Published online Oct 27, 2020. doi: 10.4240/wjgs.v12.i10.425
Peer-review started: June 19, 2020
First decision: July 30, 2020
Revised: August 12, 2020
Accepted: September 14, 2020
Article in press: September 14, 2020
Published online: October 27, 2020
Processing time: 129 Days and 5.5 Hours
Intersphincteric resection (ISR) has been increasingly used as the ultimate sphincter-preserving procedure in extremely low rectal cancer. The most critical complication of this technique is anastomotic leakage. The incidence rate of anastomotic leakage after ISR has been reported to range from 5.1% to 20%.
To investigate risk factors for anastomotic leakage after ISR based on clinicopathological variables and pelvimetry.
This study was conducted at Department of Colorectal Surgery, Japanese Red Cross Medical Center, Tokyo, Japan, with a total of 117 patients. We enrolled 117 patients with extremely low rectal cancer who underwent laparotomic and laparoscopic ISRs at our hospital. We conducted retrospective univariate and multivariate regression analyses on 33 items to elucidate the risk factors for anastomotic leakage after ISR. Pelvic dimensions were measured using three-dimensional reconstruction of computed tomography images. The optimal cutoff value of the pelvic inlet plane area that predicts anastomotic leakage was determined using a receiver operating characteristic (ROC) curve.
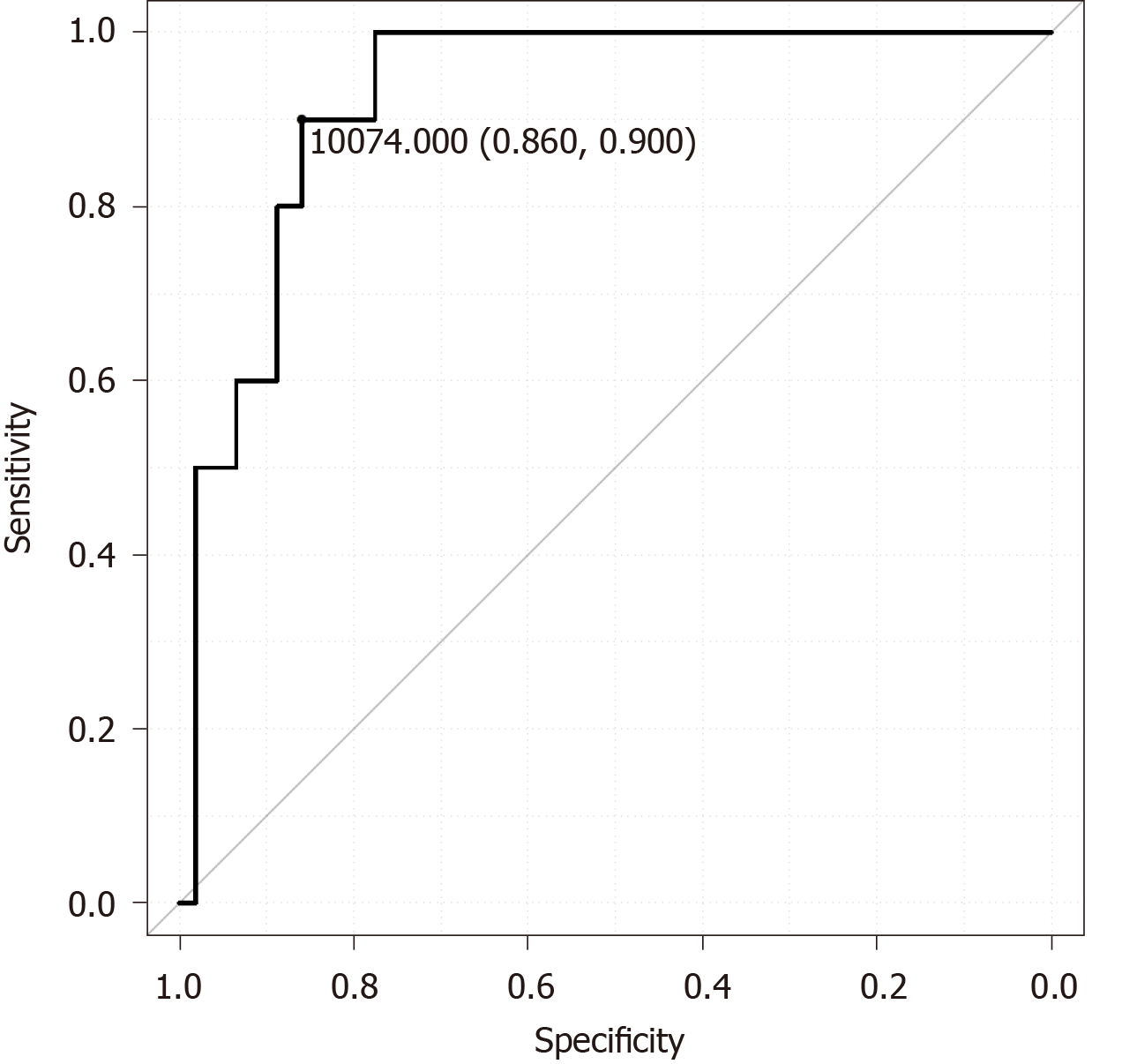
We observed anastomotic leakage in 10 (8.5%) of the 117 patients. In the multivariate analysis, we identified high body mass index (odds ratio 1.674; 95% confidence interval: 1.087-2.58; P = 0.019) and smaller pelvic inlet plane area (odds ratio 0.998; 95% confidence interval: 0.997-0.999; P = 0.012) as statistically significant risk factors for anastomotic leakage. According to the receiver operating characteristic curves, the optimal cutoff value of the pelvic inlet plane area was 10074 mm2. Narrow pelvic inlet plane area (≤ 10074 mm2) predicted anastomotic leakage with a sensitivity of 90%, a specificity of 85.9%, and an accuracy of 86.3%.
