Published online Mar 27, 2020. doi: 10.4240/wjgs.v12.i3.93
Peer-review started: October 17, 2019
First decision: December 4, 2019
Revised: December 19, 2019
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 27, 2020
Processing time: 134 Days and 1.1 Hours
Laparoscopic cholecystectomy (LC) is a minimally invasive procedure, often performed by surgical residents (SRs). Fluorescence cholangiography (FC) enables real-time identification of biliary anatomy.
To investigate the benefit of FC for enhancing SRs’ identification skills.
Prospective data was collected from January 2018 to June 2018 at our hospital. The study cohorts were the SRs (study group, n = 15) and the surgical staff (SS; control group, n = 9). Participants were assigned to watch videos of LCs with FC from five different patients who had gallbladder disease, and identify structures in the video clips (including cystic duct, common bile duct, common hepatic duct, and cystic artery), first without FC, and then with FC.
In the without-FC phase, the overall misidentification rate by SRs (21.7%) was greater than that of the SS (11.8%; P = 0.018), However, in the FC phase, the two groups did not significantly differ in misidentification rates (23.3% vs 23.3%, P = 0.99). Paired-structure analysis of the without-FC and with-FC phases for the SR group found a significantly higher misidentification rate in the without-FC phase than the with-FC phase (21.9% vs 10.9%; P < 0.01). However, misidentification rates in the with-FC phase did not significantly differ between SRs and SS.
FC enhanced identification skills of inexperienced surgeons during LC compared with conventional training. Combined with simulation-based video training, FC is a promising tool for enhancing technical and decision skills of trainees and inexperienced surgeons.
Core tip: Laparoscopic cholecystectomy (LC) is often performed by surgical residents. Avoiding bile duct injury (BDI) is a critical aspect of learning to perform this procedure safely. Landmark misperception is a high-risk factor for bile duct injury. Fluorescence cholangiography (FC) enables real-time identification of biliary anatomy during LC. We studied changes in biliary identification skills among surgical residents when FC was applied during LC, with staff surgeons as the control group. FC is a promising tool for enhancing biliary identification skills of surgeons-in-training.
- Citation: Rungsakulkij N, Thewmorakot S, Suragul W, Vassanasiri W, Tangtawee P, Muangkaew P, Mingphruedhi S, Aeesoa S. Fluorescence cholangiography enhances surgical residents’ biliary delineation skill for laparoscopic cholecystectomies. World J Gastrointest Surg 2020; 12(3): 93-103
- URL: https://www.wjgnet.com/1948-9366/full/v12/i3/93.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i3.93
The adoption of minimally invasive surgery has significantly affected training of surgical residents (SRs). Laparoscopic cholecystectomy (LC) is an minimally invasive surgery procedure that is performed by SRs in nearly 50% of cases[1]. According to the Accreditation Council of Graduated Medical Education, LC is a core-level surgery, of which a graduate should possess significant knowledge and procedural competency[2]. In our center, where the general residency training is based on Accreditation Council of Graduated Medical Education standards, LC is a core procedure that SRs are required to master, which includes avoiding bile duct injury (BDI) or other major serious complications[3]. Although various techniques and tools described in the literature and expert consensus can facilitate trainees’ performance[4,5], achieving a critical view of safety (CVS), proposed by Strasberg et al[6], is widely regarded as the most crucial step. It has three criteria, including (A) dissecting and clearing the hepatocystic triangle of fat and fibrous tissue; (B) identifying two, and only two, structures [cystic duct (CD) and cystic artery] entering the gallbladder; and (C) dissecting the gallbladder off and away from the liver, exposing at least the bottom third of the cystic plate[5,7,8].
Way et al[9], reported that the principal risk factor associated with BDI during LC was misperception, rather than errors of skill, knowledge, or judgement. The surgeon’s experience is reportedly a risk factor for BDI[10]. Optical or real-time surgery is being increasingly reported in the literature. Fluorescence cholangiography (FC) enables real-time identification of biliary anatomy during dissection of Calot’s triangle[11,12]. FC involves administering indocyanine green (ICG) by intravenous injection before surgery. ICG is taken up by the liver, then excreted exclusively in the bile. The excitation of protein-bound ICG by near-infrared light causes it to fluoresce, thereby delineating components of the biliary system for the surgeon. FC is a feasible, low-cost and effective imaging modality[13]. Conrad et al[4], reported that FC may prove beneficial in preventing BDI. Recently, FC is considered as one of the supporting imaging techniques for achieving safe LC in the rationale of FC would reduce the misinterpretation rate of the biliary tree[14]. Thus, whereas the benefits of enhanced visualization through FC would be limited for experienced surgeons, its identification benefits for less experienced surgeons might be very helpful. To our knowledge, no studies have been conducted on FC use during LC for SRs and less-experienced surgeons. Thus, the aim of this pilot study is to investigate the benefit of FC for enhancing the abilities of SRs to identify important structures during LC, compared with experienced surgeons.
Prospective data were collected between October 2018 to March 2019 at Department of Surgery, Faculty of Medicine Ramathibodi Hospital, Bangkok, Thailand. Inclusion criteria for the SR group were (A) was an in-training general surgery resident during that period; (B) had been first surgeon in fewer than 10 LC procedures; and (C) had not worked with FC before. Inclusion criteria for the control group were surgical staff members who had performed at least 50 LC procedures and had not previously performed an LC with FC.
A standardized setup was applied for all procedures. ICG was injected intravenously immediately after induction phase of anesthesiology (about 15 min before skin incision), at a dose of 1 mL of 10 mL dilution of a 2.5 mg/mL stock solution. The patient was positioned supine with the surgeon standing on the left side of the patient. A zero-degree telescope (10 mm diameter, 31 cm length, Karl Storz) was inserted through a 11-mm subumbilical trocar. Two or three further trocars were inserted with a 5-mm epigastric port, followed by a 5-mm port in the right upper quadrant. A 5-mm port was additionally inserted in the right lumbar region of the abdomen if a difficult situation was encountered during surgery. The dissection of Calot’s triangle was routinely performed. FC was periodically applied during surgery, but was always applied before dissecting Calot’s triangle and after dissecting Calot’s triangle. All procedures were video-recorded.
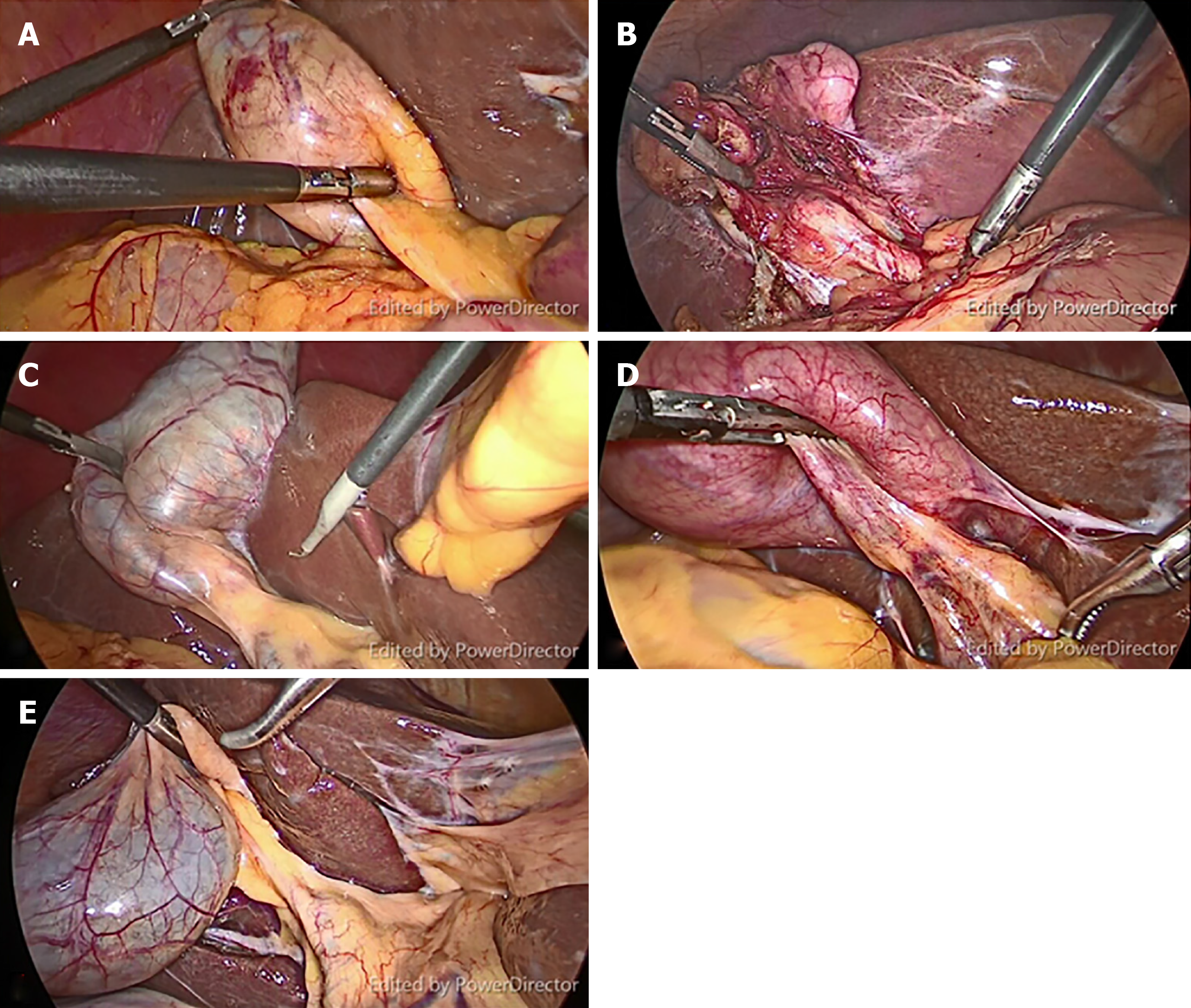
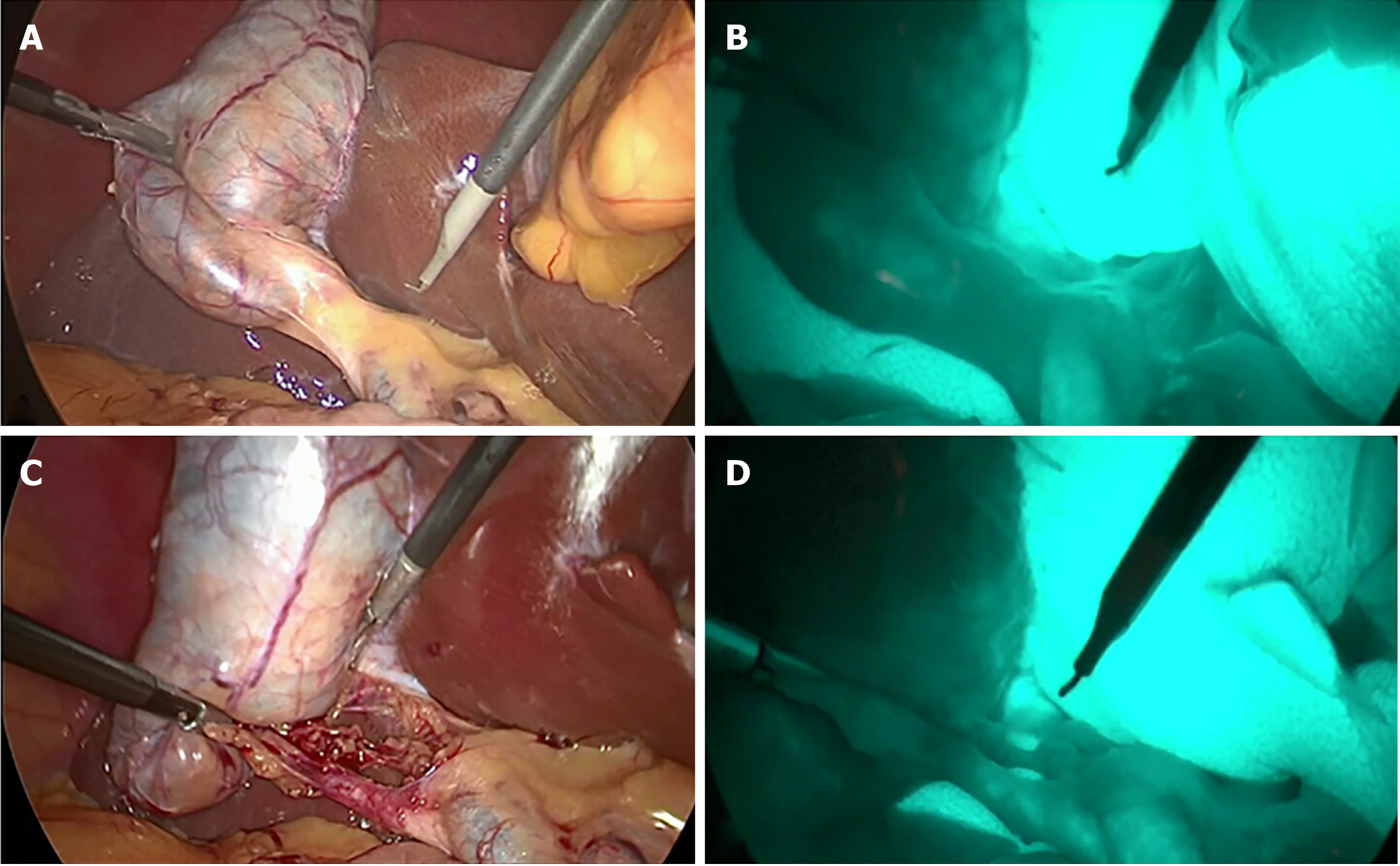
We collected video-recordings from five patients with different gallbladder diseases who underwent LC with FC. Their diagnoses were (A) gallbladder polyp in an obese patient; (B) history of biliary pancreatitis; (C) symptomatic gallstone; (D) acute cholecystitis; and (E) gallbladder polyp in a non-obese patient (Figure 1). Inform consent was applied to all populations as standard of care. All procedures were performed by the same surgeon (Rungsakulkij N). There was no BDI in these patients. The unedited video-recordings were analyzed for their quality by a blinded assessor. Consequently, each video-recording was divided into four short clips (for a total of 20 clips from the 5 patients) by the blinded assessor into the following segments: (A) Before dissecting Calot’s triangle without FC; (B) Before dissecting Calot’s triangle with FC; (C) After dissecting Calot’s triangle without FC; and (D) After dissecting Calot’s triangle with FC (Figure 2).
The correct identifications in each video clip were reviewed by two experienced surgeons. They defined “identified structures” as structures which one or both reviewers could identify from the video clips; and “unidentified structures” as structures which these two reviewers could not clearly identify; the latter were excluded from the scoring system.
For the examinations, first, the mechanism of FC was briefly reviewed by all participants in the study. The participants then watched all twenty video clips in random order. For identified structures, they pointed to the pictures to indicate the CD, common bile duct (CBD), common hepatic duct, and cystic artery in each video clip, for a total of 80 points. If participants could not clearly see or were uncertain of a structure in the video, they answered “unidentified.” Accuracy of their identifications in each video clip were reviewed by two reviewers. Whether the identifications were “true” or “false” were judged by the assessors. For structures that were clearly shown in the video and correctly identified by the participant, were considered “true”; structures that were clearly shown but identified incorrectly or “unidentified”, were considered “false.” The flow chart for the video preparation and examination method is shown in Figure 3.
An independent χ2 test was used to determine possible significant differences between false identification rate of extrahepatic bile ducts; comparing SS with SRs. A paired McNemar’s test was used to determine possible significant differences between false identification rates of extrahepatic bile ducts; comparing without FC with FC. P ≤ 0.05 was considered significant. All statistical analysis was performed with STATA software (version 14).
There were twenty-four participants including in this study, including nine experienced surgeons (staff group) and fifteen SRs (resident group). We excluded forty-five points from the analysis, for structures were not clearly identified, either with or without FC; all participants considered them to be “unidentified.” Thus, thirty-five points, based on clear structures, were included for the analysis.
Table 1 shows the overall answers of staff and residents during with-FC and without-FC phases. In the without-FC phase, the incorrect rate of the resident group was significantly greater than staff group (21.78% vs 11.85%, P = 0.018), However, in the FC phase, the two groups did not significantly differ (23.3% vs 23.3%, P = 0.99). Among the SRs, the misidentification rate did not significantly differ between with- and without-FC phases (23.3% vs 21.7%, P = 0.674) is shown in Table 2.
| Without FC | With FC | |||||||
| n = 360 | Staff | Residents | P value | n = 480 | Staff | Residents | P value | |
| False | 65 (18.0) | 16 (11.8) | 49 (21.7) | 0.018 | 112 (23.3) | 42 (23.3) | 70 (23.3) | 0.999 |
| True | 295 (81.9) | 119 (88.1) | 176 (78.2) | 368 (76.6) | 138 (76.6) | 230 (76.6) | ||
| Total | 360 (100) | 135 (100) | 225 (100) | 480 (100) | 180 (100) | 300 (100) | ||
| Identified | n (%) | Resident group, n = 525 | ||
| Without-FC | With-FC | P value | ||
| False | 119 (22.67) | 49 (21.78) | 70 (23.33) | 0.674 |
| True | 406 (77.33) | 176 (78.22) | 230 (76.67) | |
| Total | 525 (100) | 225 (100) | 300 (100) | |
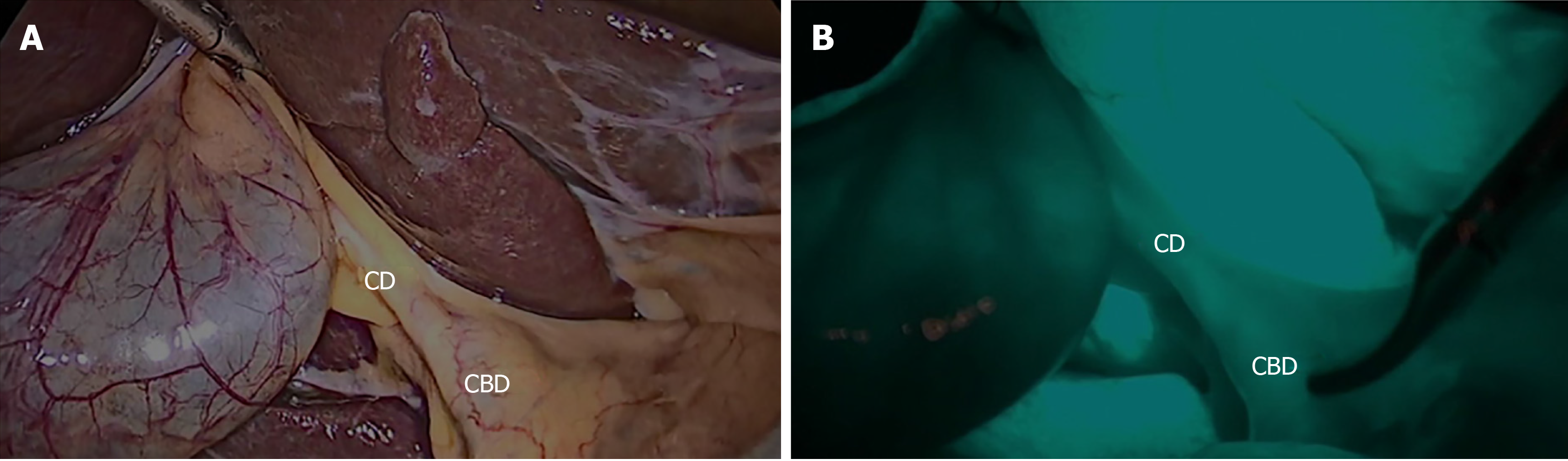
The major limitation of FC is that the delineation of the deeply located bile ducts might fail because near-infrared light can penetrate tissues only to a depth of about 5 mm. Therefore, in patients with thick connective tissue or severe cholecystitis, FC may fail to elucidate the extrahepatic bile ducts. However, we proposed that analyzing paired structures would increase the accuracy of the analysis. We defined paired structures as those structures that could be identified in both with- and without-FC phases, in the same patient, for each dissection phase (before or after dissection phase); for example, the CD in Patient 1, seen in with-FC and without-FC phases, before dissection (Figure 4). Eventually, only fourteen points were included as match-paired structures. Table 3 shows the effect of the FC between paired and unpaired structures. The result was in the without-FC phase have significantly higher misidentification rate than with-FC phase (17.8% vs 10.4%, P < 0.001) in paired group. Nevertheless, in the unpaired group, the with- and without-FC groups did not significantly differ (18.0% vs 23.3%, P = 0.063). Thus, for the analysis of the accuracy of this study, we included only paired structures. The analysis of accuracy between the with- and without-FC phases in each participant groups showed the misidentification rate was significantly higher in the without-FC phase than the with-FC phase in both the RS group (21.9% vs 10.9%, P < 0.01) and the SS group (11.1% vs 9.5%, P < 0.01; Table 4).
| Identified | Match-paired | Non-match-paired | ||||
| Without FC | With FC | P value | Without FC | With FC | P value | |
| False | 60 (17.86) | 35 (10.42) | < 0.01 | 65 (18.06) | 112 (23.33) | 0.063 |
| True | 276 (82.14) | 301 (89.58) | 295 (81.94) | 368 (76.67) | ||
| Total | 336 (100) | 336 (100) | 360 (100) | 480 (100) | ||
| Identified | Staff, n = 126 | Resident, n (%) n = 210 | ||||
| Without FC | With FC | P value | Without FC | With FC | P value | |
| False | 14 (11.11) | 12 (9.52) | < 0.01 | 46 (21.90) | 23 (10.95) | < 0.01 |
| True | 112 (88.89) | 114 (90.48) | 164 (78.10) | 187 (89.05) | ||
| Total | 126 (100) | 126 (100) | 210 (100) | 210 (100) | ||
Table 5 showed the analysis of the performance of the SR group. The without-FC phase had a significantly greater misidentification rate than the with-FC phase, in both before dissection (55.5% vs 22.2%, P < 0.01) and after dissection (12.73% vs 7.88%, P = 0.045). Table 6 showed the analysis between the two participant groups. In the without-FC phase, the SR group had significantly greater misidentification rate than the SS group (21.9% vs 11.1%, P = 0.012). Nevertheless, the two groups did not significantly differ in the with-FC phase (10.9% vs 9.5%, P = 0.67; Table 6).
| Resident group | ||||||
| Identified | Before dissection (n = 45) | After dissection (n = 165) | ||||
| Without FC | With FC | P value | Without FC | With FC | P value | |
| False | 25 (55.56) | 10 (22.22) | < 0.001 | 21 (12.73) | 13 (7.88) | 0.045 |
| True | 20 (44.44) | 35 (77.78) | 144 (87.27) | 152 (92.12) | ||
| Total | 45 (100) | 45 (100) | 165 (100) | 165 (100) | ||
| Identified | Without FC, n (%), n = 336 | With FC, n (%), n = 336 | ||||
| Staff | Residents | P value | Staff | Residents | P value | |
| False | 14 (11.11) | 46 (21.90) | 0.012 | 12 (9.52) | 23 (10.95) | 0.678 |
| True | 112 (88.89) | 164 (78.10) | 114 (90.48) | 187 (89.05) | ||
| Total | 126 (100) | 210 (100) | 126 (100) | 210 (100) | ||
LC is one of the most common procedures performed by general surgeons. It is essential that the SRs are adequately trained and competent, and are able to deliver high-standard care to patient after graduation[15]. Surgical training commonly sets minimum numbers of operations required during general surgical training[15]. However, the minimal procedural numbers of LC are not standardized worldwide for general surgical training and varies in each country[16]. Moreover, evidence that correlates the numbers of a specific procedure that trainees must perform and the achievement of procedural competency is weak[16]. Various tools for improving and assessing clinical performance of LC by SRs have been reported[17-20]. Harrysson et al[17], reported three core elements of the curriculum and framework for LC training: knowledge, technical skill, and attitudes and behaviors. The technical skills are the mainstay of surgical education and can be taught in many different ways[17]. An emerging technique for training is simulator-based training. SRs who practice on simulators before performing procedures and operations on actual patients deliver better patient safety[20,21]. Nagendran et al[22], reported the virtual reality training appears to decrease operating time and improve the performance of surgical trainees with limited laparoscopic experience, compared with no training or with box-trainer training. Skills acquired by simulation-based training seem to be transferable to the operative setting for LCs[19]. From our result, the misidentification rate did not differ between with- and without-FC phase from overall answer in resident group. However, we proposed the analysis of paired structures in order to avoid the limitation of FC which deeply located bile ducts might fail to be demonstrated. The result of paired structure analysis showed the significant ability of the FC in enhancing the skill of the SR. Eventually, our pilot study indicates that FC increases the delineation of the biliary tree significantly for SRs. Thus, FC, which is considered to be a tool of real-time surgery, can be easily applied to simulation-based training as preoperative preparation tool for trainees. Ultimately, FC might be used as the adjunct to the clinical operative setting for LC. However, the further well-designed prospective study should be conducted to confirm this hypothesis.
From our results, even after dissecting Calot’s triangle, SRs still had a high rate of misidentifying structures. However, the misidentification rate declined when FC was applied. Moreover, in term of ability to identify biliary structures, FC use decreased the misidentification rate for both SS and SRs. Consistent with previous reports, FC used during LC are increasing used in clinical practice[13]. FC can delineate the extrahepatic biliary tree, especially in difficult clinical situations[23-25]. However, FC is only one method to achieve safer LCs; the CVS is still crucial. From our result, CDs and CBDs were better seen after dissecting Calot’s triangle. Consistent with the report of Kono et al[26], FC improved identification of the CD, common hepatic duct, and CBD. They concluded that FC is a simple navigation tool for obtaining a biliary roadmap to reach the CVS during LC. However, this procedure needs sufficient extension of connective tissues around the bile ducts[26]. Osayi et al[27] reported using FC to identify biliary anatomy during LC compared with conventional intra-operative cholangiogram; they concluded that FC is a safe and effective alternative for imaging extrahepatic biliary structure.
Although LC is a safe procedure with very low mortality (< 1%), it has some associated major morbidity[28]. BDI is the most serious complication of LC. Although BDI reportedly has very low incidence (0.3%-0.5%)[29,30], it incurs significant costs, including increased hospital cost, need for additional interventions, prolonged hospital stays, and readmission rate. The sequelae of major BDI is a catastrophic occurrence and is associated with a 1-year mortality of 1.7%-3.9%[7]. The factors most associated with BDI are reported to be the surgeon’s misidentification/perception and experience[9,10]. Way et al[9] and Schwaitzberg et al[10] report that surgeons in their learning curve periods have a higher rate of BDI than experienced surgeon, and surgeons with certificates for fundamental laparoscopic skill have a lower rate of BDI than surgeons who do not have the certificate. Nevertheless, some studies reported different results[31,32]. However, current literature supports use of FC with respect to improved identification of biliary structure, feasibility, cost effectiveness, safety, and simplicity[13]. Little evidence supports the use of FC in preventing BDI. A randomized controlled trial (RCT) protocol is currently underway to establish the clinical efficacy of FC for prevention of BDI[33]. Thus, to prove the effect of FC in preventing BDI by less-experienced surgeons, a well-designed RCT should be conducted. However, the number of patients required for the RCT to prove this hypothesis would be overwhelming because of the very low incidence of BDI.
This study has some limitations. Firstly, this study uses video-based material that does not affect SRs’ psychomotor skills. Second, structure identification with FC may depend on the thickness of the soft tissue; in some situations, the video could not clearly delineate the structure in question. We excluded 45 points from the analysis, because of unclear structures. Third, this study had only a few participants.
In conclusion, FC is a surgery navigation tool that can be easily applied to simulation-based video training for SR to improve identification and decision analysis. In the simulation video, FC enhanced identification skills of surgeons-in-training during LC, especially for biliary structures, and seems to be an useful adjunct to clinical operative training. However, further prospective studies should be conducted to confirm our findings.
Fluorescence cholangiography (FC) is considered as one of the supporting imaging techniques for achieving safe laparoscopic cholecystectomy (LC) in the rationale of FC would reduce the misinterpretation rate of the biliary tree.
The identification benefit of FC might be very helpful for inexperienced surgeons.
To investigate the benefit of FC for enhancing the skill of surgical resident (SR) to identify the important structure during LC when comparing with experienced surgeon.
The prospective observatory study in university hospital. The data collected from participants including surgical staff and resident which were assigned to watch videos of LC with FC from different patients, and identify structures in the video clips.
The result indicates that FC increases the delineation of the biliary tree significantly for SR.
FC enhanced identification skills of surgeons-in-training during LC, especially for biliary structures.
The further well-designed prospective study should be conduct to confirm the ability of FC which enhancing the skill of SR.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Augustin G, Iwasaki T S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Richards MK, McAteer JP, Drake FT, Goldin AB, Khandelwal S, Gow KW. A national review of the frequency of minimally invasive surgery among general surgery residents: assessment of ACGME case logs during 2 decades of general surgery resident training. JAMA Surg. 2015;150:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Cortez AR, Winer LK, Katsaros GD, Kassam AF, Shah SA, Diwan TS, Cutler Quillin R. Resident Operative Experience in Hepatopancreatobiliary Surgery: Exposing the Divide. J Gastrointest Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Parikh SP, Szczech EC, Castillo RC, Moskowitz R, Zuberi J, Sori A, Elsawy O. Prospective Analysis of Laparoscopic Cholecystectomies Based on Postgraduate Resident Level. Surg Laparosc Endosc Percutan Tech. 2015;25:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Conrad C, Wakabayashi G, Asbun HJ, Dallemagne B, Demartines N, Diana M, Fuks D, Giménez ME, Goumard C, Kaneko H, Memeo R, Resende A, Scatton O, Schneck AS, Soubrane O, Tanabe M, van den Bos J, Weiss H, Yamamoto M, Marescaux J, Pessaux P. IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2017;24:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Pucher PH, Brunt LM, Fanelli RD, Asbun HJ, Aggarwal R. SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc. 2015;29:3074-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-125. [PubMed] |
| 7. | Chen CB, Palazzo F, Doane SM, Winter JM, Lavu H, Chojnacki KA, Rosato EL, Yeo CJ, Pucci MJ. Increasing resident utilization and recognition of the critical view of safety during laparoscopic cholecystectomy: a pilot study from an academic medical center. Surg Endosc. 2017;31:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Iwashita Y, Hibi T, Ohyama T, Umezawa A, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Han HS, Hwang TL, Suzuki K, Yoon YS, Choi IS, Yoon DS, Huang WS, Yoshida M, Wakabayashi G, Miura F, Okamoto K, Endo I, de Santibañes E, Giménez ME, Windsor JA, Garden OJ, Gouma DJ, Cherqui D, Belli G, Dervenis C, Deziel DJ, Jonas E, Jagannath P, Supe AN, Singh H, Liau KH, Chen XP, Chan ACW, Lau WY, Fan ST, Chen MF, Kim MH, Honda G, Sugioka A, Asai K, Wada K, Mori Y, Higuchi R, Misawa T, Watanabe M, Matsumura N, Rikiyama T, Sata N, Kano N, Tokumura H, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Delphi consensus on bile duct injuries during laparoscopic cholecystectomy: an evolutionary cul-de-sac or the birth pangs of a new technical framework? J Hepatobiliary Pancreat Sci. 2017;24:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Way LW, Stewart L, Gantert W, Liu K, Lee CM, Whang K, Hunter JG. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 448] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Schwaitzberg SD, Scott DJ, Jones DB, McKinley SK, Castrillion J, Hunter TD, Michael Brunt L. Threefold increased bile duct injury rate is associated with less surgeon experience in an insurance claims database: more rigorous training in biliary surgery may be needed. Surg Endosc. 2014;28:3068-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Schols RM, Bouvy ND, Masclee AA, van Dam RM, Dejong CH, Stassen LP. Fluorescence cholangiography during laparoscopic cholecystectomy: a feasibility study on early biliary tract delineation. Surg Endosc. 2013;27:1530-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Dip F, Roy M, Lo Menzo E, Simpfendorfer C, Szomstein S, Rosenthal RJ. Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc. 2015;29:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | van de Graaf FW, Zaïmi I, Stassen LPS, Lange JF. Safe laparoscopic cholecystectomy: A systematic review of bile duct injury prevention. Int J Surg. 2018;60:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Elsey EJ, Griffiths G, Humes DJ, West J. Meta-analysis of operative experiences of general surgery trainees during training. Br J Surg. 2017;104:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Stride HP, George BC, Williams RG, Bohnen JD, Eaton MJ, Schuller MC, Zhao L, Yang A, Meyerson SL, Scully R, Dunnington GL, Torbeck L, Mullen JT, Mandell SP, Choti M, Foley E, Are C, Auyang E, Chipman J, Choi J, Meier A, Smink D, Terhune KP, Wise P, DaRosa D, Soper N, Zwischenberger JB, Lillemoe K, Fryer JP. Relationship of procedural numbers with meaningful procedural autonomy in general surgery residents. Surgery. 2018;163:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Harrysson I, Hull L, Sevdalis N, Darzi A, Aggarwal R. Development of a knowledge, skills, and attitudes framework for training in laparoscopic cholecystectomy. Am J Surg. 2014;207:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Watanabe Y, Bilgic E, Lebedeva E, McKendy KM, Feldman LS, Fried GM, Vassiliou MC. A systematic review of performance assessment tools for laparoscopic cholecystectomy. Surg Endosc. 2016;30:832-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Dawe SR, Windsor JA, Broeders JA, Cregan PC, Hewett PJ, Maddern GJ. A systematic review of surgical skills transfer after simulation-based training: laparoscopic cholecystectomy and endoscopy. Ann Surg. 2014;259:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Willis RE, Van Sickle KR. Current Status of Simulation-Based Training in Graduate Medical Education. Surg Clin North Am. 2015;95:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Nilsson C, Sorensen JL, Konge L, Westen M, Stadeager M, Ottesen B, Bjerrum F. Simulation-based camera navigation training in laparoscopy-a randomized trial. Surg Endosc. 2017;31:2131-2139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Nagendran M, Gurusamy KS, Aggarwal R, Loizidou M, Davidson BR. Virtual reality training for surgical trainees in laparoscopic surgery. Cochrane Database Syst Rev. 2013;CD006575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Liu YY, Kong SH, Diana M, Lègner A, Wu CC, Kameyama N, Dallemagne B, Marescaux J. Near-infrared cholecysto-cholangiography with indocyanine green may secure cholecystectomy in difficult clinical situations: proof of the concept in a porcine model. Surg Endosc. 2016;30:4115-4123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Rungsakulkij N, Tangtawee P. Fluorescence cholangiography during laparoscopic cholecystectomy in a patient with situs inversus totalis: a case report and literature review. BMC Surg. 2017;17:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Vlek SL, van Dam DA, Rubinstein SM, de Lange-de Klerk ESM, Schoonmade LJ, Tuynman JB, Meijerink WJHJ, Ankersmit M. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc. 2017;31:2731-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Kono Y, Ishizawa T, Tani K, Harada N, Kaneko J, Saiura A, Bandai Y, Kokudo N. Techniques of Fluorescence Cholangiography During Laparoscopic Cholecystectomy for Better Delineation of the Bile Duct Anatomy. Medicine (Baltimore). 2015;94:e1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Osayi SN, Wendling MR, Drosdeck JM, Chaudhry UI, Perry KA, Noria SF, Mikami DJ, Needleman BJ, Muscarella P 2nd, Abdel-Rasoul M, Renton DB, Melvin WS, Hazey JW, Narula VK. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc. 2015;29:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Archer SB, Brown DW, Smith CD, Branum GD, Hunter JG. Bile duct injury during laparoscopic cholecystectomy: results of a national survey. Ann Surg. 2001;234:549-58; discussion 558-9. [PubMed] |
| 29. | Kohn JF, Trenk A, Kuchta K, Lapin B, Denham W, Linn JG, Haggerty S, Joehl R, Ujiki MB. Characterization of common bile duct injury after laparoscopic cholecystectomy in a high-volume hospital system. Surg Endosc. 2018;32:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Pekolj J, Alvarez FA, Palavecino M, Sánchez Clariá R, Mazza O, de Santibañes E. Intraoperative management and repair of bile duct injuries sustained during 10,123 laparoscopic cholecystectomies in a high-volume referral center. J Am Coll Surg. 2013;216:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Fahrner R, Turina M, Neuhaus V, Schöb O. Laparoscopic cholecystectomy as a teaching operation: comparison of outcome between residents and attending surgeons in 1,747 patients. Langenbecks Arch Surg. 2012;397:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Maqsood H, Buddensick TJ, Patel K, Ferdosi H, Sautter A, Setiawan L, Sill AM, Kowdley GC, Cunningham SC. Effect of Residents on Operative Time and Complications: Focus on Laparoscopic Cholecystectomy in the Community. J Surg Educ. 2016;73:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | van den Bos J, Schols RM, Luyer MD, van Dam RM, Vahrmeijer AL, Meijerink WJ, Gobardhan PD, van Dam GM, Bouvy ND, Stassen LP. Near-infrared fluorescence cholangiography assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy (FALCON trial): study protocol for a multicentre randomised controlled trial. BMJ Open. 2016;6:e011668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |