 4), she presented to the emergency room with low-grade fever, chills, chest pains, and shortness of breath. She was noted to be febrile and hypoxic on room air with an oxygen saturation of 83%. CT angiogram showed extensive, bilateral pulmonary ground glass opacities (Figure 1B). White blood cell and C-reactive protein were normal. She rapidly deteriorated requiring endotracheal intubation, and was admitted to the intensive care unit (ICU) for acute respiratory distress syndrome. Initial rapid influenza testing was negative and echocardiogram was not suggestive of heart failure. Infectious workup was negative for streptococcus, legionella, mycoplasma, chlamydia, Bordetella, and aspergilla species. Respiratory virus polymerase chain reaction (PCR) testing could not detect any common subtypes of adenovirus, coronavirus, human metapneumovirus, rhinovirus, enterovirus, influenza, parainfluenza, or respiratory syncytial virus. Bronchoscopy with broncheoalveolar lavage (BAL) and blood cultures remained without growth of bacterial or fungal organisms. Specific PCR testing for COVID-19 was eventually obtained, however this returned negative.
During her early ICU course she required 100% FiO2 and high positive end-expiratory pressures. Over the next week, patient’s hypoxia gradually improved, she was able to be liberated from mechanical ventilation, and was transferred out of the intensive care unit. As of March 28th, 2020 she remains on the regular nursing floor and continues to improve, but with persistent respiratory symptoms.
Our health network is based in Pittsburgh, Pennsylvania but serves multiple counties in western Pennsylvania, eastern Ohio, and northern West Virginia. The patient in this case resides in a region neighboring Pittsburgh. The surgery was performed at a community hospital near Pittsburgh. In the weeks prior to surgery on March 13th, 2020, the patient did not travel more than several miles away from her home, and her family and close contacts did not report illness or recent travel. March 14th, 2020 marked the first confirmed cases of COVID-19 in the county where the surgery occurred, and as of March 28, there have been 158 confirmed cases in this county with one death reported in the hospital that the patient was treated. The extensive evaluation in this patient did not provide any explanation for development of her acute respiratory distress syndrome. Although, COVID-19 PCR testing for this patient was negative, the highly suggestive presentation in the setting of a growing pandemic with no other identifiable etiology questions the reliability of this test.
Influenza viruses have unusual genomic properties that enables rapid spread resulting in, ubiquitous infection on a global scale. Modern medicine has witnessed three influenza pandemics in the last century (1918, 1957 and 1968). In the past few years, every expert on influenza warned about another inevitable pandemic. This pandemic started with identification of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as the causative agent from a cluster of pneumonias in the Hubei providence of China in December 2019. There number of cases worldwide continues to grow and the United States is now the global epicenter of this pandemic. This has forced surgical providers in our country to make adjustments in their practice to minimize the impact of disease and reserve resources for the high clinical demand of the outbreak. We review some aspects of the COVID-19 pandemic that will be relevant to surgeons and surgical practice in this context.
Aerosol and fomite transmission are the main modes of spreading SARS-CoV-2, but studies on similar airborne organisms such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), and growing evidence on SARS-CoV-2, supports the idea of COVID transmission through other routes. There is evidence that these two recent coronavirus diseases can be transmitted through the fecal-oral route[6,7]. Studies reported the presence of SARS-CoV-2 in anal or oral swabs obtained from patients, therefore raising the possibility of fecal-oral transmission[8,9].
Blood may not be an established route for transmission of COVID-19. SARS-CoV-1 the closest related virus strain to the pathogen of COVID-19 is now also considered to be a transfusion transmitted-disease[11]. Studies reported the presence of SARS-CoV-2 in blood samples of a small portion of patients[11]. As such, blood donors are now screened for the COVID-19 in Wuhan, China.
Large respiratory droplets are considered to be the primary transmission vector of the SARS-CoV-2 virus. These droplets can take a finer, aerosolized form with manipulation of the upper aerodigestive tract, thus increasing the danger of spread. This primary mode of transmission has caused increased concern for several procedures including endotracheal intubation, bronchoscopy, and upper endoscopy which have been deemed high risk. Upon entry into the airway, the virus has been found to bind to the angiotensin converting enzyme-2 (ACE2) receptor in the lower respiratory tract[12]. ACE2 receptors are also located in the enterocytes of the small intestine which is considered to be an explanation for digestive symptoms in COVID-19 patients[13,14]. Several studies have highlighted the presence of virus in the feces of COVID-19 patients, and now fecal-oral transmission has also been proposed as a mechanism of spread[8,9], which may profoundly impact underdeveloped countries.
One major concern for surgical practices is COVID-19 transmission during the asymptomatic incubation period. The incubation period for COVID-19 may range between 2-14 d, with a median time of approximately 5 d[10]. If these patients undergo surgery during this period they can infect hospital staff and contaminate the physical space of the operating room and multiple other areas of the hospital. Because conventional personal protection equipment (PPE) does not provide adequate protection against COVID-19, the surgical team is at risk. A patient undergoing surgery in a hospital in Wuhan infected 14 health-care workers even before the manifestation of symptoms[16]. In another report, a patient who travelled from Shanghai to attend a business meeting in Munich, Germany was asymptomatic until her flight back to China. However, two of this patient's close contacts and another two patients attending the meeting with no close contact were found to be infected with COVID-19. Three of these patients became symptomatic before the result of PCR test of the index patient was returned positive[17].
The “serial interval” is an epidemiologic term that refers to the time between successive cases in a chain of transmission of an infectious disease. The serial interval of COVID-19 is close to or shorter than its median incubation period. This suggests that a substantial proportion of secondary transmission may occur prior to illness onset[18]. Data obtained from the Diamond Princess cruise ship in which all quarantined passengers were screened, showed that 619 (17%) of passengers tested positive, and over half of cases were asymptomatic at the time of diagnosis[19].
Due to the rapid progression of the pandemic, suspected (clinical) and confirmatory (etiology) diagnostic methods have been employed. The primary clinical diagnostic modalities for COVID-19 have been clinical history (potential exposure and symptoms) and CT scanning. Nasopharyngeal swab reverse transcription PCR (RT-PCR) is the most commonly employed confirmatory test, while BAL is the preferred source in an intubated patient. Serum RT-PCR and viral gene sequencing has also been used. Recently there is also increased attention to the value of rectal and stool RT-PCR in patients with and without digestive symptoms.
There is a report of a symptomatic patient with a CT finding of bilateral “ground-glass” pulmonary opacities. This patient was found to have a negative SARS-CoV-2 RT-PCR on pharyngeal swab sample. During 7 d after admission, the test was negative on two more pharyngeal swabs and two sputum samples. The RT-PCR test on a fecal sample was however positive. This case highlights the limitations of current testing and also shows that the virus can proliferate in the digestive tract and therefore allows potential fecal-oral transmission[20].
Early studies from China have identified the most common CT finding to be bilateral pneumonia. More specifically bilateral ground-glass opacities with peripheral distribution were seen in the majority of patients[21,22]. CT scans in critically ill ICU patients have noted consolidations, but less severe cases may not have this finding. Nodules, cavitations, lymphadenopathy, or pleural effusions were not typically observed in any COVID-19 patients[23]. Generally speaking, similar radiographic characteristics were observed in previous studies of SARS patients. The sensitivity of CT scan in detection of SARS-CoV-2 is shown in Table 1.
CT scan findings have been shown to correlate in up to 92%-98% of PCR positive COVID-19 patients in China[24,25]. Despite this Chung et al[26] showed that CT scanning was not suggestive of viral pneumonia in 3 of 21 during the early course of their disease. In a study of 51 COVID-19 patients, the initial RT-PCR was 71% positive on the initial test, with the remaining 29% becoming positive between 1 and 7 days later in those who were eventually confirmed to have the disease[25].
Several other studies have shown variable nasopharyngeal RT-PCR sensitivities. In a study of 1070 specimen collected from 205 COVID-19 patients (ages 5-67), BAL showed the highest sensitivity of 93% (14/15), while sputum, nasal swab, bronchoscopic brunshings, pharyngeal swab, feces, and blood sampling showed positive rates of 72% (72/104), 63% (5/8), 46% (6/13), 32% (126/398), 29% (44/153), and 1% (3/307) respectively[12]. Variability is likely due to multiple factors including viral load, sampling techniques, as well as the heterogeneity in specimen processing. Guo and colleagues investigated the role of serum humoral response in COVID-19 testing and found that after 5.5 d from symptom onset, serum enzyme-linked immunosorbent assay (ELISA) for IgM was positive in 75.6% of RT-PCR confirmed and 93.1% of suspected cases. The combination of serum RT-PCR and IgM ELISA had a sensitivity of 98.6%[27]. Lan et al[28] presented 4 healthcare workers who recovered from COVID-19 with 2 consecutive negative RT-PCR tests prior to discharge, but were again positive by RT-PCR 5-13 d after quarantine period had ended. In a study of 10 children with confirmed COVID-19 by nasopharyngeal RT-PCR, 8 had positive rectal RT-PCR tests at the time of confirmatory diagnosis. Additionally, all 8 patients exhibited positive rectal swab PCR even after nasopharyngeal RT-PCR testing was negative[29]. Table 2 shows the detection rate of SARS-CoV-2 based on the types of specimens and laboratory methods used. These studies further highlight our limited, but rapidly developing understanding of the viral pathophysiology, and that multiple tests are currently needed to improve detection. Table 3 summarizes the variation in reported sensitivity of different modalities used for diagnosis of SARS-CoV-2.
| Ref. | Biochemistry method | Specimen source | Detection rate (%) | n | Collection time from symptom onset |
| Wang et al[12], 2020 | PCR | BAL | 93 | 15 | |
| Sputum | 72 | 104 | |||
| Nasopharygeal | 63 | 8 | |||
| Brushings | 46 | 46 | |||
| Oropharyngeal | 32 | 398 | |||
| Fecal | 29 | 153 | |||
| Xiao et al[15], 2020 | PCR | Feces | 53 | 73 | |
| Xie et al[22], 2020 | PCR | Pharyngeal | 97 | 167 | |
| Fang et al[25], 2020 | PCR | Sputum Oropharyngeal | 71 | 51 | 3 +/- 3 d |
| Ai et al[24], 2020 | PCR | Pharyngeal | 59 | 1014 | |
| Guo et al[27], 2020 | PCR | Serum | 52 | 140 | mean 5.5 d |
| IgM ELISA1 | Serum | 93 | 58 | mean 5.5 d | |
| IgM ELISA2 | Serum | 75 | 82 | mean 5.5 d |
Health care providers who are on the front lines of the fight against COVID-19 are most at risk. Frequent contact and exposure to a higher viral load not only increases their risk of infection, but of getting the most severe form of disease. In Italy, 8.3% of the infected population was health care personnel. This ratio was 3.8% in China, of whom 14.8% were classified as severe or critical cases[30]. Physicians who are performing any procedures are at an even higher rate of infection. Of Italian physicians treating COVID-19 patients, 61 have died of the disease and this list continues to grow[31].
As surgeons performing more invasive procedures, theoretically they are even at higher risk. Since COVID-19 is primarily transmitted through aerosol, specific surgical specialists such as ear, nose and throat, thoracic, foregut surgeons and surgical endoscopists are at higher risk of contracting the disease. In fact, there are reports from around the globe of several surgeons, across multiple specialties, who died or developed very severe disease[32].
Frequent handling of sharp instruments or use of power tools, hammers and other implements during certain operations can lead to spread of infectious material and contaminate the operating room. Experimental studies show SARS-CoV-2 remains on surfaces for up to 72 h[33]. Use of high-powered electric tools can turn infectious tissue or bone to airborne particles, facilitating its transmission.
Alister Hart, Chair of Orthopedic Surgery at University College London is working with his colleagues to understand the impact of COVID-19 in a surgical setting. He has expressed concern that if this matter doesn’t get addressed appropriately, the operating rooms will soon be turned to “viral labs in a wind tunnel”. The combination of power tools, high-velocity blood splatter and ventilation systems can produce this “viral wind tunnel” that he is referring to. This will result in a catalog of casualties among the operating room staff and patients[34].
In addition to the direct surgical implications, there are significant anesthesia considerations necessary for operating during the COVID-19 pandemic. Intubation in of itself converts the primary droplet transmission of the virus to a higher risk, aerosolized vector. Viral particles can be widely disseminated, especially in difficult to clean locations. Elective intubations in particular may place unnecessary risk to patients and staff, as well as burden the already inadequate supply of PPE. A minimum of a fitted, N-95 mask coupled with eye protection, cap, and standard contact protective coverings are recommended during intubation of patients with suspected or confirmed COVID-19. A powered air-purifying respirator has been proposed to provide improved protection compared to an N-95 grade mask, during similar viral outbreaks, but the improper fitting of N-95 masks may confound this observation. Negative pressure rooms should be considered for intubation if available and safely feasible. While standard liquid-resistant surgical masks provide suboptimal aerosol protection, they should be utilized if N-95 mask or powered air-purifying respirators are unavailable. Surgical masks may also be placed over N-95 masks to minimize contamination for N-95 re-use. There is also the theoretical concern that mechanically ventilating patients who are actively shedding virus can contaminate the ventilator circuit including the anesthesia machine itself. The Anesthesia Patient Safety Foundation released recommendations to reduce inoculation of the internal circuit including use of a heat and moisture exchanging filter between the external circuit, which removes 99.97% of particles 0.3 microns or greater in size. Overall these concepts closely mirror measures taken from prior work with tuberculosis, influenza, SARS, and MERS outbreaks[35].
Immunological function has been found to undergo a myriad of changes during the perioperative time period. These alterations affect both the innate and adaptive systems, and are further confounded in patients with conditions requiring pharmacological immunosuppression, malignancy, and nutritional deficiencies, as well as underlying cardiac, pulmonary, hepatic, and renal impairments. Limited published data on COVID-19 patients who underwent urgent on non-urgent surgeries shows an unexpectedly high morbidity and mortality[36].
The early post-operative period will exhibit a pro-inflammatory state characterized by upregulation of innate inflammatory mediators including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α. This initial phase is soon followed by compensatory immunosuppression with production of IL-4, IL-10, sTNFR1, IL-1 receptor antagonist (IL-1Ra), transforming growth factor-β. The anti-inflammatory state is further prolonged by cellular functions of dendritic and T-regulatory (Treg) cells. The intensity and duration of immunosuppression is proportional to the amount of surgical trauma and presence of complications[37].
In a study by Gentile et al[38] investigating physiological changes in ICU patients with surgical complications, they identified patients who exhibited significant organ dysfunction but did not meet criteria of multiorgan dysfunction syndrome. These patients exhibited low-grade inflammation coupled with prolonged immunosuppression, protein catabolism, muscle wasting, and impaired wound healing. They referred to this as persistent inflammation, immunosuppression and catabolism syndrome. Ultimately patients with persistent inflammation, immunosuppression and catabolism syndrome were found to be significantly higher risk for nosocomial infections as well as morbidity[38].
Proponents of continuing elective surgeries have cited multiple reasons for the need for elective surgeries including increased morbidity and mortality of patients whose procedures were delayed, as well as the socioeconomic ramifications on the health system and its employees. Restriction on non-urgent hospital utilization is however proven to be a safe public health strategy in the setting of infectious outbreaks. The most comparable experience, although in a much smaller scale is the policy implemented during the 2003 outbreak of SARS in Toronto, Canada. To limit the spread of SARS and allocate the hospital resources to SARS patients, a provincial emergency was declared and widespread restrictions were imposed, restricting admissions to only urgent cases. Studies showed that mortality, readmission, and complication rates did not change for any of the non-SARS medical or surgical conditions during or after these restrictions. Of note, a large decrease in elective cardiac procedures (50%-65%) and moderate decrease (11%-37%) in invasive cardiac procedures for acute myocardial infarction were observed during this time frame. However, these decreases did not adversely affect the outcomes of acute myocardial infarctions[39].
Our surgical community should also be prepared for handling the high demand for surgical care during the post-outbreak period as the result of the current suspension of non-urgent care. One study looked at the impact of 2003 SARS epidemic on the volume of a colorectal surgery practice. In this study from Prince of Wales Hospital in Hong Kong, although there was no formal order for restriction of the non-urgent care during the outbreak, there was a 52% decrease in outpatient attendance, 32% in surgical procedures and 48% in colonoscopies during the outbreak. Major emergency procedures and cancer resections were unaffected. This led to a subsequent 200% increase in outpatient appointments and an increase in waiting time for colonoscopy by 3-9 wk in the post outbreak period. The waiting time for minor elective colorectal surgery was extended by 5 mo[40]. It would be intuitive conclude that increased wait times following a pandemic may affect outcomes. A hypothetical example of this may be delay in diagnosis of malignancy on screening or surveillance endoscopy, but to our knowledge there is no prior data to support this notion.
On March 25, 2000, American College of Surgeons released the guidelines for emergency general surgery in COVID-19 positive patients or those at high clinical suspicion for COVID infection. The goal is to provide timely surgical care when indicated while optimizing patient care resources such as PPE, hospital and intensive care unit beds and ventilators and also protect the health care team. The guideline favors non-operative management such as antibiosis, interventional radiology techniques when it’s feasible and safe. Therefore in conditions such as suspected bowel perforation, intestinal ischemia, closed loop obstruction, or obstruction secondary to incarcerated hernia emergent surgery should proceed[41].
Concern has been expressed about the shortage of providers in the care of COVID patients in the intensive care setting. There are at least 30000 surgeons in the United States based on 2018 national estimates[42]. Critical care has always been a cornerstone of surgical training. Surgical residents now have several months of training dedicated to the ICU and trauma, as well as continuous exposure on nearly every surgical service. The Accreditation Council for Graduate Medical Education also recently increased the number of required critical care cases for residents. During the 2018-2019 academic year there were 8844 active general surgery residents with 284 trainees in surgical critical care programs[43]. This does not include the hundreds of additional trainees in other surgical residencies and fellowships who also receive substantial critical care training. With growing concern regarding physician shortages and provider burnout in regions of high COVID-19 prevalence, surgeons represent a formidable force to assume care of COVID-19 patients, if and when the need arises.
The COVID-19 pandemic is a “stress test” to evaluate the capacity and resilience of our surgical community in dealing with the challenges imposed to our health system and society. The focus of media is on the predictive epidemiologic curves and models. While epidemiologists and infectious disease physicians are the cornerstone in the fight against COVID-19, our article highlights the significant role that the surgeons should have in this pandemic. Many surgical procedures increase the risk of transmission due to their aerosol generating potential. Routine elective surgeries also place a considerable burden on available PPE supplies and ventilators which may be utilized for the care of COVID-19 patients. The presented case demonstrates the potentially devastating risks incurred by patients and healthcare workers during a viral pandemic, even despite successful routine practices. As recently pointed out by Dr. Anthony Fauci, the virus decides about the timelines in the models. However, the models can also change based on our decisions and behavior. The actions of healthcare systems and providers are a major factor in “flattening the curve”, and it is our role as surgeons, to make every effort to bend the curves against the virus’ will.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Desjardins MR S-Editor: Wang JL L-Editor: A E-Editor: Qi LL
| 1. | Desjardins MR, Hohl A, Delmelle EM. Rapid surveillance of COVID-19 in the United States using a prospective space-time scan statistic: Detecting and evaluating emerging clusters. Appl Geogr. 2020;118:102202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Control and Prevention. Interim Guidance for Healthcare Facilities: Preparing for Community Transmission of COVID-19 in the United States. Available from: www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/guidance-hcf.html. |
| 3. | American College of Surgeons. COVID-19: Recommendations for Management of Elective Surgical Procedures. Available from: https://www.facs.org/covid-19/clinical-guidance/elective-surgery. |
| 4. | United States, Congress, Centers for Medicare and Medicaid Services. CMS Adult Elective Surgery and Procedures Recommendations: Limit All Non-Essential Planned Surgeries and Procedures, Including Dental, until Further Notice, 15 Mar 2020. Available from: http://www.cms.gov/files/document/31820-cms-adult-elective-surgery-and-procedures-recommendations.pdf. |
| 5. | O'Donnell J. Elective Surgeries Continue at Some US Hospitals during Coronavirus Outbreak despite Supply and Safety Worries. USA Today. [published 21 March 2020]. Available from: http://www.usatoday.com/story/news/health/2020/03/21/hospitals-doing-elective-surgery-despite-covid-19-risk-short-supplies/2881141001/. |
| 6. | Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767-1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1737] [Cited by in RCA: 1787] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 7. | Goh GK, Dunker AK, Uversky V. Prediction of Intrinsic Disorder in MERS-CoV/HCoV-EMC Supports a High Oral-Fecal Transmission. PLoS Curr. 2013;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3822] [Article Influence: 764.4] [Reference Citation Analysis (1)] |
| 9. | Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1120] [Cited by in RCA: 1127] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 10. | Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172:577-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4286] [Cited by in RCA: 3370] [Article Influence: 674.0] [Reference Citation Analysis (1)] |
| 11. | Chang L, Yan Y, Wang L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus Med Rev. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 388] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 12. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92:595-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 443] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 14. | Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, Hamie QM, Meier CF, Hunziker S, Forras-Kaufmann Z, Kuyumcu S, Fox M, Schwizer W, Fried M, Lindenmeyer M, Götze O, Verrey F. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 15. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1995] [Article Influence: 399.0] [Reference Citation Analysis (1)] |
| 16. | Chang, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med. 2020;8:e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 388] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 17. | Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2799] [Cited by in RCA: 2491] [Article Influence: 498.2] [Reference Citation Analysis (0)] |
| 18. | Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 19. | Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1527] [Cited by in RCA: 1472] [Article Influence: 294.4] [Reference Citation Analysis (0)] |
| 20. | Chen L, Lou J, Bai Y, Wang M. COVID-19 Disease With Positive Fecal and Negative Pharyngeal and Sputum Viral Tests. Am J Gastroenterol. 2020;115:790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;200490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1106] [Cited by in RCA: 952] [Article Influence: 190.4] [Reference Citation Analysis (1)] |
| 22. | Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020;200343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1213] [Cited by in RCA: 1211] [Article Influence: 242.2] [Reference Citation Analysis (1)] |
| 23. | Ng M, Lee E, Yang J, Yang F, Li X, Wang H, Lui M, Lo C, Leung B, Khong P, Hui C, Yeun K, Kuo M. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol Cardiothorac Imaging. 2020;2. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 474] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 24. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;200642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3285] [Article Influence: 657.0] [Reference Citation Analysis (0)] |
| 25. | Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;200432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2088] [Cited by in RCA: 1873] [Article Influence: 374.6] [Reference Citation Analysis (2)] |
| 26. | Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1827] [Cited by in RCA: 1696] [Article Influence: 339.2] [Reference Citation Analysis (0)] |
| 27. | Guo L, Ren L, Yang S, Xiao M, Chang, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang Q, Xu S, Zhu H, Xu Y, Jin Q, Sharma L, Wang L, Wang J. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020;ciaa310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1043] [Cited by in RCA: 1085] [Article Influence: 217.0] [Reference Citation Analysis (0)] |
| 28. | Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 827] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 29. | Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502-505. [PubMed] |
| 30. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11507] [Article Influence: 2301.4] [Reference Citation Analysis (0)] |
| 31. | Zosia C; Centers for Disease Control and Prevention. More Than 60 Doctors in Italy Have Died in COVID-19 Pandemic. Medscape Medical News. [published 30 March 2020]. Available from: https://www.medscape.com/viewarticle/927753. |
| 32. | Ing EB, Xu AQ, Salimi A, Torun N. Physician Deaths from Corona Virus Disease (COVID-19). 2020 Preprint. Available from: medRxiv:2020.04.05.20054494. . [DOI] [Full Text] |
| 33. | van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5894] [Cited by in RCA: 5666] [Article Influence: 1133.2] [Reference Citation Analysis (0)] |
| 34. | Raphael T. Why Surgeons Don't Want to Operate Right Now. Bloomberg. [published 24 March 2020]. Available from: https://www.bloomberg.com/opinion/articles/2020-03-24/the-coronavirus-crisis-is-putting-surgeons-at-risk-too. |
| 35. | Zucco L, Levy N, Ketchandji D, Aziz M, Ramachandran S. Perioperative Considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. [published 12 February 2020]. Available from: https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. |
| 36. | Aminian A, Safari S, Razeghian-Jahromi A, Ghorbani M, Delaney CP. COVID-19 Outbreak and Surgical Practice: Unexpected Fatality in Perioperative Period. Ann Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 37. | Dąbrowska AM, Słotwiński R. The immune response to surgery and infection. Cent Eur J Immunol. 2014;39:532-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 552] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 39. | Stukel TA, Schull MJ, Guttmann A, Alter DA, Li P, Vermeulen MJ, Manuel DG, Zwarenstein M. Health impact of hospital restrictions on seriously ill hospitalized patients: lessons from the Toronto SARS outbreak. Med Care. 2008;46:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Bradford IM. Tales from the frontline: the colorectal battle against SARS. Colorectal Dis. 2004;6:121-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | American College of Surgeons. COVID-19 Guidelines for Triage of Emergency General Surgery Patients. Available from: https://www.facs.org/covid-19/clinical-guidance/elective-case/emergency-surgery. |
| 42. | U.S. Bureau of Labor Statistics. 29-1067 Surgeons. [published 29 March 2019]. Available from: www.bls.gov/oes/2018/may/oes291067.htm#nat. |
| 43. | Accreditation Council for Graduate Medical Education. ACGME Data Resource Book Academic Year 2018-2019. Illinois: Accreditation Council for Graduate Medical Education, 2019. |