Narrow pelvic inlet and obesity were independent risk factors for anastomotic leakage after ISR. Anastomotic leakage after ISR may be predicted from a narrow pelvic inlet plane area (≤ 10074 mm2).
Core Tip: Intersphincteric resection (ISR) is the ultimate sphincter-preserving procedure in extremely low rectal cancer. We investigated risk factors for anastomotic leakage after ISR based on clinicopathological variables and pelvimetry. Narrow pelvic inlet and obesity were independent risk factors for anastomotic leakage after ISR. Anastomotic leakage after ISR may be predicted from a narrow pelvic inlet plane area (≤ 10074 mm2).
- Citation: Toyoshima A, Nishizawa T, Sunami E, Akai R, Amano T, Yamashita A, Sasaki S, Endo T, Moriya Y, Toyoshima O. Narrow pelvic inlet plane area and obesity as risk factors for anastomotic leakage after intersphincteric resection. World J Gastrointest Surg 2020; 12(10): 425-434
- URL: https://www.wjgnet.com/1948-9366/full/v12/i10/425.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i10.425
Since Schiessel et al[1] first introduced intersphincteric resection (ISR) in 1994, the procedure has been increasingly accepted as the ultimate sphincter-preserving procedure in extremely low rectal cancer. ISR preserves the natural anus and avoids permanent colostomy. However, ISR is performed in the deep and funnel-shaped pelvic cavity, where access and visualization of the narrow pelvis is difficult[2,3]. Anastomotic leakage is the most critical complication that can cause reduced function or narrowing of the anal sphincter, possibly warranting a permanent colostomy[4-6]. According to a report from a committee (chaired by N Saito) sponsored by the Ministry of Health, Labour and Welfare of Japan, anastomotic leakage occurred in 23 (10.2%) of 225 patients after ISR[7]. The incidence rate of anastomotic leakage after ISR has been reported to range from 5.1% to 20%[8-10].
Akasu et al[11] have reported on the risk factors for anastomotic leakage after ISR but have not analyzed pelvic size. Pelvic anatomy is a determinant factor of rectal dissection, as the narrow pelvic cavity and bony structures surrounding the rectum may hinder dissection maneuvers[12]. Pelvimetry is famous for its use in predicting obstetric risk. In this study, we used pelvimetry to measure the inlet and outlet plane areas, particularly pelvic dimensions, to investigate risk factors for anastomotic leakage after ISR.
This retrospective study was approved by the ethics review board of the Japanese Red Cross Medical Center on July 31, 2019.
This study included subjects who underwent laparotomic and laparoscopic ISR as treatment for extremely low rectal adenocarcinoma with inferior margins less than 5 cm from the anal verge at the Japanese Red Cross Medical Center between 2005 and 2019. The spread of the rectal cancer was quantified in accordance with the clinical tumor-node-metastasis (TNM) classification (8th edition)[13]. Liver metastases were detected in all patients with stage IV cancer. The patients with peritoneal metastasis, multivisceral resection, gastrointestinal stromal tumor, and carcinoid were excluded.
Our indications for ISR included: (1) An inferior tumor margin less than 5 cm from the anal verge; (2) A moderate- to well-differentiated adenocarcinoma; (3) Local spread restricted to the internal sphincter without involvement of striated muscle; (4) The presence of some amount of T4 tumor (vaginal invasion); (5) Patients with resectable metastases to the liver or lungs; and (6) Normal sphincter function.
The surgical procedure was performed in accordance with the method by Schiessel et al[1]. Laparotomy was performed with a small incision below the umbilicus, considering minimally invasive surgery. Under either laparotomy or laparoscopy, the inferior mesenteric artery (IMA) root was generally preserved, and the superior rectal artery was severed at the branching site of the left colic artery (LCA) or first branch of the sigmoid colic artery (SCA). When the IMA root was preserved, the lymph node around IMA root was separately resected to improve the curability. If tension was present at the anastomotic site or the color of the colon indicated an inadequate blood supply, the IMA was severed at the root and splenic flexure was mobilized. The rectum was divided transanally, removing a part or the entirety of the internal sphincter. Reconstruction was performed with a hand-sewn coloanal anastomosis from the anus. A closed suction drain was inserted in the left side of the pelvis and brought out at the lower angle of the wound. In some cases, an anal bougie (transanal drain) was inserted. Anastomotic leakage occurred in the second patient; thus, in all the subsequent patients, we constructed a covering stoma. The diverting stoma was closed 3-6 mo after surgery. Before the closure, we performed colonoscopy, contrast enema radiography, or computed tomography (CT). CT was performed each time there was a complication. The operations were performed by 3 staff surgeons (A, B, and C).
Anastomotic leakage was defined as the presence of an anastomosis fistula during the first postoperative endoscopy or gastrografin enema. In addition, anastomotic leakages were classified into three categories according to the clinical management by Rahbari et al[14] as follows: Grade A requires no active therapeutic intervention; Grade B, an active therapeutic intervention without operation; and Grade C, re-laparotomy.
We conducted retrospective univariate and multivariate regression analyses on 33 items to elucidate the risk factors for anastomotic leakage after ISR. These items were categorized into three groups as follows: Surgery, tumor, and patient related. The surgery-related factors were operation type (laparotomy or laparoscopic surgery), circumferential resection margin (CRM +/−), site of IMA ligature (IMA/LCA or SCA), splenic flexure mobilization (with/without), construction of a diverting stoma (with/without), insertion of an anal bougie (with/without), blood loss, transfusion (with/without)[15], surgeon (A, B, and C), surgical duration, and curability (A, B, or C). Tumor-related factors were tumor size, distance from the anal verge to the inferior margin of the tumor, patient categorization as clinical TNM classification, preoperative radiotherapy (with/without), and neoadjuvant chemotherapy (with/without). The patient related factors were age, sex, body mass index (BMI), American Society of Anesthesiologists Physical Status (ASA-PS; I, II, or III), serum total protein level, hemoglobin level, prognostic nutritional index[7], diabetes (hemoglobin A1c >/≤ 5.8 g/dL), and nine pelvimetry measurements.
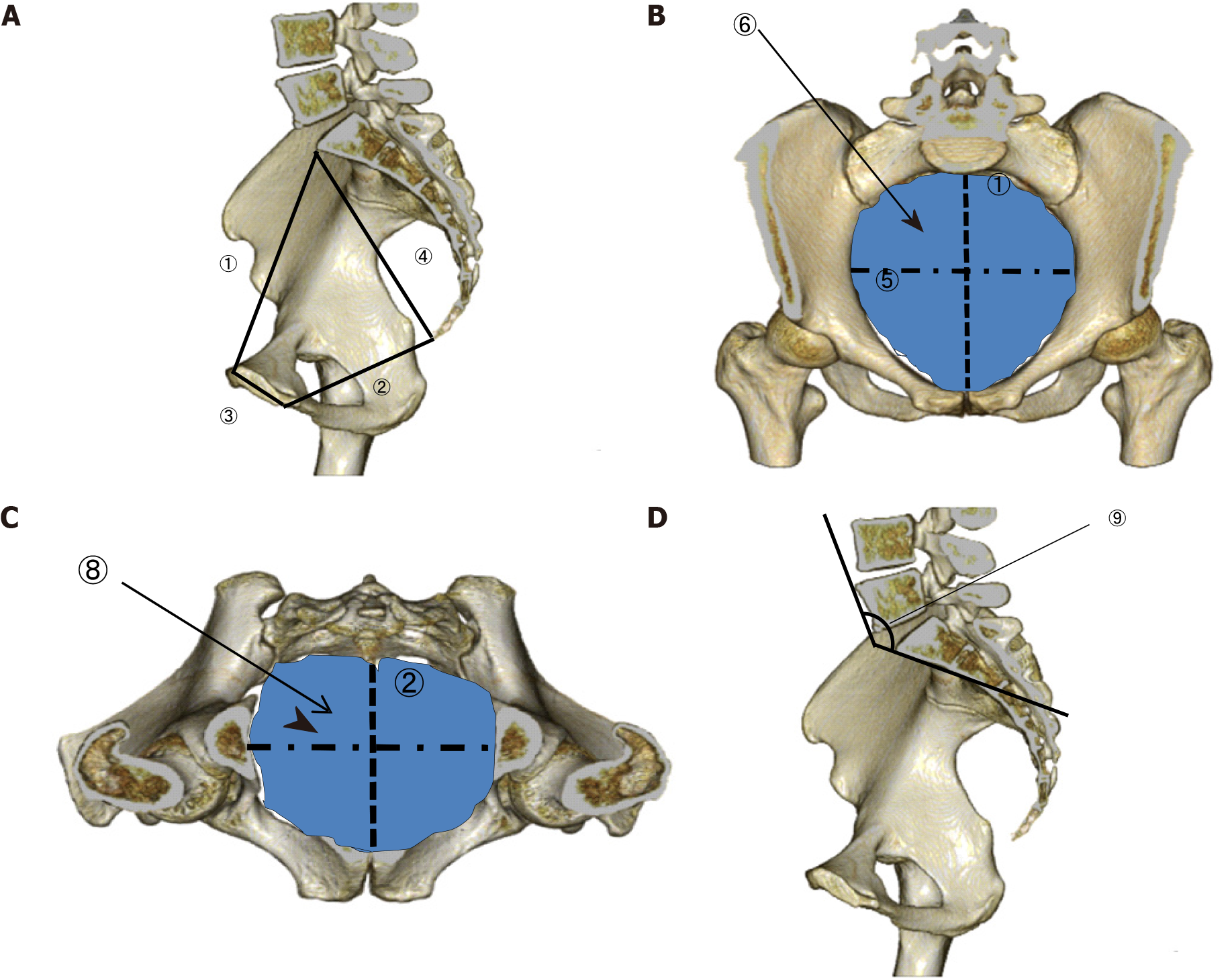
We used the Synapse Vincent magnifying viewer (Fujifilm Medical Co., Ltd., Tokyo, Japan) to convert CT images into three-dimensional images. By using this imaging system, the anteroposterior (AP) diameter, transverse diameter, and total area were measured at the pelvic inlet and outlet. The length of the pubic symphysis and distance from the sacral promontory to the coccyx were measured to determine the anterior and posterior depths of the pelvis (Figure 1A-C). The lumbosacral (tortuosity) angle at the sacral promontory was also measured (Figure 1D).
Differences between the leakage and non-leakage groups were detected using the Welch’s t-test or Student t-test for continuous data. The χ2 test was used for categorical secondary outcomes. The predictors found to be associated with anastomotic leakage in the univariate analysis (P < 0.05) were analyzed by subsequent multiple logistic regression method to identify independent factors. If both diameter and area were statistically significant, the area was preferentially used in the multivariate analysis. The optimal cutoff value of the pelvic inlet plane area for predicting anastomotic leakage was determined using the receiver operating characteristic curve. P values of < 0.05 were considered statistically significant in this study.
We enrolled 117 patients [89 men; mean age, 61.3 years (range, 26-86 years)]. We observed anastomotic leakage in 10 (8.5%) of the 117 patients as follows: Grade A in 3 patients (30%), grade B in 5 (50%), and grade C in 2 (20%). In the 3 cases with grade A leakage, it was cured by conservative treatment such as antibiotic treatment. In the 5 patients with grade B leakage, the fistulae were closed using an alpha-cyanoacrylate monomer (Aron Alpha A Sankyo, Toagosei Co., Ltd., Tokyo, Japan), a tissue adhesive. Two patients with grade C leakage needed re-operation. One patient underwent permanent colostomy, and the other underwent nephrostomy for vesicorectal fistula. Closure of the diverting stoma was possible in 8 (80%) of the 10 patients. No operative deaths occurred.
Table 1 shows the correlation between the postoperative anastomotic leakage and clinicopathological variables. The univariate analysis revealed a statistically significant relationship between anastomotic leakage and higher BMI, smaller pelvic inlet area, shorter AP diameter of the inlet plane, shorter transverse diameter of the inlet plane, shorter AP diameter of the outlet plane, longer pubic symphysis, greater lumbosacral angle, and larger amount of bleeding.
| No leakage | Leakage | P value | |
| Patients number | 107 | 10 | |
| Age in yr | 61.1 ± 12.7 | 64.3 ± 9.9 | 0.436 |
| Male sex, n (%) | 80 (74.8) | 9 (90) | 0.489 |
| Body mass index | 22.4 ± 3.3 | 25.6 ± 2.7 | 0.004 |
| Anesthesiologists physical status | 0.569 | ||
| 1 | 29 | 3 | |
| 2 | 75 | 7 | |
| 3 | 3 | 0 | |
| Serum total protein level, g/dL | 6.9 ± 0.5 | 7.1 ± 0.5 | 0.351 |
| Blood hemoglobin level, g/dL | 13.5 ± 1.8 | 14.6 ± 1.4 | 0.055 |
| Prognostic nutritional index | 49.7 ± 6.3 | 52.4 ± 4.9 | 0.205 |
| Diabetes mellitus | 0.326 | ||
| No | 93 | 7 | |
| Yes | 14 | 3 | |
| Inlet plane: Antero-posterior diameter, mm | 110.3 ± 10.8 | 100 ± 7.9 | 0.002 |
| Inlet plane: Transverse diameter, mm | 156.3 ± 35.4 | 136.4 ± 17.7 | 0.008 |
| Area of the inlet plane, mm2 | 11330 ± 1279 | 9428 ± 636.1 | < 0.001 |
| Outlet plane: Antero-posterior diameter, mm | 97.8 ± 8.9 | 90.6 ± 10.4 | 0.018 |
| Outlet plane: Transverse diameter, mm | 103.4 ± 10.1 | 102.8 ± 4.4 | 0.739 |
| Area of the outlet plane, mm2 | 9572 ± 1501 | 9323 ± 999 | 0.487 |
| Length of the pubic symphysis, mm | 41.9 ± 5.6 | 45.7 ± 5.2 | 0.044 |
| Distance from the sacral promontry to the coccyx, mm | 125.5 ± 13.3 | 131.3 ± 14.8 | 0.193 |
| Tortuosity angle | 131.7 ± 8.7 | 140.4 ± 11.4 | 0.004 |
| Tumors | |||
| Tumor size, mm | 41.3 ± 25.2 | 33.3 ± 19.7 | 0.335 |
| Distance from the anal verge, mm | 16.8 ± 19.2 | 20.4 ± 14.6 | 0.563 |
| TNM stage | 0.056 | ||
| 0 | 3 | 1 | |
| I | 35 | 6 | |
| II | 23 | 1 | |
| III | 34 | 1 | |
| IV | 12 | 1 | |
| Preoperative radiotherapy | 0.972 | ||
| No | 90 | 9 | |
| Yes | 17 | 1 | |
| Neoadjuvant chemotherapy | 0.341 | ||
| No | 89 | 10 | |
| Yes | 18 | 0 | |
| Surgery | 0.341 | ||
| Laparotomy | 89 | 10 | |
| Laparoscopic surgery | 18 | 0 | |
| Circumferential resection margin | 0.809 | ||
| No | 100 | 9 | |
| Yes | 7 | 1 | |
| Ligated site of the artery | 0.583 | ||
| Inferior mesenteric artery | 5 | 0 | |
| Left colonic artery | 41 | 3 | |
| Sigmoid colon artery | 61 | 7 | |
| Mobilization of the splenic flexure | 0.738 | ||
| No | 99 | 9 | |
| Yes | 8 | 1 | |
| Diverting stoma | 0.401 | ||
| No | 1 | 1 | |
| Yes | 106 | 9 | |
| Anal bougie | 0.341 | ||
| No | 89 | 10 | |
| Yes | 18 | 0 | |
| Bleeding amount, mL | 645 ± 709 | 1380 ± 1166 | 0.004 |
| Blood transfusion, mL | 8 ± 60 | 292 ± 621 | 0.181 |
| Operator | 0.418 | ||
| X | 87 | 9 | |
| T | 9 | 1 | |
| S | 11 | 0 | |
| Operation time in min | 367 ± 156 | 387 ± 167 | 0.698 |
| Curativity | 0.54 | ||
| A | 86 | 9 | |
| B | 8 | 0 | |
| C | 13 | 1 |
In the multivariate analysis, we identified high BMI (odds ratio 1.674; 95% confidence interval: 1.087-2.58; P = 0.019) and smaller pelvic inlet plane area (odds ratio 0.998; 95% confidence interval: 0.997-0.999; P = 0.012) as statistically significant risk factors for anastomotic leakage (Table 2).
| Variables | Multivariate analysis | ||
| Odds ratio | 95%CI | P value | |
| Body mass index | 1.674 | 1.087-2.58 | 0.019 |
| Area of the inlet plane | 0.998 | 0.997-0.999 | 0.012 |
| Outlet plane: Antero-posterior diameter | 0.905 | 0.811-1.008 | 0.07 |
| Length of the pubic symphysis | 1.125 | 0.883-1.435 | 0.341 |
| Tortuosity angle | 1.049 | 0.941-1.17 | 0.39 |
| Bleeding amount | 1.001 | 0.999-1.003 | 0.144 |
According to the receiver operating characteristic curves, the optimal cutoff value of the pelvic inlet plane area was 10074 mm2 (Figure 2). Narrow pelvic inlet area (≤ 10074 mm2) predicted anastomotic leakage with a sensitivity of 90%, a specificity of 85.9%, and an accuracy of 86.3%. The positive predictive value was 37.5%, and the negative predictive value was 98.9%.
Narrow pelvic inlet and obesity were independent risk factors for anastomotic leakage after ISR. This is the first report of increased anastomotic leakage due to narrow pelvic inlet. Narrow pelvic inlet hinders the surgical procedures with the approach from the abdominal cavity, and the difficulty might lead to anastomotic leakage.
Rullier et al[16] demonstrated that male sex and obesity were independent risk factors for anastomotic leakage after low anterior resection (LAR). The male pelvis is narrower than the female pelvis; thus, male sex as a risk factor for anastomotic leakage might be due to the narrower pelvis in males.
Zhou et al[17] evaluated the technical difficulties in LAR or abdominoperineal resection using three-dimensional reconstruction of CT images. Their multivariate analyses revealed that the AP diameter of the pelvic inlet, AP diameter of the pelvic outlet, and height of the pubic symphysis were factors that affect operative time. These results indicate that narrower and deeper pelvises could increase operating times.
Zur Hausen et al[18] also evaluated the clinical outcomes in LAR or abdominoperineal resection using CT pelvimetry. A shorter AP pelvic inlet diameter was associated with a higher rate of incomplete mesorectal excision. However, the number of cases were relatively small (n = 74), and the study failed to show the association between pelvic diameters and incidence of anastomotic leakage. The authors concluded that preoperative pelvimetry may help identify difficult pelvic dissections preoperatively.
The present study shows that narrow pelvic inlet significantly increased the incidence of anastomotic leakage after ISR. Furthermore, narrow pelvic inlet area (≤ 10074 mm2) predicted anastomotic leakage with a sensitivity of 90%, a specificity of 85.9%, and an accuracy of 86.3%. Identification of patients at high risk for anastomotic leakage may allow for the selective use of the indocyanine green fluorescence method or more-advanced access methods such as transanal total mesorectal excision or robotic-assisted laparoscopic surgery[19] for improving surgical outcomes.
In our study, obesity was also an independent risk factor for anastomotic leakage after ISR. Surgery for obese patients is generally more difficult because obesity contributes to inadequate exposure of the surgical field, which results in accidental injury. Yang et al[20] and Yamamoto et al[21] reported that obesity was a risk factor for anastomotic leakage after LAR. Rullier et al[16] reported that obesity was a risk factor for anastomotic leakage after ISR. A protective stoma is, therefore, suitable after ISR, particularly in obese patients. The first limitation of our study is that it was conducted within a single specialized institution. Second, this study was consecutive but retrospective. Third, the number of laparoscopic cases was small, and the effect of laparoscopic surgery was not determined. A follow-up study should be performed to confirm and clarify the characteristics of anastomotic leakage after ISR including laparoscopic surgery.
In conclusion, narrow pelvic inlet and high BMI were associated with anastomotic leakage after ISR. Anastomotic leakage after ISR may be predicted from a narrow pelvic inlet plane area (≤ 10074 mm2).
Intersphincteric resection (ISR) has been increasingly used as the ultimate sphincter-preserving procedure in extremely low rectal cancer.
Anastomotic leakage is the most critical complication that can cause reduced function or narrowing of the anal sphincter, possibly warranting a permanent colostomy.
This study investigated risk factors for anastomotic leakage after ISR based on clinicopathological variables and pelvimetry.
We enrolled 117 patients with extremely low rectal cancer who underwent laparotomic and laparoscopic ISRs. Risk factors for anastomotic leakage after ISR that were analyzed using a multivariate analysis. Pelvic dimensions were measured using three-dimensional reconstruction of computed tomography images. The optimal cutoff value of the pelvic inlet plane area that predicts anastomotic leakage was determined using a receiver operating characteristic curve.
Higher body mass index and small pelvic inlet plane area were independently associated with anastomotic leakage after ISR. According to the receiver operating characteristic curves, the optimal cutoff value of the pelvic inlet plane area was 10074 mm2. Narrow pelvic inlet plane area (≤ 10074 mm2) predicted anastomotic leakage with a sensitivity of 90%, a specificity of 85.9%, and an accuracy of 86.3%.
Narrow pelvic inlet and obesity were independent risk factors for anastomotic leakage after ISR.
A follow-up study should be performed to confirm and clarify the characteristics of anastomotic leakage after ISR including laparoscopic surgery.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Japan Surgical Society, No. 0413910; and The Japanese Society of Gastroenterology, No. 032796.
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fakhr I, Vignali A, Zhao WT S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumours. Br J Surg. 1994;81:1376-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 320] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Chin CC, Yeh CY, Huang WS, Wang JY. Clinical outcome of intersphincteric resection for ultra-low rectal cancer. World J Gastroenterol. 2006;12:640-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Dimitriou N, Michail O, Moris D, Griniatsos J. Low rectal cancer: Sphincter preserving techniques-selection of patients, techniques and outcomes. World J Gastrointest Oncol. 2015;7:55-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Daams F, Wu Z, Lahaye MJ, Jeekel J, Lange JF. Prediction and diagnosis of colorectal anastomotic leakage: A systematic review of literature. World J Gastrointest Surg. 2014;6:14-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Yamada K, Saiki Y, Takano S, Iwamoto K, Tanaka M, Fukunaga M, Noguchi T, Nakamura Y, Hisano S, Fukami K, Kuwahara D, Tsuji Y, Takano M, Usuku K, Ikeda T, Sugihara K. Long-term results of intersphincteric resection for low rectal cancer in Japan. Surg Today. 2019;49:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Olavarria OA, Kress RL, Shah SK, Agarwal AK. Novel technique for anastomotic salvage using transanal minimally invasive surgery: A case report. World J Gastrointest Surg. 2019;11:271-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Saito N, Moriya Y, Shirouzu K, Maeda K, Mochizuki H, Koda K, Hirai T, Sugito M, Ito M, Kobayashi A. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum. 2006;49:S13-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Schiessel R, Novi G, Holzer B, Rosen HR, Renner K, Hölbling N, Feil W, Urban M. Technique and long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2005;48:1858-1865; discussion 1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Barisic G, Markovic V, Popovic M, Dimitrijevic I, Gavrilovic P, Krivokapic Z. Function after intersphincteric resection for low rectal cancer and its influence on quality of life. Colorectal Dis. 2011;13:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Akasu T, Takawa M, Yamamoto S, Fujita S, Moriya Y. Incidence and patterns of recurrence after intersphincteric resection for very low rectal adenocarcinoma. J Am Coll Surg. 2007;205:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Akasu T, Takawa M, Yamamoto S, Yamaguchi T, Fujita S, Moriya Y. Risk factors for anastomotic leakage following intersphincteric resection for very low rectal adenocarcinoma. J Gastrointest Surg. 2010;14:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M. Can we predict immediate outcome after laparoscopic rectal surgery? Ann Surg. 2008;247:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Fisseler-Eckhoff A. [New TNM classification of malignant lung tumors 2009 from a pathology perspective]. Pathologe. 2009;30 Suppl 2:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1033] [Article Influence: 68.9] [Reference Citation Analysis (4)] |
| 15. | Rutegård M, Rutegård J. Anastomotic leakage in rectal cancer surgery: The role of blood perfusion. World J Gastrointest Surg. 2015;7:289-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 643] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Zhou XC, Su M, Hu KQ, Su YF, Ye YH, Huang CQ, Yu ZL, Li XY, Zhou H, Ni YZ, Jiang YI, Lou Z. CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol Lett. 2016;11:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Zur Hausen G, Gröne J, Kaufmann D, Niehues SM, Aschenbrenner K, Stroux A, Hamm B, Kreis ME, Lauscher JC. Influence of pelvic volume on surgical outcome after low anterior resection for rectal cancer. Int J Colorectal Dis. 2017;32:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furutani A, Manabe S, Yamaoka Y, Hino H. Oncological outcomes of robotic-assisted laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer. Surg Endosc. 2018;32:4498-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Yang L, Huang XE, Zhou JN. Risk assessment on anastomotic leakage after rectal cancer surgery: an analysis of 753 patients. Asian Pac J Cancer Prev. 2013;14:4447-4453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Yamamoto S, Fujita S, Akasu T, Inada R, Moriya Y, Yamamoto S. Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech. 2012;22:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |