Copyright
©2013 Baishideng Publishing Group Co.
World J Gastrointest Surg. Apr 27, 2013; 5(4): 83-96
Published online Apr 27, 2013. doi: 10.4240/wjgs.v5.i4.83
Published online Apr 27, 2013. doi: 10.4240/wjgs.v5.i4.83
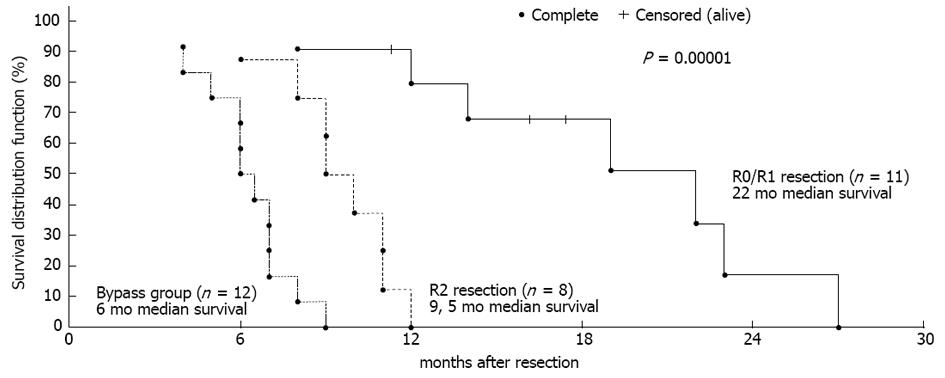
Figure 1 Differences in survival between the groups were significant.
The explanation is in the text.
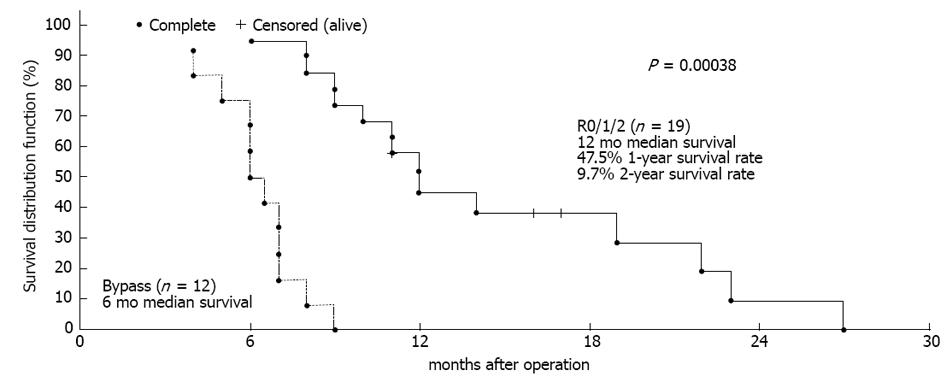
Figure 2 Median survival following palliative operations was 6 mo (95%CI: 5-7 mo) and there was a significant difference in survival between the palliative group (C) and the united resection group (group A + group B).
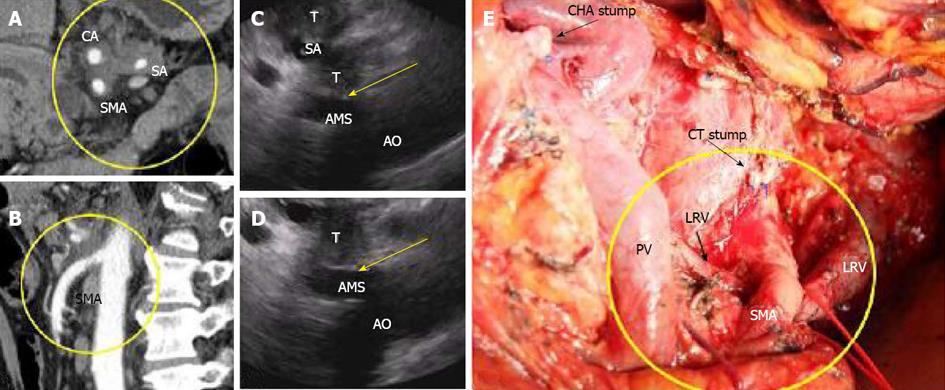
Figure 3 In this 65-year-old man (case #10), pancreatic body DAC with 360° celiac (CA), splenic (SA) and superior mesenteric artery (SMA) encasement was established on CT (A, B), but endoUS data did not confirm this conclusion, finding a plane between the tumor and the SMA (C, D, arrows).
