Copyright
©The Author(s) 2024.
World J Gastrointest Surg. Mar 27, 2024; 16(3): 882-892
Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.882
Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.882
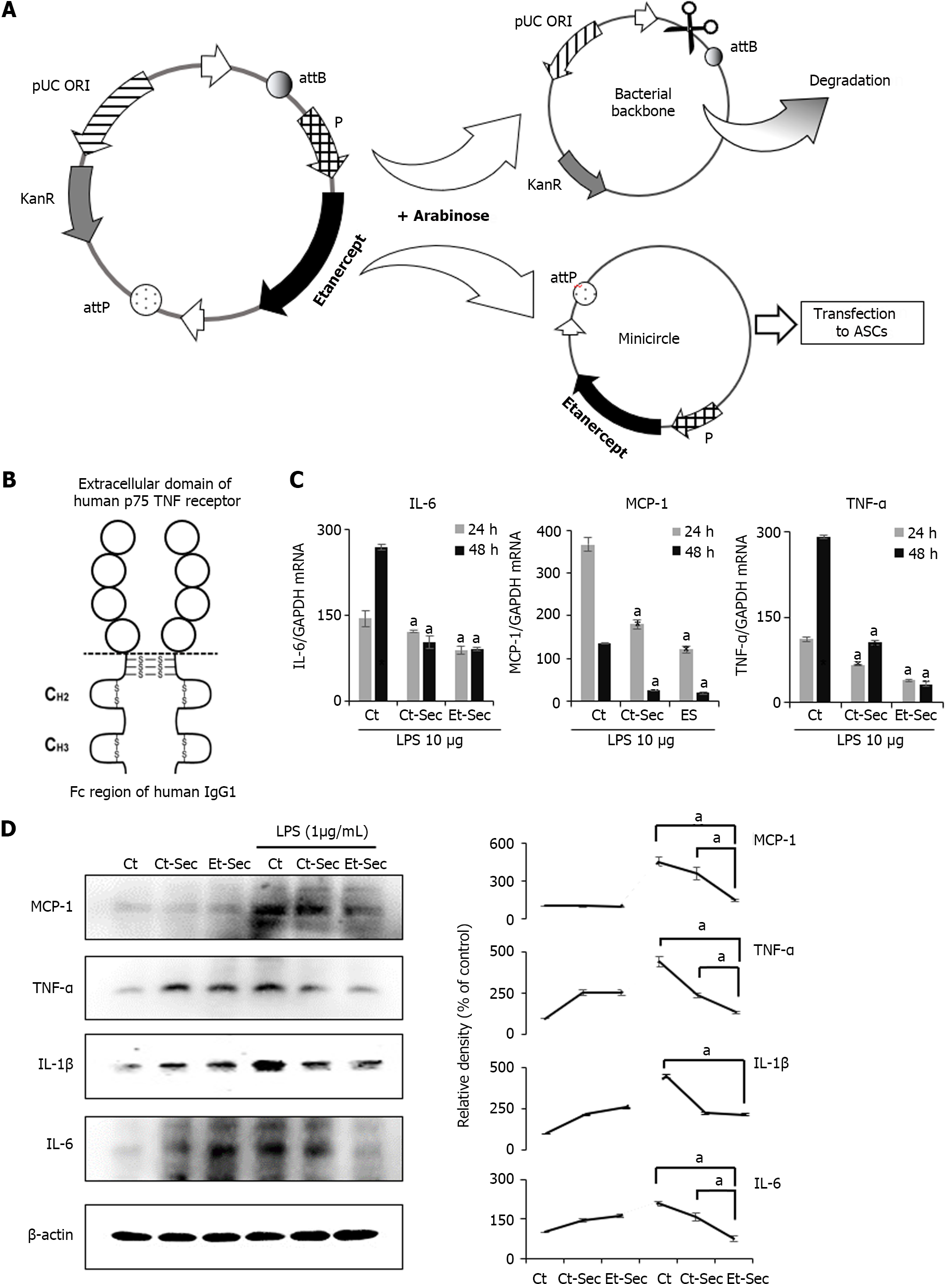
Figure 1 Generation of etanercept-secretome and assessment of its in vitro anti-inflammatory effects.
A: Schematic representation of the mini-circle plasmid encoding etanercept derived from the parental plasmid through arabinose treatment; B: Etanercept Structure. Etanercept, a protein-based drug with tumor necreosis factor (TNF)-α receptor and IgG1 Fc domains, effectively binds and neutralizes TNF-α, a critical inflammatory cytokine; C: Real-time polymerase chain reaction of inflammatory markers. In the etanercept-secretome (Et-Sec) group, mRNA expression levels of TNF-α and interleukin (IL)-6 were significantly lower compared to other groups [control group and control-secretome (Ct-Sec) group] at 24 and 48 h, after lipopolysaccharide (LPS) treatment (P < 0.05); D: Western blot analysis of inflammatory markers. Et-Sec treatment led to a significant reduction in Monocyte Chemoattractant Protein-1, TNF-α, and IL-6 expression levels when compared to the Ct-Sec treatment in LPS-induced inflammation in CCD-18Co colon normal cells (P < 0.05). Relative densities of individual markers had been quantified using Image J software and then were normalized to that of β-actin in each group. Values are presented as mean ± SD of three independent experiments. aP < 0.05. Et-Sec: Etanercept-secretome; Ct-Sec: Control-secretome; LPS: Lipopolysaccharide; TNF: Tumor necreosis factor; MCP-1: Monocyte chemoattactant protein-1; IL: Interleukin; IBD: Inflammatory bowel disease; DSS-treated group: Dextran sulfate sodium-treated group; ASCs: Adipose-derived stem cells; Ct: Control group.
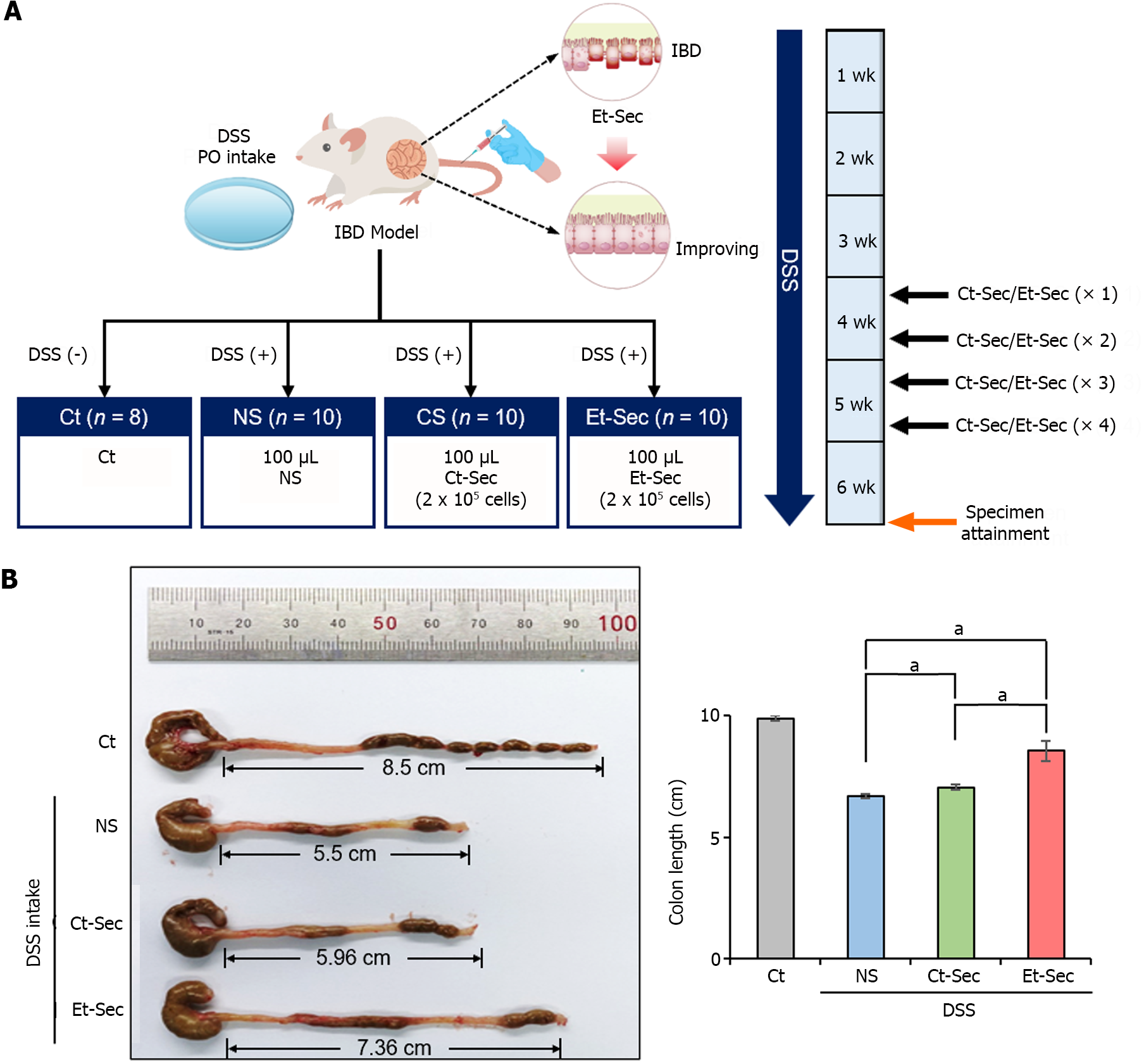
Figure 2 In vivo modeling and macroscopic anti-inflammatory effects of etanercept-secretome.
A: Experimental setup. The left panel illustrates the generation of the inflammatory bowel disease (IBD) mouse model and the configuration of treatment groups, while the right panel depicts the treatment process and tissue acquisition timing on a weekly basis; B: Determination of efficacy by evaluating bowel length. A representative illustration (left) and a comparison of each group (right) were presented. Bowel length served as an indicator of inflammation and gut health. The dextran sulfate sodium-treated groups demonstrated shorter bowel length compared to the control group. Both control-secretome (Ct-Sec) and etanercept-secretome (Et-Sec) groups exhibited significantly longer bowel length, with Et-Sec showing a more pronounced elongation compared to Ct-Sec (P < 0.05), indicating stronger anti-inflammatory effects in IBD mouse model. Values are presented as mean ± SD of three independent experiments. aP < 0.05. Et-Sec: Etanercept-secretome; Ct-Sec: Control-secretome; LPS: Lipopolysaccharide; TNF: Tumor necreosis factor; MCP-1: Monocyte chemoattactant protein-1; IL: Interleukin; IBD: Inflammatory bowel disease; DSS-treated group: Dextran sulfate sodium-treated group; ASCs: Adipose-derived stem cells.
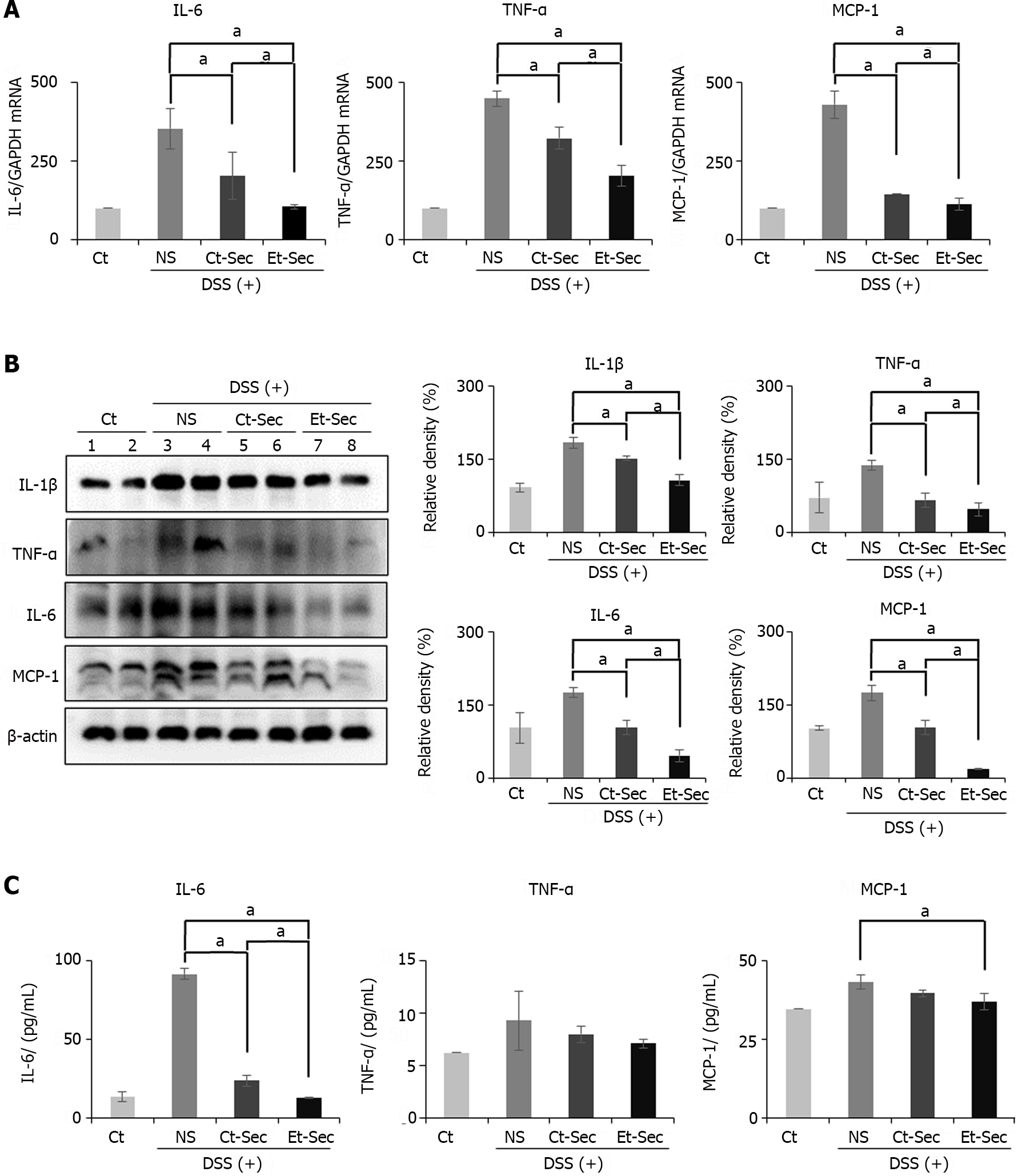
Figure 3 Determination of in vivo anti-inflammatory effects of etanercept-secretome.
A: Realtime polymerase chain reaction of inflammatory markers. Secretome-treated groups [control-secretome (Ct-Sec) and etanercept-secretome (Et-Sec)] showed reduced expression compared to normal saline (NS) (P < 0.05). Et-Sec group exhibited a more significant reduction than Ct-Sec group (P < 0.05); B: Western Blot Analysis of inflammatory markers. The Secretome-treated groups (Ct-Sec and Et-Sec) demonstrated reduced expression compared to NS (Ps < 0.05), with the Et-Sec group exhibiting a more significant reduction than the Ct-Sec group (P < 0.05); C: ELISA determing the levels of serum inflammatory markers. The Et-Sec group exhibited the most significant reduction in serum interleukin-6 and monocyte chemoattactant protein-1 levels in the inflammatory bowel disease mouse model (P < 0.05). Relative densities of individual markers had been quantified using Image J software and then were normalized to that of β-actin in each group. Values are presented as mean ± SD of three independent experiments. aP < 0.05. Et-Sec: Etanercept-secretome; Ct-Sec: Control-secretome; LPS: Lipopolysaccharide; TNF: Tumor necreosis factor; MCP-1: Monocyte chemoattactant protein-1; IL: Interleukin; IBD: Inflammatory bowel disease; DSS-treated group: Dextran sulfate sodium-treated group; ASCs: Adipose-derived stem cells; Ct: Control group; NS: Normal saline.
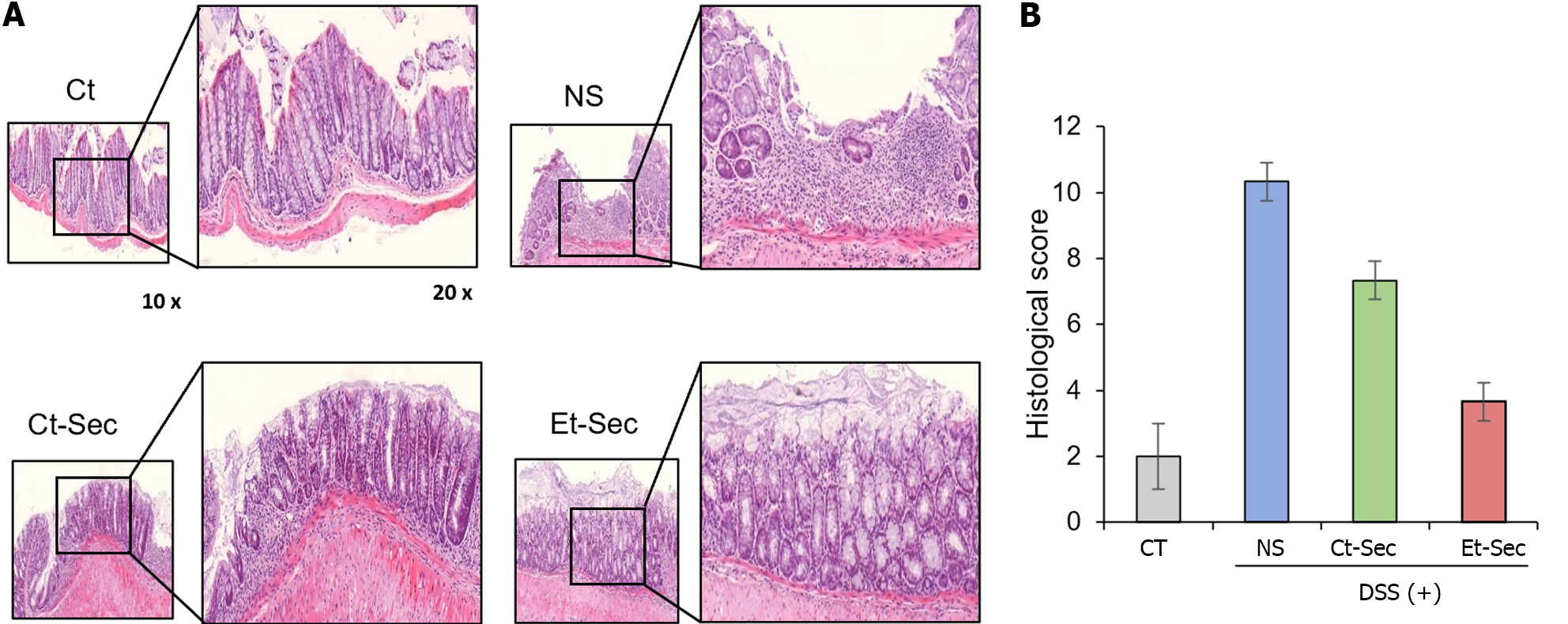
Figure 4 Histological and immunohistochemical evaluation of the in vivo anti-inflammatory effects of etanercept-secretome.
A: Left panel: Hematoxylin and eosin (H&E) staining of intestinal tissue samples. The dextran sulfate sodium (DSS)-treated group displayed tissue disorganization, while the etanercept-secretome (Et-Sec)-treated group exhibited a marked recovery of tissue architecture, suggesting a potential therapeutic effect of Et-Sec on inflammatory bowel disease. Right panel: Comparison of histological scores reflecting inflammation in H&E stains. The Et-Sec group demonstrated significantly lower scores compared to all DSS-treated groups (P < 0.05); B: Immunohistochemical analysis of inflammatory markers, including tumor necreosis factor-α, PECAM-1, F4/80, and monocyte chemoattactant protein-1. DSS administration led to a significant increase in these inflammatory markers, while treatment with secretome (Ct-Sec and Et-Sec) resulted in a significant reduction in their levels (P < 0.05). Importantly, Et-Sec treatment demonstrated a more pronounced reduction in the all inflammatory markers compared to Ct-Sec treatment (P < 0.05). Values are presented as mean ± SD of three independent experiments. Percentages of immunoreactive areas were measured using NIH image J and expressed as relative values to those in control tissues. aP < 0.05. Et-Sec: Etanercept-secretome; Ct-Sec: Control-secretome; LPS: Lipopolysaccharide; TNF: Tumor necreosis factor; MCP-1: Monocyte chemoattactant protein-1; IL: Interleukin; IBD: Inflammatory bowel disease; DSS-treated group: Dextran sulfate sodium-treated group; ASCs: Adipose-derived stem cells; Ct: Control group; NS: Normal saline.
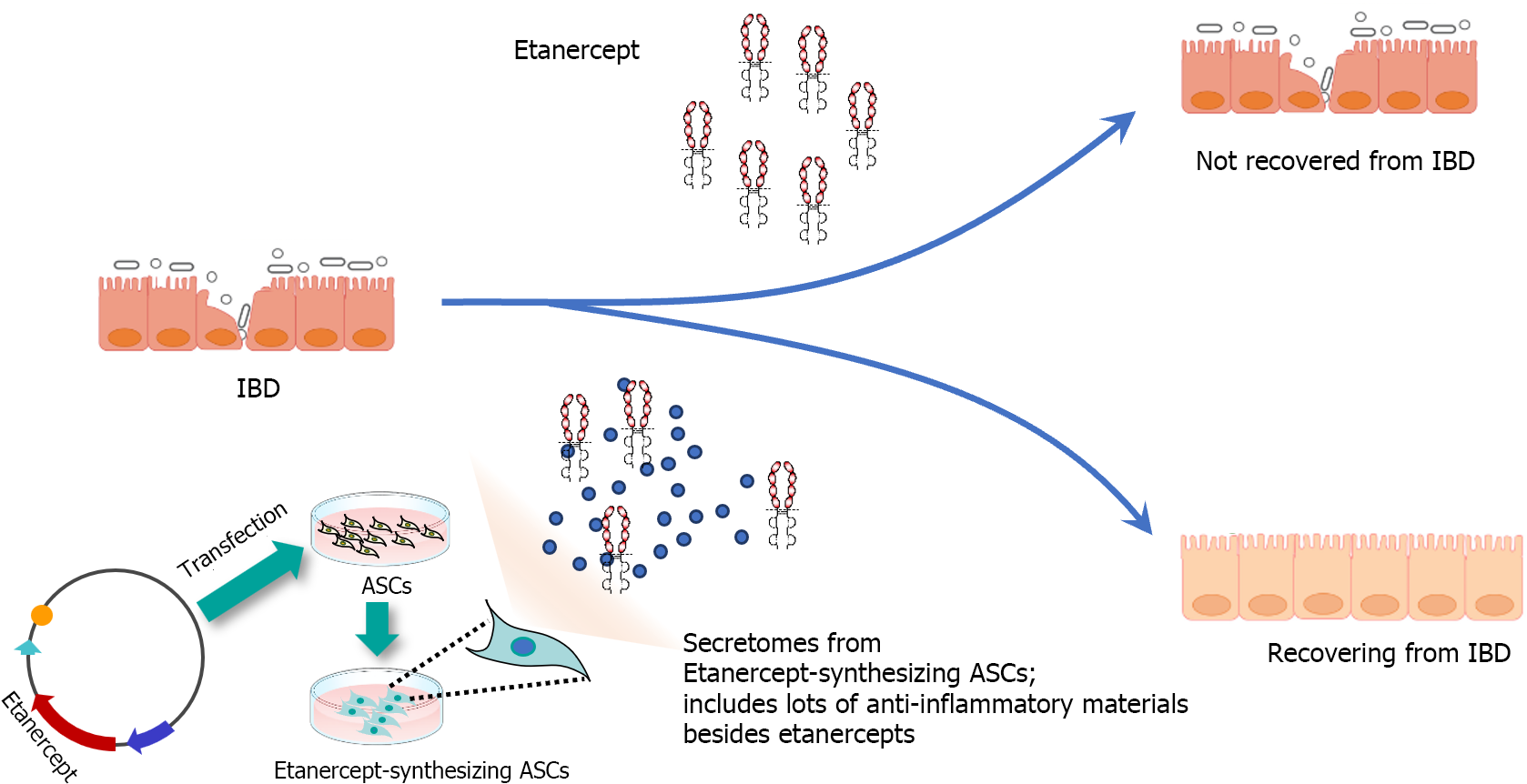
Figure 5 Possible anti-inflammatory mechanism of etanercept-secretome in inflammatory bowel disease.
The therapeutic efficacy of Etanercept in treating inflammatory bowel disease (IBD) exhibits variability, likely due to the complex and multifactorial nature of IBD, which involves a multitude of pro-inflammatory factors beyond tumor necreosis factor-α. This complexity suggests the need for a multi-targeted therapeutic approach. In contrast, etanercept-secretome (Et-Sec) is obtained from genetically modified adipose-derived stem cells capable of producing both etanercept and a diverse secretome characterized by anti-inflammatory and immunomodulatory attributes. This unique composition raises the possibility that Et-Sec may possess enhanced effectiveness in suppressing the inflammatory mechanisms associated with IBD when compared to the use of etanercept alone. IBD: Inflammatory bowel disease; ASCs: Adipose-derived stem cells.
- Citation: Kim SJ, Kim OH, Hong HE, Ju JH, Lee DS. Etanercept-synthesizing adipose-derived stem cell secretome: A promising therapeutic option for inflammatory bowel disease. World J Gastrointest Surg 2024; 16(3): 882-892
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/882.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.882