AMS: Arteria mesenterica superior, AO: Aorta, T: Tumor. Distal pancreatectomy with excision of the celiac artery (CA) and left adrenalectomy were performed, and no SMA involvement was identified during surgery (E). The level of resection was R1 because of the contact of the SMA with the tumor. CHA: Common hepatic artery; CT: Celiac trunk; LRV: Left renal vein; PV: Portal vein.
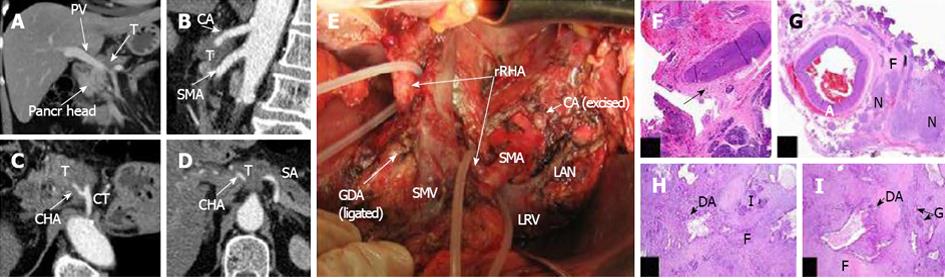
Figure 4 In this 64-year-old woman (case 7), circular encasement of the celiac, common hepatic, left gastric, left hepatic and splenic arteries by PDA on the background of aberrant arterial anatomy; a replaced right hepatic artery (rRHA, Michels, type VIIIb) was identified on CT (A-D).
E. Photograph of operating field after distal pancreatectomy (R0 resection), with excision of the celiac, common, left gastric, left hepatic arteries and gastroduodenal artery resection in the absence of any evidence for major arterial invasion, either during surgery or on histopathology; the blood supply to the stomach was routed from the SMA via pancreaticodudenal arcades and then through the GDA with the latter’s proximal segment being resected and ligated. F-I: Removed specimen under microscope. The tumor (DA) was smaller than 2 cm and was surrounded by a thick layer of fibrotic tissue (H,I). There were no signs of involvement of the major peripancreatic (CHA, GDA, LHA) arteries; H: CHA section obtained from close to the point of its transection (white arrow) in the fibrotic zone (black arrow) along the pancreas margin. No evidence of tumor growth x 5. G: Celiac plexus and trunk area of diffuse fibrosis (F) x 5; A: Artery; N: Nerve plexus with large ganglion; H: Pancreatic tissue with apparent diffuse fibrosis (F), groups of islets remaining (I) and groups of glandular formations of ductal adenocarcinoma (DA) of the pancreas x 50. d: Structures of DA throughout the fibrotic tissue (F) containing remnants of pancreatic tissue (atrophic islets and ductules) x 50, hematoxylin + eosin. PV: Portal vein; T: Tumor; CA: CT-celiac artery (celiac trunk); SMA: Superior mesenteric artery; GDA: Gastroduodenal artery; rRHA: Replaced right hepatic artery; PDA: Pancreato-duodenal arcade; LRV: Left renal vein; PV: Portal vein; SMV: Superior mesenteric vein; LAV: Left adrenal vein.
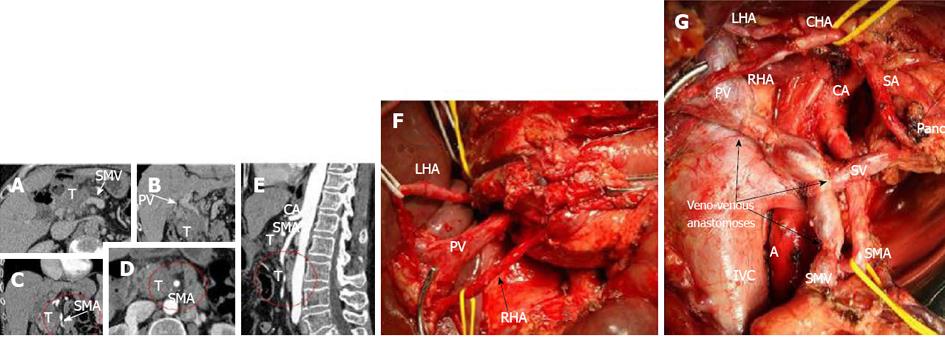
Figure 5 In this 61-year-old woman (case 6), 260° and 360° pancreatic ductal adenocarcinoma encasement of SMA segments was diagnosed on computed tomography (A-E), while endoUS data described only tumor abutment with the superior mesenteric artery.
A, B: Venous phase. Sagittal view. Computed tomography provided evidence of circumferential involvement of the SMV and PV; C, E: Arterial phase. Sagittal view. The distal SMA segment (6-7 cm from the origin) presented circumferential adjacency to pancreatic head ductal adenocarcinoma. The celiac artery (CA) was unaffected; D: Arterial phase. Axial image. At least 260° of the proximal SMA segment (2.5-3 cm from the origin) was circumscribed by tumor. An extended Whipple procedure with pancreatic body, portal, splenic and superior mesenteric vein resection was performed with the use of a superficial femoral vein autograft (F, G). Notwithstanding “organoleptic” signs of unresectability (both hepatic arteries were embedded in the tumor) (F), there were no signs of superior mesenteric artery (SMA) or hepatic artery involvement during surgery (G). The level of resection was R1 because of the contact of the SMA with the tumor. A: Aorta; CHA: Common hepatic artery; RHA: Right hepatic artery; LHA: Left hepatic artery; SA: Splenic artery; SMV: Superior mesenteric; PV: Portal vein; LRV: Left renal veins; T: Tumor; Pancr: Pancreatic tail stump.
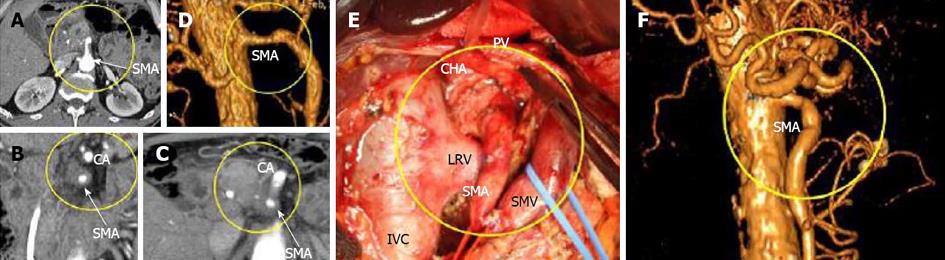
Figure 6 In this 59-year-old man (case 4), 360°pancreatic ductal adenocarcinoma encasement of the superior mesenteric artery was diagnosed on computed tomography (A-D), while endoUS data described only tumor abutment with the superior mesenteric artery.
A-C: Computed tomography (CT), Arterial phase, Axial images. CT showed circumferential infiltration of the SMV. The CA was intact; D: CTA. Local narrowing of the SMA at the site at which it was circumscribed by the tumor; E: Intraoperative photograph. An extended Whipple procedure was performed. There were no signs of SMA involvement during surgery. The level of resection was R1 because of the contact of the SMA with the tumor; F: CT angiography. Three months postsurgery. No relapse and no narrowing of the SMA. SMA: Superior mesenteric artery; SMV: Superior mesenteric; PV: Portal; IVC: Inferior caval; LRV: Left renal veins; CA: Celiac artery.
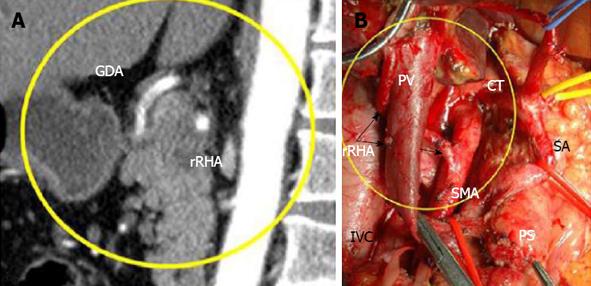
Figure 7 In 75-year-old woman (case 1), 360° PDAC encasement of the replaced right hepatic artery was diagnosed on computed tomography (A), while endoUS data described only tumor abutment with the artery.
Arterial phase, Sagittal images: CT showed circumferential infiltration of the rRHA, B: Intraoperative photograph. An extended Whipple procedure was performed. There were no signs of rRHA or SMA involvement during surgery (arrows). The level of resection was R1 because of the contact of the rRHA with the tumor. SMA: Superior mesenteric artery; rRHA: Replaced right hepatic; LHA: Left hepatic; RGEA: Right gastro-epiploic arteries; CT: Celiac trunk; SMV: Superior mesenteric; PV: Portal; LRV: Left renal vein; T: Tumor; PS: Pancreatic stump.
- Citation: Egorov VI, Petrov RV, Solodinina EN, Karmazanovsky GG, Starostina NS, Kuruschkina NA. Computed tomography-based diagnostics might be insufficient in the determination of pancreatic cancer unresectability. World J Gastrointest Surg 2013; 5(4): 83-96
- URL: https://www.wjgnet.com/1948-9366/full/v5/i4/83.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i4.83