Published online Jun 25, 2015. doi: 10.4239/wjd.v6.i6.807
Peer-review started: November 17, 2014
First decision: December 12, 2014
Revised: May 4, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: June 25, 2015
Processing time: 215 Days and 11 Hours
Long-acting glucagon-like peptide-1 (GLP-1) analogues marketed for type 2 diabetes (T2D) treatment have been showing positive and protective effects in several different tissues, including pancreas, heart or even brain. This gut secreted hormone plays a potent insulinotropic activity and an important role in maintaining glucose homeostasis. Furthermore, growing evidences suggest the occurrence of several commonalities between T2D and neurodegenerative diseases, insulin resistance being pointed as a main cause for cognitive decline and increased risk to develop dementia. In this regard, it has also been suggested that stimulation of brain insulin signaling may have a protective role against cognitive deficits. As GLP-1 receptors (GLP-1R) are expressed throughout the central nervous system and GLP-1 may cross the blood-brain-barrier, an emerging hypothesis suggests that they may be promising therapeutic targets against brain dysfunctional insulin signaling-related pathologies. Importantly, GLP-1 actions depend not only on the direct effect mediated by its receptor activation, but also on the gut-brain axis involving an exchange of signals between both tissues via the vagal nerve, thereby regulating numerous physiological functions (e.g., energy homeostasis, glucose-dependent insulin secretion, as well as appetite and weight control). Amongst the incretin/GLP-1 mimetics class of anti-T2D drugs with an increasingly described neuroprotective potential, the already marketed liraglutide emerged as a GLP-1R agonist highly resistant to dipeptidyl peptidase-4 degradation (thereby having an increased half-life) and whose systemic GLP-1R activity is comparable to that of native GLP-1. Importantly, several preclinical studies showed anti-apoptotic, anti-inflammatory, anti-oxidant and neuroprotective effects of liraglutide against T2D, stroke and Alzheimer disease (AD), whereas several clinical trials, demonstrated some surprising benefits of liraglutide on weight loss, microglia inhibition, behavior and cognition, and in AD biomarkers. Herein, we discuss the GLP-1 action through the gut-brain axis, the hormone’s regulation of some autonomic functions and liraglutide’s neuroprotective potential.
Core tip: Glucagon-like peptide-1 (GLP-1) physiological responses are dependent on a gut-brain axis and receptor (GLP-1R) activation. GLP-1Rs are widely expressed throughout the body, including several brain areas. GLP-1 may readily diffuse across the blood-brain-barrier, activating neuroprotective pathways. Given the native GLP-1 short half-life, liraglutide has been developed with a highly increased half-life, allowing its use to treat type 2 diabetes (T2D). Given T2D patients increased risk for obesity and dementia [e.g., Alzheimer disease (AD)], and evidence from preclinical studies, whereby liraglutide showed impressive neuroprotective effects, clinical studies are underway to test the role of liraglutide on weigh control and AD.
- Citation: Candeias EM, Sebastião IC, Cardoso SM, Correia SC, Carvalho CI, Plácido AI, Santos MS, Oliveira CR, Moreira PI, Duarte AI. Gut-brain connection: The neuroprotective effects of the anti-diabetic drug liraglutide. World J Diabetes 2015; 6(6): 807-827
- URL: https://www.wjgnet.com/1948-9358/full/v6/i6/807.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i6.807
The incretin effect was first discovered when experiments were conducted to evaluate the possibility that food ingestion would lead to hormone secretion by the gut into the bloodstream to modulate pancreatic insulin secretion and lower blood glucose levels[1-3]. Shortly after, glucose-dependent insulinotropic polypeptide (GIP) and gastrointestinal glucagon-like peptide-1 (GLP-1) were described as the incretin hormones secreted by intestinal cells that were responsible for a potent insulinotropic activity upon elevated plasma glucose (70% of the postprandial insulin secretion)[4-7]. More recently, in an increased number of studies directed to the analysis of incretins effects in patients with type 2 diabetes (T2D), impaired interactions between mediators such as insulin, glucagon and incretin hormones have been increasingly suggested to underlie the development of T2D[8].
In this review, we will focus primarily on the complex interaction between gut and central nervous system (CNS), particularly in the regulation of appetite and body weight. More specifically, we will briefly overview the role of the increasingly used anti-T2D drug from the incretin/GLP-1 mimetics class liraglutide in brain, with a special emphasis on its anorectic and potential neuroprotective effects on T2D-associated neurodegeneration.
Despite the known GIP action in inhibiting gastric acid secretion and gastrointestinal motility, as well as in the stimulation of insulin release[6,7], it was observed that not only GLP-1 is more insulinotropic in hyperglycemic conditions than GIP[9,10], but also that GIP insulinotropic activity was diminished in T2D patients[11,12]. Thus, as the GIP secretion was maintained normal or even increased, such apparently reduced β-cell response to GIP might result from a down-regulation of GIP receptor expression/activity[13,14]. This was further reinforced by the observation that, despite no changes in GLP-1-related insulinotropic activity in diabetic patients, their impaired insulin secretion could be associated with a decreased incretin effect[15-17]. Importantly, in addition to its insulinotropic effects, GLP-1 has been also involved in the suppression of postprandial glucagon secretion, delaying gastric emptying, promoting early satiety (and the subsequent decrement in food intake), slowing the rate of endogenous glucose production and, ultimately, promoting weight loss, particularly in diabetic conditions[4,18,19]. Moreover, it has been reported that, by stimulating cell proliferation and protecting against apoptosis, GLP-1 also enhanced pancreatic β-cell mass[20,21]. In this perspective, it has been suggested that the combination of these effects may contribute to the normalization of blood glucose levels in T2D patients[19,22], thus rendering the GLP-1 hormone (instead of GIP) a very attractive target for the treatment of T2D.
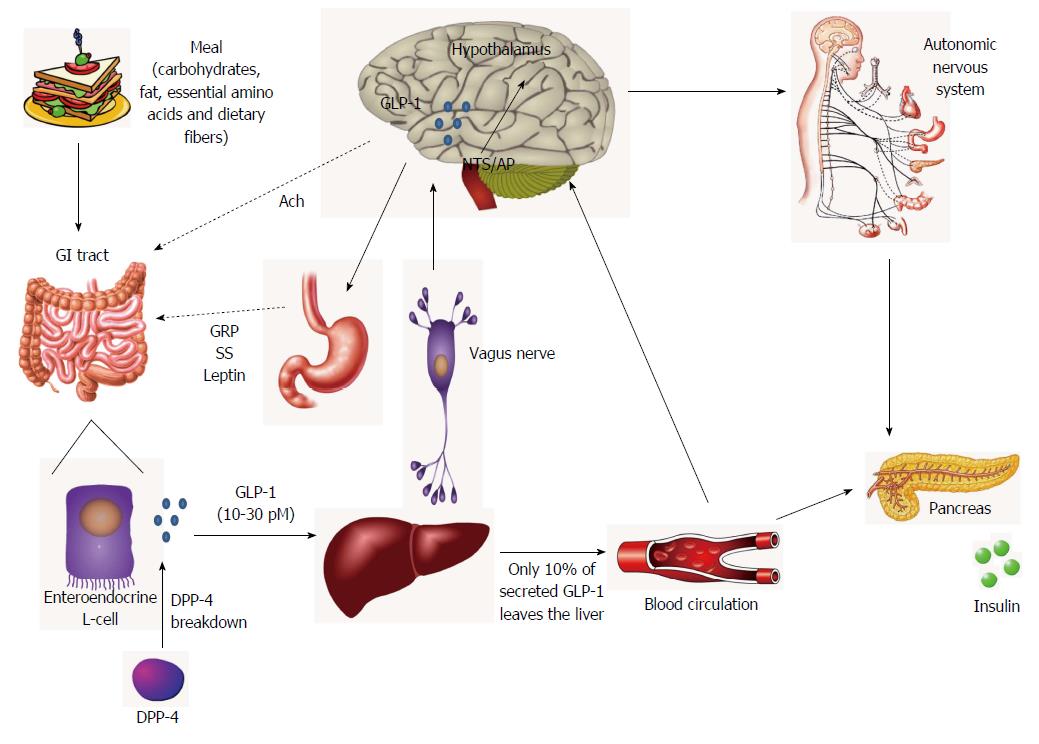
As previously referred, GLP-1 is primarily synthesized and secreted from the intestine (ileum and colon) enteroendocrine L cells and, to a lesser extent, from pancreatic α-cells and from neurons located at the nuclei of brainstem [solitary tract nucleus (NTS), caudal brainstem and area postrema (AP)][23-25].
This hormone arises from the post-translational cleavage of proglucagon (catalyzed by the prohormone convertase) and, depending on the tissue, proglucagon may originate different products[26]. For instance, in pancreas the major products are glucagon, glycentin related polypeptide and a major proglucagon fragment, containing the GLP-1 and GLP-2 sequences, whilst in brain and gut proglucagon processing liberates GLP-1, GLP-2 (which is not an incretin, as it is deprived from insulinotropic and glucose lowering properties), IP-2, glicentin, and oxyntomodulin[23,26]. Additionally, recent studies showed that multiple forms of GLP-1 are secreted by humans, including GLP-1 (1-37) and GLP-1 (1-36) amides (synthesized as immature forms), as well as the bioactive forms glycine-extended form GLP-1 (7-37)-amide and the GLP-1 (7-36)-amide (this being the predominant form in plasma and brain)[23,27].
Most GLP-1 is secreted postprandially, particularly after fat- and carbohydrate-rich meals. Interestingly, this secretion is proportional to the size of the meal and may reach 10-30 pM[4,19]. Individual nutrients, including glucose and other sugars, fatty acids, essential amino acids and dietary fibers also stimulate GLP-1 release[28]. Amongst these, the glucose and fructose mechanism of stimulation have been the more explored and it has been shown that, in humans, oral (but not intravenous) glucose administration stimulates GLP-1 secretion[4,26]. Moreover, basal secretion of GLP-1 may even occur as a product of glucagon secretion in fasting state and reach 5-10 pM, being essential for maintaining glucose homeostasis[4,25]. Interestingly, plasma GLP-1 (7-36)-amide increases rapidly (within just a few minutes) through a biphasic pattern of secretion and release after oral glucose absorption, composed by an early phase within 10-15 min followed by a prolonged second phase at 30-60 min[28,29].
Although the majority of secreting L-cells are located in the distal small intestine, they can be found also throughout the entire length of the small intestine[30,31]. Interestingly, these cells may contact with different regions, being stimulated by a variety of mediators. For instance, L-cells can contact directly with nutrients at their luminal surface and with vascular tissue through their basolateral surface, as well as with the enteric and the CNS via the vagus nerve[31,32]. Hence, evidence suggests that early and late phases of GLP-1 secretion may be generated either through (1) the direct nutrient stimuli to L-cells (particularly those located in more proximal regions of the small intestine, being at least partially responsible to induce the first phase of GLP-1 secretion); or (2) via the indirect action of neural and endocrine factors[19,30,32]. More specifically, it has been hypothesized that the early GLP-1 secretion in rodents and humans may be indirectly regulated by the autonomic nervous system and neurotransmitters and peptides [e.g., gastrin-releasing peptide, acetylcholine, γ-aminobutyric acid (GABA), calcitonin gene-related peptide and GIP], with the vagus nerve playing an essential role herein[31,33]. Additionally, others proposed that non-nutrient factors, as leptin and insulin, could also contribute to the rapid release of GLP-1[33,34].
Importantly, brain GLP-1 can be peripherally originated (from the intestine), reaching the CNS through leaks in the blood-brain barrier (BBB) (at the level of area postrema and subfornical organs), whereby it may readily diffuse across the BBB (GLP-1 is a relatively small molecule), and may also influence the activity of afferent vagal neurons[35]. However, as previously referred, GLP-1 synthesis can also occur locally in brain, in a process dependent on the complex brainstem-hypothalamic-preproglucagon system. More specifically, in preproglucagon neurons from the CNS, proglucagon is processed to GLP-1 in neuronal cell bodies[36]. First evidence showed that the largest population of GLP-1 immunoreactive innervations occurred in the dorsomedial and paraventricular nuclei of the hypothalamus, and to a lesser extent in the cortex and hindbrain[37,38]. Then, others reported that preproglucagon neurons are primarily located in the lower brainstem, particularly in the caudal NTS and AP, some cell bodies being also found in the dorsomedial part of the medullary reticular nucleus[37,39,40]. Besides this, both the NTS and AP appear to receive visceral sensory inputs generated by the vagal nerves that innervate the gastroduodenal tract[41]. Indeed, it has been described that sensors in the hepatic portal vein may activate the vagus nerve, initiating a neural signal to the NTS/AP in the brainstem, which in turn transmits the information through axons to the hypothalamic nuclei[42]. Intracellularly, GLP-1 secretion may be mediated by several signaling pathways. In general, these include the activation of protein kinases A (PKA) and C (PKC) and mitogen-activated protein kinase (MAPK), as well as an increase in intracellular calcium (Ca2+)[43]. More specifically, upon a meal, the increase in blood glucose is accompanied by its uptake into the cells (namely via sodium/glucose transporters) and subsequent metabolization. As a result, the increment in ATP levels may lead to the closure of ATP-linked potassium channels and, ultimately, GLP-1 secretion[33,43]. Conversely, inhibition of GLP-1 secretion in gut has been described to involve a negative feedback, probably via GLP-1-mediated stimulation of somatostatin secretion[34,44]. Interestingly, the neuropeptide galanin has been also identified as an inhibitor of GLP-1 secretion from intestinal L-cells, both in vitro and in vivo[26,34].
Physiological responses of GLP-1 are elicited upon binding of the hormone to its receptors belonging to the class B family of 7-transmembrane heterotrimeric expressed G-protein-coupled receptors, a family that also includes receptors for glucagon, GLP-2, and GIP[45,46]. Increasing evidence points towards an ubiquitous expression of GLP-1 receptor (GLP-1R), in tissues ranging from pancreas (α, β, and δ cells), lung, heart, kidney, stomach, intestine, pituitary, skin and ganglion neurons of the vagus nerve, to multiple regions of CNS (including brainstem, hypothalamus, hippocampus and cortex)[26,30]. Importantly, GLP-1R expression was detected in mammalian brain neurons, astrocytes, microglia and endothelial cells[47] and, even more strikingly, GLP-1Rs have been identified in lipid rafts, where they interact with caveolin-1, thereby regulating receptor subcellular localization, trafficking, and signaling[48].
Interestingly, rat and human GLP-1Rs are polypeptiede chains with 463 amino acids and share 90% sequence homology[19]. Structurally, GLP-1R possesses a long N-terminal extracellular region responsible for peptide recognition and binding, and a cytoplasmic C-terminal containing the components for specific G protein coupling, thus having a major influence in signaling specificity and transmission[49,50]. Once activated, GLP-1R stimulates the adenylyl cyclase system, increasing intracellular cyclic adenosine monophosphate (cAMP) levels and, subsequently, activating the downstream PKA and exchange protein activated by cAMP-2 (Epac2) pathways[4,29]. Alternatively, GLP-1R activation may also increase intracellular Ca2+ and phospholipase C levels, or stimulate other signal transduction pathways, depending on the activated tissues, including phosphoinositide 3-kinase (PI3K), insulin receptor substrate-2, epidermal growth factor receptor transactivation, PKC, MAPK, cyclic AMP response element binding protein (CREB), pancreatic duodenal homeobox-1, and glucose transporter-2[26,51,52].
Importantly, some authors suggested that at least part of GLP-1-associated endocrine effects (e.g., GLP-1R-dependent insulin secretion) may be indirectly mediated by neural mechanisms[42]. This appears to be supported by the increasing notion that GLP-1R activation may generate new signals to guide the energetic flux towards tissues (via the autonomic nervous system) and ultimately regulating a diverse array of homeostatic functions (Figure 1)[23,53,54].
Concerning the use of incretin-based anti-T2D therapy, we must bear in mind that a continuous GLP-1 administration would be required to effectively maintain glucose homeostasis. In fact, given the native GLP-1 short half-life of less than 2 min [the hormone is rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4)][55,56], this would render its therapeutic use unfeasible, as we will discuss later.
DPP-4 is a ubiquitous and multifunctional enzyme that can be found either solubilized in blood or membrane-anchored in many cell types[57]. This glycoprotein is widely expressed in multiple tissues, including kidney, lung, adrenal gland, pancreas, liver, thymus, lymph node, uterus, placenta, prostate and on the surface of lymphocytes, macrophages and endothelial cells[58,59]. More relevant herein, DPP-4 appears to be also expressed in several brain areas (e.g., hypothalamus, hippocampus, circumventricular organs, choroid plexus, and leptomeninges)[60,61]. And besides its well known role in GLP-1 inactivation, DPP-4 has been also implicated in numerous pleiotropic cellular processes involving cell cycle regulation, proliferation, adhesion, immunomodulation and apoptosis[62-64].
Molecularly, DPP-4 is able to specifically cleave different dipeptides possessing an alanine, proline or hydroxyproline in the penultimate N-terminal position. These substrates include fibronectin, substance P, chemokines, neuropeptide Y (NPY), peptide YY (PYY), and the best validated in vivo substrates: GLP-1, GLP-2 and GIP[62,63]. The resulting GLP-1 (7-36)-amide is metabolized to GLP-1 (9-37) or GLP-1 (9-36)-amide, which has a 1000-fold reduced affinity for GLP-1R and thus completely blunts its insulin-releasing activity[56,57]. Besides DPP-4, another relevant step in GLP-1 inactivation process can be catalyzed by the neutral endopeptidase (NEP), a membrane-bound zinc metallopeptidase expressed in both the periphery and CNS, that is responsible for GLP-1 (7-36)-amide hydrolysis into smaller peptides[65,66]. Therefore, as most of GLP-1 passing the portal circulation has been already degraded by DPP-4, GLP-1 (9-37) and GLP-1 (9-36)-amide constitute the major circulating forms of the hormone (with an estimated half-life of 8-10 min, as a result of renal clearance)[25]. Apparently, this suggests that, after GLP-1 secretion and release by intestinal L-cells, DPP4 starts to continuously degrade the incretin hormone, thus accounting for 50% of GLP-1 inactivation[30,67]. Then, after its passage through the liver, another large amount of the remaining intact bioactive form of the peptide is further inactivated, thus culminating in less than 10% of active GLP-1 reaching the blood circulation[30].
The management of T2D through a patient’s lifetime is often difficult and frequently renders the achievement of therapeutic goals unsuccessful. However, this scenario has been increasingly challenged in the recent years, due not only to the promising results obtained with GLP-1-related therapy in T2D, but also to its widespread beneficial effects on body weight and metabolic parameters where other promising anti-T2D approaches failed[68]. Altogether, this rendered the GLP-1R-mediated intracellular signaling one of the most appealing targets in the development of therapies for diabetes management. Importantly, and despite GLP-1’s pharmaceutical promise, the first crucial step was the need to overcome its rapid degradation by DPP-4, thereby enhancing the hormone’s action time[69-71]. In this regard, two novel classes of glucose lowering agents, the DPP-4-inhibitors and the GLP-1R agonists (GLP-1RAs) have been intensively developed over the last years[72].
DPP-4-inhibitors are orally-given small molecules that compete with DPP-4 substrates for the active site of the enzyme, thus avoiding the inactivation of native bioactive GLP-1 and, therefore, extending its half-time and increasing its levels in circulation[73,74]. Currently, there are three inhibitors approved for treatment of T2D in the United States and Europe: sitagliptin, saxagliptin and linagliptin[74]. Besides these, vildagliptin is another DPP-4 inhibitor also available only in Europe[73].
On the other hand, given the peptidic nature of GLP-1RAs, it is necessary to administer them by subcutaneous injection[75]. These molecules have been developed based on the effects of native GLP-1 (binding to GLP-1R and activating similar glucoregulatory effects), but with a high resistance to DPP-4 degradation, thereby increasing the systemic GLP-1 activity[73]. Currently, three GLP-1RAs are commercially available: exenatide twice daily (EBID), liraglutide once daily, and exenatide once weekly (EQW)[74]. Importantly, most of these incretin-based therapies have been approved as a second line therapy, in dual or triple combination with other anti-diabetic therapies, including metformin, sulphonylureas (SU) and thiazolidinediones (TZD)[75].
Since the endogenous levels of incretin hormones appear to be reduced in T2D patients, increasing evidence points towards a higher effectiveness of GLP-1RAs-based therapies in glycemic control than the use of DPP-4-inhibitors, particularly in reducing glycated hemoglobin A1C (HbA1C) and postprandial glucose levels[8,11]. Furthermore, contrary to GLP-1RAs, DPP-4-inhibitors appear to be weight neutral, with no effect in gastric emptying, and with less described positive cardiovascular effects[76,77]. And although some adverse events have been reported in both therapeutic subclasses [namely an increased risk for infections with DPP-4 inhibitors and common gastrointestinal side effects (predominantly nausea) with GLP-1RAs], the overall tolerability was comparable and general positive results were described for both classes of incretin-related therapy, with no episodes of major hypoglycemia documented in patients on either therapy[8,78]. Thus, it is not surprising that, in the last years, the incretin-based therapies (and particularly GLP-1RAs) have been increasingly faced as the potential “dream team” not only for the treatment of T2D and its associated complications, but also for other disorders involving changes in glucose homeostasis and metabolism [e.g., neurodegenerative diseases, as Alzheimer disease (AD)].
As previously referred, the main advantage of GLP-1RAs over DPP-4 in terms of T2D treatment appears to be the fact that these patients often present lower circulating incretins’ levels[8,11]. Although comparative clinical efficacy data are still limited to support the use of one GLP-1RA molecule over another, some comparative studies have already shown some differences between the two main GLP-1RAs, exenatide and liraglutide. First, liraglutide has been considered a true GLP-1 agonist, sharing 97% sequence identity with human GLP-1, while exenatide is a mimetic isolated from the saliva of the Gila monster (Heloderma suspectum) that shares only 53% structural similarity with native GLP-1[79-83]. Additionally, the long-acting GLP-1 analog liraglutide, Arg34,Lys26-{N-ε-[γ-Glu(N-α-hexadecanoyl)]}-GLP-1 (7-37), has a substitution of a lysine residue with arginine at position 34 and a 16-carbon fatty acid chain via a glutamic acid spacer attached to lysine at position 26, thereby promoting the noncovalent binding of liraglutide to serum albumin that not only confers its DPP-4 enzyme resistance and protection from renal clearance, but also allows liraglutide molecules to form heptamers, slowing its absorption rate from injection site and increasing its half-life in plasma to 13 h (in contrast with the 2 h half-life of exenatide)[84-86]. And although the efficacy of peptide injection into the organism may be partially compromised by the formation of antibodies against GLP-1RAs, given its protein sequence differences liraglutide appears to be less immunogenic than exenatide[75,87,88]. Indeed, the Liraglutide Effect and Action in Diabetes-6 (LEAD-6) study reported that 61% of T2D patients treated with exenatide developed antibodies compared to only 2.6% of patients given liraglutide[89,90]. Importantly, this trial also showed that, during the 26-wk study, liraglutide was significantly more efficient than exenatide BID in reducing HbA1c levels (1.12% vs 0.79% respectively) and in improving HOMA-β (homeostasis model assessment of β-cell function) index in T2D patients, thereby suggesting that liraglutide may also induce a better improvement in β-cell function[89,90]. But, to us, the most striking point from the LEAD-6 clinical trial was that, in a 14-wk extension, patients who started and responded well to exenatide BID treatment could even further ameliorate some parameters when switching to liraglutide. For instance, these patients further reduced HbA1C levels by 0.32%, body weight by 0.9 kg and systolic blood pressure by 3.8 mmHg[89,90]. In another comparative study between liraglutide and exenatide QW - the DURATION-6 trial -, the first was shown to decrease HbA1C levels by 1.48% in T2D patients compared to the 1.28% lowering achieved with exenatide QW[91]. Additionally, in this study more patients submitted to liraglutide therapy were able to achieve HbA1C < 7% than with exenatide QW[91]. And, as in LEAD-6, weight loss was greater among patients receiving liraglutide (-3.58 kg vs -2.68 kg). Interestingly, 94% of the liraglutide-treated T2D patients from the DURATION-6 trial were satisfied with their treatment compared with 86% of those receiving exenatide[91]. To further complete this comparative overview on both drugs, we must refer that, since the primary route of exenatide clearance from the body is through renal excretion, this may pose some risk of accumulation in patients with renal disease, and, thus, exenatide is not recommended for patients with hepatic impairment[79,92,93]. Conversely, liraglutide (as GLP-1) is almost exclusively enzymatically degraded by DPP-4 and NEP, and therefore, renal impairment should not affect liraglutide efficacy[94].
The actions of liraglutide in peripheral diseases: T2D is mainly characterized by hyperglycemia and an impaired insulin action. However, T2D most dangerous and devastating consequences may arise from the development of long-term complications (e.g., retinopathy, nephropathy, cardiovascular disease, stroke, neuropathy, cerebrovascular disease), which in most cases are already installed by the time of diagnosis[95].
As previously discussed, the first characteristic that turned GLP-1 into an ideal candidate for T2D treatment was its property to enhance glucose- induced insulin release and overcome insulin desensitization[6,7]. However, over time GLP-1 has been shown to exert many other interesting (and potentially therapeutically relevant) effects in the organism[96] and, in this perspective, the GLP-1RA liraglutide may have also a significant impact (rather than the “mere” GLP-1R activation), not only in periphery, but also in CNS and in other pathologies besides T2D (Table 1), as we will further discuss.
| Liraglutide effects | Study | Ref. |
| Pancreas | Review | Davies et al[99] |
| Preclinical | Shao et al[97] | |
| Yosida et al[98] | ||
| Heart | Review | Davies et al[99] |
| Martín-Timón et al[100] | ||
| Vilsbøll et al[101] | ||
| Seufert and Gallwitz[104] | ||
| Preclinical | Liu et al[103] | |
| Noyan-Ashraf et al[106] | ||
| Shiraki et al[107] | ||
| Clinical | Russell-Jones et al[102] | |
| Marso et al[105] | ||
| Kidney | Preclinical | Fujita et al[114] |
| Clinical | Armstrong et al[110] | |
| Davidson et al[111] | ||
| Liver | Clinical | Eguchi et al[116] |
| Armstrong et al[117] | ||
| Muscle | Preclinical | Ji et al[109] |
| Li et al[118] | ||
| Weight and appetite | Review | Davies et al[99] |
| Seufert and Gallwitz[104] | ||
| Rigato and Fadini[119] | ||
| Sjoholm[121] | ||
| van Bloemendaal et al[123] | ||
| Ng and Wilding[135] | ||
| Clinical | Toft-Nielsen et al[17] | |
| Horowitz et al[133] | ||
| Wadden et al[136] | ||
| Wadden et al[137] | ||
| Senda et al[138] | ||
| [139] | ||
| Astrup et al[140] | ||
| Rosenstock et al[141] | ||
| Neuroprotection/T2D brain | Review | Hölscher[205] |
| Duarte et al[206] | ||
| Hölscher[210] | ||
| Preclinical | Hou et al[47] | |
| Hunter et al[204] | ||
| Hamilton et al[207] | ||
| Agrawal et al[208] | ||
| Cummings et al[209] | ||
| Parthsarathy et al[212] | ||
| AD | Preclinical | McClean et al[213] |
| Han et al[214] | ||
| McClean et al[215] | ||
| Long-Smith et al[216] | ||
| McClean et al[217] | ||
| Parthsarathy et al[218] | ||
| Yang et al[219] | ||
| Clinical | [220] | |
| [221] | ||
| [222] | ||
| Stroke | Preclinical | Sato et al[227] |
Regarding the role of liraglutide in the periphery and the progressive loss and dysfunction of pancreatic β-cells that constitutes one of the key features of T2D, several recent studies have shown that the drug was able not only to protect (through anti-apoptotic effects), preserve and enhance β-cell mass, but also to improve β-cell function [seen in the db/db and in the transient receptor potential melastatin 2 (TRPM2)-deficient mice models], thereby improving glucose control, and insulin secretion and sensitivity[97-99].
It is well known that chronic hyperglycemia and poor blood glucose control may also underlie cardiovascular dysfunction and development of cardiovascular disease, thus rendering T2D a risk factor for cardiovascular disease[100]. Therefore, it is not surprising that emerging data from animal and human studies also point towards a liraglutide-mediated reduction in systolic blood pressure and improvement in lipid profiles (decreased low-density lipoprotein cholesterol and triglycerides levels, and increased high-density lipoprotein cholesterol)[99,101,102]. Moreover, Liu et al[103] demonstrated that liraglutide improves cardiac function in diabetic rats, probably due to a decreased expression of proteins involved in the endoplasmatic reticulum stress pathway. Accordingly, recent studies also associated liraglutide treatment with a significant decrease in several cardiovascular risk biomarkers, such as plasminogen activator inhibitor-1, B-type natriuretic peptide [104-106], interleukin-6 (IL-6) and tumor necrosis factor-α[107,108]. Besides cardiovascular function, liraglutide was also shown to reduce endothelial cell dysfunction in a process probably mediated by its anti-oxidant and anti-inflammatory effects[107]. Moreover, other tissues can be directly or indirectly affected by GLP-1RA therapies, including muscle, liver and kidney[109-111], thus rendering them highly promising against other pathologies than T2D. However, some caution must be used and further clarification is needed as, e.g., was recently reported that, albeit rare the aggravation of an existing nephropathy in T2D patients submitted to GLP-1RA treatment may be due to external factors (e.g., other medications), but if related with this therapy it may probably arise from gastrointestinal problems[112,113]. Nevertheless, recent data also showed that liraglutide was able to counteract renal impairment in a mouse model that also displayed chronic hyperglycemia and increased renal oxidative stress[114]. According to these authors, such protection was due to liraglutide-mediated inhibition of NAD(P)H oxidase and activation of cAMP-PKA pathway, thus suppressing the progression of renal failure[114].
Concerning the effects of GLP-1 analogues in hepatic impairment, there is currently an increasing interest mostly due to the lack of effective therapies against hepatic diseases. Amongst them, the non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver diseases, being strongly related with insulin resistance, metabolic syndrome, cardiovascular disease, cerebral vessel disease and T2D[115]. In a recent pilot study involving patients diagnosed with diabetes complicated by NAFLD/non-alcoholic steatohepatitis (NASH), liraglutide (0.9 mg/body per day) was given for 24 wk[116]. Data suggested that liraglutide significantly improved liver function (as given by the decreased serum levels of alanine aminotransferase, aspartate aminotransferase, ferritin and C-reactive protein) and histological changes in NASH patients[116]. Besides this, data from LEAN-2 study demonstrated that liraglutide combined with metformin also exerted positive effects in liver enzymes and hepatic steatosis in T2D patients[117].
Regarding the impact of liraglutide on muscle, Li et al [118] showed recently that this drug increases cAMP and AMP-activated protein kinase (AMPK) signaling pathways, which may in turn induce GLUT4 translocation in mouse skeletal muscle cells.
The role of liraglutide in appetite and weight loss: A centrally-regulated peripheral anorectic effect: Unhealthy lifestyle, weight gain and obesity are intimately related with the increased prevalence of T2D worldwide, being estimated that 60%-90% of T2D patients are overweight[119-121]. Importantly, weight gain has been also described as a common side-effect of standard anti-T2D therapies. Therefore, weight management should be an important issue herein, as it not only may interfere with treatment, but may also increase the risk for the development of long-term complications (e.g., cardiovascular disease)[120,122]. However, the control of body weight and energy balance is a complex process, involving different tissues and pathways, such as gut, adipose tissue, pancreas and brain[30,123]. For instance, gut-derived hormones (e.g., GLP-1, PYY, ghrelin, cholecystokinin and oxyntomodulin) are known to play a major role in feeding regulation, at least partially by relaying to the CNS information on nutritional status[123]. On the other hand, GLP-1’s well known anoretic properties have been suggested to arise from the combination of both central and peripheral effects, via another gut-brain connection[23,124]. More specifically, GLP-1-mediated satiating effect requires primarily the activation of peripheral GLP-1Rs located on vagal sensory afferents in the gut and in the hepatoportal region of the liver; after the transmission of the metabolic signals into the brain c-fos expression is increased in NTS neurons[124,125]. Once activated, these neurons act on brain neuronal circuits from the brainstem and several other areas involved in appetite control, where GLP-1R are also expressed. These include the ventral tegmental area (VTA), nucleus accumbens (NAc) and hypothalamus[37,39,123]. In the hypothalamus, GLP-1R are highly expressed in the paraventricular nucleus, dorsomedial hypothalamus and the arcuate nucleus (ARC) [where they overlap with the pro-opiomelanocortin (POMC) neurons (anorexigenic neurons)], being present at a lesser extent in agouti-related peptide (AgRP)/NPY neurons (orexigenic neurons)[23,126,127]. At this respect, Seo et al[128] demonstrated that intracerebroventricular (icv) infusion of GLP-1 stimulates the synthesis of anorexigenic peptides (POMC and cocaine- and amphetamine-regulated transcript), and simultaneously decreased the synthesis of NPY and AgRP in rodents. Accordingly, while effects on hypothalamic and brainstem circuits may regulate food intake, the inhibitory effect of GLP-1 on the rewarding value of food appears to be regulated by the regions of VTA and NAc (mesolimbic reward system)[123,124]. This hypothesis was supported by the finding that the administration of the GLP-1R antagonist, exendin 9-39, into the NAc promoted hyperphagia in rats[129]. After hypothalamic and brainstem (dorsal motor nuclei) stimulation, efferent impulses depart from these brain areas to regulate the gastrointestinal tract, pancreas and other peripheral organs, by slowing gastric emptying and acid secretion, and decreasing gut motility, thereby further controlling the feeding behavior and glucose metabolism[39,124]. Although such slowed gastric emptying may also arise from a direct activation of gastric inhibitory GLP-1Rs, this appears to be more visible with long-acting GLP-1RAs, particularly liraglutide (whose potent gastric emptying capacity has been increasingly described)[104]. Importantly, the rate of gastric emptying may also influence stomach distension, thereby stimulating gastric mechanoreceptors, with the subsequent activation of the nodose ganglion (inferior ganglion of vagus nerve) that may per se culminate in the activation of NTS neurons to induce satiety[123]. Besides this, a direct central action of GLP-1 in satiation signaling was also demonstrated by an inhibition of food intake by pathways independent of the vagal afferents[130-132].
As T2D-associated postprandial hyperglycemia may be further aggravated by an accelerated gastric emptying, it is not surprising that GLP-1RAs drugs have been successful in attenuating postprandial blood glucose levels also by slowing gastric emptying[133]. And although this is not considered the main mechanism by which these drugs regulate appetite and weight loss, it has been widely described that the incidence of gastrointestinal adverse events (such as nausea and vomiting, which albeit transient may affect nearly 50% of treated patients) is often accompanied by a decrease in food intake and body weight. Despite the limited knowledge on the mechanisms involved, it is plausible that a direct effect on gastrointestinal system and a central action producing conditioned taste aversion may play a role herein[104,123,134].
In recent clinical trials, T2D patients treated with liraglutide (either as a monotherapy or in combination therapies) have shown a weight loss, in contrast with the weight gain associated with SU, TZD and insulin[119,121]. More strikingly, both people with or without T2D, treated with clinically relevant doses of liraglutide for 20 to 30-wk presented significant reductions in body weight (from 1 to 3 kg)[99,135]. Importantly herein, despite the increased GLP-1 levels in response to the amount of nutrient intake, obese people were shown to have lower basal GLP-1 levels[136]. Accordingly, a negative relation was also established between body mass index (BMI) and GLP-1 oral stimulation[17]. Moreover, the SCALE Maintenance study reported a loss of 5.9 kg in obese patients treated with 3.0 mg liraglutide for 56-wk vs placebo-treated ones[137]. Interestingly, even a study case involving the Prader-Willi syndrome (PWS) (a rare genetic disorder characterized by an extreme and insatiable appetite that ultimately leads to morbid obesity), described that a 25-year-old female hyperglycemic PWS patient submitted to liraglutide therapy, not only improved her glycemic control, but was also able to control hyperphagia and to decrease plasma ghrelin levels and her BMI[138].
From the above, such consistent weight loss observed with liraglutide and other GLP-1RAs, not only in T2D patients, but also in non-T2D people has aroused the interest for the use of GLP-1RAs as promising pharmacotherapies for weight management. To further potentiate this interest, we must bear in mind that, to date, only one medical therapy against obesity is approved in Europe, with most current anti-obesity pharmacological drugs having serious undesirable side effects[135]. In this regard, the use of these drugs in obese people is currently being tested in several clinical trials[139-141].
The neuroprotection in type 2 diabetic and degenerative brain: Insulin and liraglutide: (1) Pathological commonalities between T2D and neurodegenerative diseases: neuroinflammation and brain dysfunctional insulin/ insulin-like growth factor-1 (IGF-1).
Obesity, hypertension, dyslipidemia and T2D all constitute risk factors, particularly vascular, for cognitive dysfunction[142]. Numerous experimental data indicated a decrease in cognitive function in T2D patients, which is often accompanied by impairments in memory, attention, intelligence, processing speed and executive functions, as well as by brain atrophy (particularly in cortical, subcortical and hippocampal areas) and white matter abnormalities[143,144]. Interestingly, the well described cell loss that occurs in T2D pancreas appears to be accompanied by cell loss also in the CNS upon disease progression[145,146]. Indeed, the pathological progression of T2D and, more specifically, its associated glucose toxicity, insufficient insulin action, neuroinflammation and general aging, amongst others, can slowly lead to nervous damage and may ultimately result in diabetic neuropathy and diabetic encephalopathy, thus increasing the risk for dementia in diabetes[147-149]. In line with this, chronic inflammatory response in brain has been suggested to play a pivotal role in propagating T2D-mediated injury. This may probably occur via activation of microglia and other immune cells, with the subsequent release of neurotoxic amounts of proinflammatory cytokines and free radicals and ultimately leading to neurodegeneration and brain disease upon T2D progression[150-152]. Additionally, similarly to desensitization of IR from peripheral tissues of T2D patients, increasing evidence also point towards the impairment of brain insulin signaling in AD[153,154]. As a consequence, it has been increasingly suggested that brain insulin resistance could be a potential link between T2D and AD[95,151]. This hypothesis has been supported by numerous evidences, e.g., (1) a decrease in IR expression in brains from AD patients; (2) an inverse correlation found between AD Braak stage and the levels of such receptors; (3) the accumulation of hyperphosphorylated tau (a neuropathological hallmark of AD) upon insulin signaling impairment and the consequent inhibition of GSK-3β phosphorylation and of tau protein phosphatase 2A[155,156]; (4) the increased Aβ accumulation and decreased insulin degrading enzyme (IDE) levels in AD patients displaying also brain insulin resistance[157]; and (5) the memory enhancement and protection against Aβ toxicity in AD patients submitted to insulin therapy[158]. These and other in vivo and in vitro findings have been also corroborated and extended by epidemiological studies showing an increased risk for T2D patients to develop AD and vice versa. According to a very recent study, T2D patients have a 65% increased risk to develop AD later in their life[155], whereas others found that 85% of AD patients had either T2D or increased fasting glucose levels[159].
Parkinson disease (PD) is the second most common neurodegenerative disorder after AD, the most common form of parkinsonism (motor syndrome) and, pathophysiologically, is the predominant form of synucleinopathies, which are characterized by an abnormal accumulation of α-synuclein (α-syn) protein[160]. PD is characterized by the neuronal accumulation of Lewy bodies (containing deposits of α-syn) and the progressive loss of dopaminergic neurons (particularly in substantia nigra) that may culminate in multiple motor (tremor, rigidity, slowness of movement, and postural instability) and non-motor (autonomic dysfunction and neuropsychiatric problems) symptoms occurring throughout the course of the disease[161]. Nowadays, an increasing amount of evidence points towards several similar biochemical changes between PD and T2D, leading to the idea that dysfunctional insulin signaling might be at the center of those alterations[162]. Amongst such commonalities are, e.g., the increased serum IGF-1 levels in PD patients[163,164], both the insulin and IGF-1 resistance found in basal ganglia and substantia nigra from PD[165,166], the impaired dopaminergic signaling observed in a rat model of T2D[167], and the observation that activation of PI3K/Akt pathway was able to rescue α-syn toxicity in vitro[168].
Stroke (or cerebrovascular accidents) is considered the third most common cause of death in developed countries and may develop as a long-term complication in comorbid diseases, such as T2D[169,170]. Indeed, epidemiological studies point towards a 2- to 6-fold increased risk of T2D patients to development of stroke, whereas stroke victims often present impaired glucose tolerance[171,172]. It is well known that this disease arises from a disturbance in the blood supply to the brain (due to ischemia or hemorrhage), thus resulting in neurological deficits and a loss of brain function[173]. Interestingly, stroke has been regularly associated with increased neuroinflammation markers[174], thereby rendering GLP-1 analogues an appealing therapeutic strategy, as we will discuss later.
Strikingly, such correlation between T2D, AD, PD and stroke appears to be also applicable to a wide range of other neurodegenerative diseases, including vascular dementia or Huntington disease (HD)[95,170], thereby suggesting that T2D is a risk factor for at least some of these brain pathologies.
(2) Brain insulin/IGF-1 as metabolic and body weight regulators.
As it is well known, the primary regulators of glucose homeostasis and metabolism involve insulin/IGF-1 signaling pathways responsible for the control of both peripheral signals and CNS effects[155]. Although most of the brain insulin is primarily secreted by the pancreas (whereas IGF-1 comes from the liver), being then transported by cerebrospinal fluid (CSF) and crossing the BBB into the brain, numerous evidence also points towards their local synthesis (particularly in brain cortex, olfactory bulb, hippocampus, hypothalamus, and amygdala)[175]. Once bound to their receptors, the insulin and IGF-1 receptors (IR and IGF-1R, respectively) that are ubiquitously expressed in brain (particularly neurons), many physiological functions are activated, such as neuronal outgrowth and survival, enhancement of attention, memory formation and cognition, food intake and weight maintenance, synaptic protection and sexual regulation[170,176,177]. Of these, food intake and energy homeostasis are the most directly related also with GLP-1R-mediated signaling, being mediated by a specialized group of hypothalamic glucosensing neurons that appear to be able to detect and respond to even small variations in glycemia[130]. Such anorexigenic effect of insulin signaling depends on a strict control of the hypothalamic PI3K/protein kinase B (Akt) signaling pathway, on the inhibition of NPY and AgRP expression and on the induction of POMC neurons[178,179].
From the above, it is not surprising that changes in body weight, hyperinsulinemia and insulin resistance may arise from alterations in the homeostatic balance, thus culminating in injurious effects on the organism[180,181]. Indeed, hyperinsulinemia has been widely associated to a downregulation of insulin transport across the BBB, thus decreasing its uptake and levels in the brain, as well as the subsequent IR activity, ultimately leading to a brain insulin resistance[182,183]. Moreover, insulin resistance and subsequent impairment in glucose supply, transport and utilization may lead to glucose dysmetabolism that may be also accompanied by a decrease in cerebral flow and damaging effects to intracellular organelles, including mitochondria[184-186]. As brain mitochondria are particularly susceptible to metabolic impairment, situations like brain insulin resistance may lead to dysfunctional respiratory chain and phosphorylation system, and alterations in mitochondrial dynamics and biogenesis that may significantly compromise ATP production and mitochondrial membrane potential. As a result, mitochondrial permeability transition pore may open and ultimately activate apoptotic cell death[184]. Thus, defects in brain insulin/IGF-1 signaling may raise a deleterious vicious cycle involving metabolic impairment and mitochondrial dysfunction, that in turn generate a wide range of harmful effects (e.g., increased oxidative stress, advanced glycation end-products formation, inflammatory response, excitatory neurotransmitter release)[175,187]. Altogether, these alterations can be responsible for a decrease in neuronal function and survival, and impaired synaptic activity and plasticity, that may be also accompanied by a decrease in neurogenesis, leading to impaired memory formation and storage, and learning potential, culminating in cognitive decline[188]. To further intricate this subject, as we detailed above, both T2D and neurodegeneratives diseases share several common features that together with the neuroprotective effects demonstrated by many of the already marketed anti-T2D therapeutic agents have potentiated the recent investigations worldwide on the potential beneficial role of such drugs also in the context of neurodegeneration[96,189].
(3) Restoration of insulin/IGF-1 action as a potential therapeutic target in neurodegenerative diseases: what can we count on in a near future?
Preclinical studies involving insulin and IGF-1 to treat animal models of neurodegenerative diseases revealed promising results. In this regard, our group showed that insulin was able to protect against oxidative stress and a decline in mitochondrial oxidative phosphorylation efficiency induced by the amyloid β-peptide (Aβ, one of the main players in AD pathology) in streptozotocin (STZ)-induced type 1 diabetic rats[190]. Similarly, Quesada et al[191] reported that IGF-1 treatment significantly increases tyrosine hydroxylase (TH) positive neurons and improves motor performance of the medial forebrain bundle 6-hydroxydopmaine (6-OHDA) lesion rat model of PD, in a PI-3K/Akt-dependent manner. Additionally, we and others showed that by increasing blood insulin and brain IGF-1 levels, the in vivo peripheral administration of IGF-1 was not only able to protect against peripheral glucose intolerance[192], but also to rescue motor deficits and brain glucose dysmetabolism upon HD[193]. Molecularly, these observations were further supported and extended by a recent study using an in vitro model of human HD, in which insulin- and IGF-1-mediated PI3K/Akt activation was able to improve mitochondrial function and decrease mitochondrial reactive oxygen species (ROS) formation[194].
Even though insulin therapy can be very useful to prevent or slow the progression of long-term complications of diabetes and others pathologies, as diseases progress not only the hormone’s beneficial effects may be lost (including glycemic control), but also it may increase the risk for severe hypoglycemic episodes, which have been also associated with increased brain damage. As a result this may further increase neuronal and cognitive dysfunction[195]. Alternatively, the use of insulin sensitizers (another commonly prescribed class of anti-T2D drugs, whose most widely used member is metformin, a first line pharmacological approach against the pathology) could provide a reliable therapeutic alternative also in the context of neurodegenerative diseases[154,196]. Indeed, metformin was demonstrated to protect the brains of the non-obese T2D Goto-Kakizaki rat models against oxidative imbalance, by decreasing oxidative stress markers and increasing antioxidant defenses[197]. Additionally, Gupta et al[198] reported that metformin ameliorates neuronal insulin resistance and AD-associated characteristics in an in vitro model of the so-called “type 3 diabetes”. Nevertheless, we must bear in mind that metformin presents several controversial side effects, and some authors suggested that metformin therapy and subsequent AMPK activation may even exacerbate previously existing impairments[199-201].
From all the above, and given also the previously mentioned actions in the brain, the native GLP-1 hormone would be considered the “almost perfect” alternative to restore insulin action in CNS, either in T2D, neurodegenerative diseases or simply in normal aging. And this has been widely corroborated by its rapid capacity to cross the BBB when injected peripherally, acting as a growth factor in brain, whereby the hormone has been shown to stimulate the metabolism, the expression of genes associated with cell growth, repair and replacement and to protect against oxidative injury, neuroinflammatory response and apoptosis, thus exerting beneficial effects in neuronal health and cognition[155]. However, given the previously described limited half-life of the native GLP-1, recent research interests have been mostly focused on the neuroprotective potential of DPP-4 inhibitors and GLP-1RAs.
Regarding DPP-4 inhibitors, their beneficial roles in CNS have been intensively analyzed and appear to constitute promising candidates for the treatment of CNS disorders. In this regard, sitagliptin (the first DPP-4 inhibitor approved for T2D treatment), has been recently shown to positively affect working and reference memories, and to increase the hypothalamic acetylcholine content and adiponectin receptor 1 expression in T2D Sprague-Dawley rats[202]. Additionally, vildagliptin (another DPP-4 inhibitor)-mediated decrease in Aβ, tau protein phosphorylation and neuroinflammatory markers levels were also accompanied by an amelioration in memory retention capacity in a STZ-induced AD rat model[203].
On the other hand, and similarly to the native GLP-1, liraglutide and the other synthetic GLP-1RAs are able to readily cross the BBB, reaching the brain almost intact and thereby exerting neuroprotective effects[204]. Therefore, it is not surprising that most of the preclinical studies that reported such protective effects were performed in T2D, AD, PD and also in stroke models[205]. For instance, liraglutide has been increasingly suggested to prevent or attenuate T2D-associated neuronal and cognitive deficits[206]. Although the underlying mechanisms remain unclear, Hamilton et al[207] proposed that such protection could rely on the activation of stem cells and neuronal progenitor cells to counteract T2D-induced neurodegeneration. Indeed, these authors reported that liraglutide was able to promote neurogenesis [as given by the increase in the number of 5’-bromo-2’-deoxyuridine (BrdU)-positive cells dividing progenitor cells and doublecortin-positive young neurons] in the dentate gyrus of three different types of obese T2D mouse models. Additionally, other authors observed that liraglutide administration in 2 mo-old pre-diabetic UCD-T2D rats (a polygenic obese model of T2D) reduced energy intake and body weight, improved insulin sensitivity and reduced triglycerides, thus delaying diabetes onset by about 4 mo compared to controls[208,209]. Moreover, these authors reported that liraglutide normalized brain metabolic homeostasis (as given by TFAM, SIRT1, and AMPK phosphorylation), decreased hippocampal lipid oxidation (upon determination of 4-hydroxynonenal levels), improved brain mitochondrial regulation (via PGC-1α) and preserved synaptic plasticity (as given by the BDNF-TrkB signaling) in the UCD-T2D rats over the disease progression[208,209]. These results appear to be in line with previous evidences that GLP-1R activation may be involved in memory and learning[210]. In fact, During et al[211] demonstrated that GLP-1R activation was correlated with an enhancement of associative and spatial learning, as GLP-1R-deficient mice exhibited a learning deficit phenotype. Strikingly, liraglutide (25 nmol/kg, once daily i.p., for 30 d) has been also demonstrating potent anti-inflammatory effects in brains from a mouse model irradiated with 6Gy (X-ray, a model of chronic inflammation), as given by a reduction in microglia activation in cortex and dentate gyrus, and a decrease in mean astrocyte load after irradiation (by massively reducing the total GFAP load), in pro-inflammatory cytokines (IL-6, IL-12p70, IL-1β) and in total nitrite levels[212]. Moreover, liraglutide was able to decrease cerebral edema, and to ameliorate both neurobehavioral deficits (in modified Garcia test and wire hanging test) and inflammatory parameters (given by an increased brain phosphorylated AMPK and reduced neutrophil infiltration) in an intracerebral hemorrhage-induced brain injury mouse model (strongly associated with inflammatory mechanisms)[47].
Therefore, given also the pathological commonalities between T2D and AD, it is plausible that a therapeutic approach involving incretin analogues might be beneficial against the cognitive deficits occurring in AD.
The neuroprotective potential of liraglutide in AD has been increasingly analyzed. In one of the first studies, McClean et al[213] reported that liraglutide significantly affects brain neurotransmission and modulates synaptic plasticity by enhancing long-term potentiation (LTP) mechanisms. More recently, Han et al[214] observed that pre-treatment with liraglutide dose-dependently protected against the impairment in learning and memory (as given by the dysfunctional spatial memory and hippocampal late-phase LTP) induced by a bilateral intrahippocampal injection of Aβ in adult male Sprague-Dawley rats, suggesting a possible preventive strategy against the development of AD in T2D patients. Strinkingly, McClean et al[215], Long-Smith et al[216] and McClean et al[217] described that once daily intraperitoneally (i.p.)-injected liraglutide for 8 wk was able to reduce brain amyloid plaque formation by 30%-50%, as well as the levels of soluble amyloid oligomers (by 25%) and amyloid precursor protein (APP) in the APP/PS1 AD mouse model (which expresses the human Swedish mutated form of APP and a mutated human form of presenilin-1). Additionally, these authors observed that chronic liraglutide administration blunted the Aβ-associated changes in neuronal IR localization and IRS-1 phosphorylation at serine 616 (a key marker of insulin resistance), increased IDE levels, reduced microglia activation by up 50%, decreased the Aβ-associated astrocytic activation, enhanced LTP and synaptophysin levels, and increased hippocampal neuronal progenitor cells, thus indicating a protection against neuroinflammation, synapse loss and deterioration of synaptic plasticity that may culminate in the restoration of memory function, particularly in object recognition and water maze tasks. Interestingly, Parthsarathy et al[218] also reported that chronic treatment with liraglutide-mediated increase in neurogenesis in an AD mouse model was accompanied by an increased differentiation of newly generated cells into mature neurons. Concerning abnormal tau protein phosphorylation (another neuropathological hallmark of AD), liraglutide treatment has been able to reduce both brain tau protein phosphorylation at different residues (Ser199, Ser202, and Ser396) and phospho-tau immunoreactivity in T2D rats, which also displayed a reduction of HOMA-IR close to control levels and a normalization of brain insulin signaling (as given by the activation of Akt and subsequent inhibition of GSK-3β at Ser9)[219]. Altogether, these results strongly suggest that liraglutide may be able to prevent and/or reverse the major pathological hallmarks of AD. In line with this, two clinical trials are underway to assess the effects of liraglutide in AD. In a small randomized clinical trial at the University of Aarhus (Denmark)[220], 17 early-onset AD patients were treated with liraglutide (1.8 mg, once a day, 26 wk) and the already available results point towards an effect of liraglutide on cerebral amyloid deposits in the brain (assessed by Pittsburg compound B PET scan), but more novelties are expected soon. The second trial, at the Imperial College of London and launched in June 2013[221], is a large-scale Phase 2 clinical trial involving 206 early AD patients treated with liraglutide (1.8 mg/d, for 12 mo), that aims to use fluorodeoxyglucose-PET scan to detect changes in cerebral glucose metabolic rate, microglia activation, CSF markers, and amyloid and tau levels, to correlate with eventual Alzheimer Disease Assessment Scale Executive (ADAS) and Magnetic resonance imaging (MRI) changes. Interestingly, another ongoing, Phase 2, randomized, double-blind clinical trial sponsored by the National Institute on Aging and aiming at analyzing the action of the long-acting GLP-1RA exendin-4 (Ex-4) in AD[222] will end by December 2015 and involves the evaluation of patients’ performance in the Clinical Dementia Rating scale sum-of-boxes, ADAS (cognitive sub-scale), behavior and cognition, changes on structural and functional MRI and magnetic resonance spectroscopy, hormonal and metabolic changes, as well as alterations in CSF and plasma AD biomarkers.
Regarding the protective potential of GLP-1 analogues against PD, evidences are still relatively scarce and mostly relying on preclinical assessing the role Ex-4 in PD. Upon a 3 wk, twice daily i.p administration of Ex-4, it was observed a significant increase in the number of TH-immunoreactive neurons and of dopamine levels that, together with a reduction of amphetamine-induced rotations in the 6-OHDA rat model of PD, suggest that Ex-4 may have beneficial cellular and functional properties in this model of PD[223,224]. Similar effects were also reported in another rat model of PD involving a lipopolysaccaride-induced lesion to substantia nigra[224]. Moreover, Li et al[225] observed that Ex-4 protected against the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, another PD-like phenotype inducer)-associated loss of nigral neurons and striatal dopaminergic fibers, preserved dopamine levels and improved motor function. With all this in mind, in a recent randomized Phase 2 clinical trial, Ex-4 safety and efficacy was evaluated in 21 PD patients, which received 5 mg b.i.d for 1 mo or 10 mg b.i.d for 11 mo[226]. The main observations herein included a good tolerability to Ex-4, improved motor scores [from the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)] at 12 mo (a mean improvement of 2.7 vs a 2.2 point decline in control group) and cognitive efficiency (using the Mattis dementia rating scale 2) (a mean improvement of 2.8 vs a 3.5 point worsening in control group)[226].
Given the previously mentioned increasing interest on the potential therapeutic use of GLP-1 analogues for stroke treatment, in a recent study Sato et al[227] demonstrated that liraglutide was able to ameliorate behavioral scores, to reduce brain infarct volumes at 24 h, to decrease the levels of ROS derivatives and to upregulate vascular endothelial growth factor in brain cortex of a rat model for stroke (90 min transient middle cerebral artery occlusion and 1 h reperfusion). Similarly, neuroprotective effects upon stroke and ischemia were also reported for Ex-4[225,228,229].
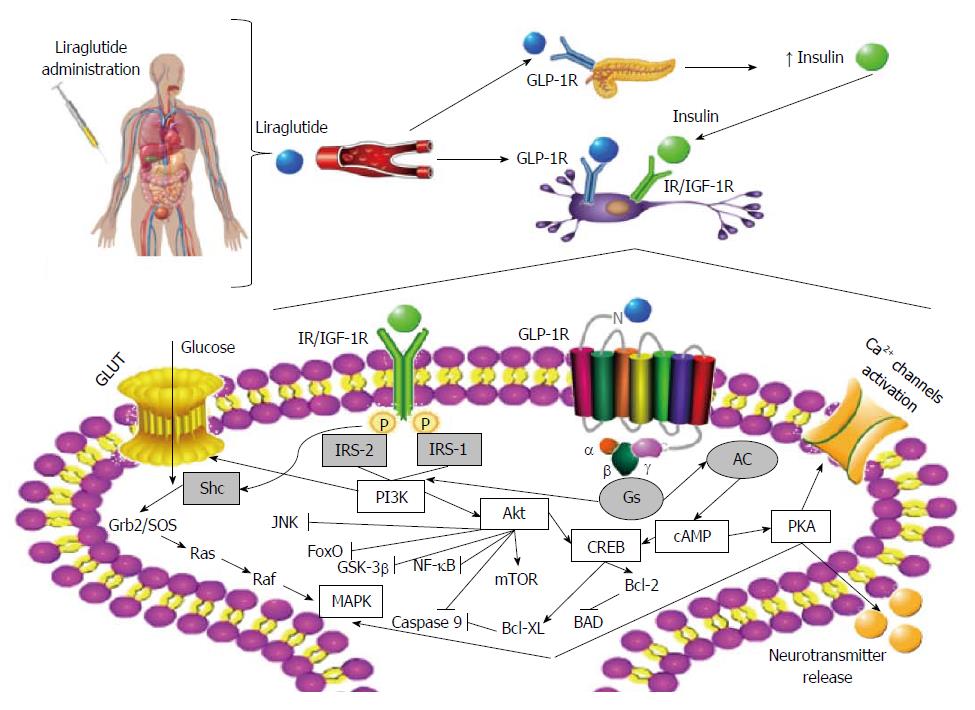
From the above, as exenatide and liraglutide are the currently marketed GLP-1RAs for the treatment of T2D, and since preclinical studies have demonstrated remarkable and consistent neuroprotective effects, some clinical trials are underway to assess the effect and efficacy of Ex-4 and liraglutide in AD and PD patients. And although the underlying molecular mechanisms (particularly those focused on the liraglutide’s neuroprotective and anti-inflammatory potential) remain mostly unknown, a recent study on human neurons shed some light on this issue. Indeed, Sharma et al[230] suggested that liraglutide-mediated neuroprotection may involve the PI3K/Akt pathway and its subsequent regulation of mammalian target of rapamycin; however, it is also plausible that GLP-1R activation under such circumstances may also activate the alternative extracellular signal-regulated kinase signaling. Traditionally, liraglutide-mediated activation of GLP-1R may further activate adenylyl cyclase and increase cAMP content, leading to the subsequent activation of PKA and CREB, and ultimately contributing to cell survival, inhibition of apoptosis, activation of Ca2+ channels, cell growth, repair and regeneration, as well as regulation of translation/transcription processes in response to stress (Figure 2).
As the quote “the key to one’s heart is through his stomach”, the gut hormone GLP-1 (and, more specifically, its long-lasting synthetic analogs) may hold the key for promising therapeutic effects against diseases affecting such different tissues, as the heart, pancreas, kidneys, liver, brain, and all the other tissues where we can find GLP-1Rs. It is plausible that this hormone, mainly secreted from L-cells, may exert its influence on the activities of several organs via complex axis involving the gut. And despite the still limited knowledge on this matter, we believe that the clarification of the molecular mechanisms involved herein might be of the outmost relevance in the context of the promising preventive/therapeutic potential of the long-acting GLP-1RAs against the development of long-term complications of several diseases. As a novel GLP-1RA and already marketed anti-T2D drug, and given the strong association between T2D and numerous neurodegenerative pathologies, liraglutide has been extensively investigated for such purpose in the recent years and has shown remarkable effects, not only peripherally, but also in CNS upon a wide range of diseases, including T2D, AD, PD and stroke. As liraglutide’s positive effects in brain, we emphasize its promotion of neuronal survival, protection from apoptosis, regulation of neuroinflammatory response and modulation of stress response, thereby suggesting a strong neuroprotective potential. And this might be of a pivotal relevance, in our ever increasingly aged world and societies, characterized by an exponential increase in age-related diseases that still lack effective (or at least with a minimum of serious side-effects) preventive or therapeutic approaches, thereby posing an enormous socio-economic burden and urging the seek for an effective cure or delay in such diseases. Thus, the limited preclinical and clinical findings already available strongly suggest that liraglutide may emerge as a potential mono- or combined therapy against most of the common diseases afflicting the brain.
P- Reviewer: Hyogo H, Liu C, Sertoglu E S- Editor: Gong XM L- Editor: A E- Editor: Wang CH
| 1. | McIntyre N, Holdsworth CD, Turner DS. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab. 1965;25:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 232] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Elrick H, Stimmler L, Hlad CJ, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 562] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Moore B. On the treatment of Diabetus mellitus by acid extract of Duodenal Mucous Membrane. Biochem J. 1906;1:28-38. [PubMed] |
| 4. | Combettes MM. GLP-1 and type 2 diabetes: physiology and new clinical advances. Curr Opin Pharmacol. 2006;6:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 581] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem. 1971;49:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 201] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Garber A, Thomsen AB, Hartvig H, Davies M; 1860-LIRA-DPP-4 Study Group. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Mentis N, Vardarli I, Köthe LD, Holst JJ, Deacon CF, Theodorakis M, Meier JJ, Nauck MA. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011;60:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1196] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 11. | Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199-E206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 407] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 12. | Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept. 1994;51:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 228] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Lynn FC, Pamir N, Ng EH, McIntosh CH, Kieffer TJ, Pederson RA. Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes. 2001;50:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 623] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Pratley RE, Gilbert M. Targeting Incretins in Type 2 Diabetes: Role of GLP-1 Receptor Agonists and DPP-4 Inhibitors. Rev Diabet Stud. 2008;5:73-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 450] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 17. | Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 544] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 19. | Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 20. | Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397-4408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600-4605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 261] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Rachman J, Gribble FM, Barrow BA, Levy JC, Buchanan KD, Turner RC. Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide 1 (7-36) amide in patients with NIDDM. Diabetes. 1996;45:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Cabou C, Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. Rev Diabet Stud. 2011;8:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic α-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? J Endocrinol. 2011;211:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010;21:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [PubMed] |
| 27. | Gejl M, Rungby J, Brock B, Gjedde A. At the centennial of Michaelis and Menten, competing Michaelis-Menten steps explain effect of GLP-1 on blood-brain transfer and metabolism of glucose. Basic Clin Pharmacol Toxicol. 2014;115:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117-126. [PubMed] |
| 29. | Koole C, Pabreja K, Savage EE, Wootten D, Furness SG, Miller LJ, Christopoulos A, Sexton PM. Recent advances in understanding GLP-1R (glucagon-like peptide-1 receptor) function. Biochem Soc Trans. 2013;41:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2055] [Cited by in RCA: 2323] [Article Influence: 129.1] [Reference Citation Analysis (1)] |
| 31. | Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550-E559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 33. | Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-Cell: The view from within. Diabetes. 2006;55:70-77. [DOI] [Full Text] |
| 34. | Bojanowska E. Physiology and pathophysiology of glucagon-like peptide-1 (GLP-1): the role of GLP-1 in the pathogenesis of diabetes mellitus, obesity, and stress. Med Sci Monit. 2005;11:RA271-RA278. [PubMed] |
| 35. | Orskov C, Poulsen SS, Møller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45:832-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 192] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept. 2005;128:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 498] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 258] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118-126. [PubMed] |
| 41. | Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 698] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 42. | Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 44. | Chisholm C, Greenberg GR. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am J Physiol Endocrinol Metab. 2002;283:E311-E317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 46. | Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 47. | Hou J, Manaenko A, Hakon J, Hansen-Schwartz J, Tang J, Zhang JH. Liraglutide, a long-acting GLP-1 mimetic, and its metabolite attenuate inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2012;32:2201-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Baggio LL, Kim JG, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes. 2004;53 Suppl 3:S205-S214. [PubMed] |
| 49. | Coopman K, Wallis R, Robb G, Brown AJ, Wilkinson GF, Timms D, Willars GB. Residues within the transmembrane domain of the glucagon-like peptide-1 receptor involved in ligand binding and receptor activation: modelling the ligand-bound receptor. Mol Endocrinol. 2011;25:1804-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Al-Sabah S, Donnelly D. A model for receptor-peptide binding at the glucagon-like peptide-1 (GLP-1) receptor through the analysis of truncated ligands and receptors. Br J Pharmacol. 2003;140:339-346. [PubMed] |
| 51. | Holz GG, Leech CA, Habener JF. Activation of a cAMP-regulated Ca(2+)-signaling pathway in pancreatic beta-cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem. 1995;270:17749-17757. [PubMed] |
| 52. | Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE. Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology. 1993;133:57-62. [PubMed] |
| 53. | Knauf C, Cani PD, Kim DH, Iglesias MA, Chabo C, Waget A, Colom A, Rastrelli S, Delzenne NM, Drucker DJ. Role of central nervous system glucagon-like Peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Burcelin R, Dolci W, Thorens B. Portal glucose infusion in the mouse induces hypoglycemia: evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes. 2000;49:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Vilsbøll T, Agersø H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 56. | Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 526] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 57. | Green BD, Flatt PR. Incretin hormone mimetics and analogues in diabetes therapeutics. Best Pract Res Clin Endocrinol Metab. 2007;21:497-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Matheeussen V, Baerts L, De Meyer G, De Keulenaer G, Van der Veken P, Augustyns K, Dubois V, Scharpé S, De Meester I. Expression and spatial heterogeneity of dipeptidyl peptidases in endothelial cells of conduct vessels and capillaries. Biol Chem. 2011;392:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356-5363. [PubMed] |
| 60. | Alponti RF, Frezzatti R, Barone JM, Alegre Vde S, Silveira PF. Dipeptidyl peptidase IV in the hypothalamus and hippocampus of monosodium glutamate obese and food-deprived rats. Metabolism. 2011;60:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92:442-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Kim NH, Yu T, Lee DH. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors. Biomed Res Int. 2014;2014:368703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 432] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 64. | Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 733] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 65. | Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Göke R, Göke B, Thole H, Zimmermann B, Voigt K. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458-E464. [PubMed] |
| 68. | Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G, Zhang R, Zhang S, Lin L, Chen K. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci USA. 2007;104:943-948. [PubMed] |
| 70. | Green BD, Flatt PR, Bailey CJ. Inhibition of dipeptidylpeptidase IV activity as a therapy of type 2 diabetes. Expert Opin Emerg Drugs. 2006;11:525-539. [PubMed] |
| 71. | Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia. 2006;49:253-260. [PubMed] |
| 72. | Campbell RK, White JR. More choices than ever before: emerging therapies for type 2 diabetes. Diabetes Educ. 2008;34:518-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Stolar MW, Grimm M, Chen S. Comparison of extended release GLP-1 receptor agonist therapy versus sitagliptin in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:435-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Montanya E. A comparison of currently available GLP-1 receptor agonists for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2012;13:1451-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Blonde L, Montanya E. Comparison of liraglutide versus other incretin-related anti-hyperglycaemic agents. Diabetes Obes Metab. 2012;14 Suppl 2:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, Ceriello A, Giugliano D. Dipeptidyl peptidase-4 inhibitors and HbA1c target of & lt; 7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2011;13:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 78. | Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE; DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 79. | Eli Lilly Nederland B. V. Byetta® (exenatide) European SPC. [accessed 2014 Oct 7]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf. |
| 80. | Novo Nordisk A/S. Victoza® (liraglutide) European SPC. [accessed 2014 Oct 7]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. |
| 81. | Rossi MC, Nicolucci A. Liraglutide in type 2 diabetes: from pharmacological development to clinical practice. Acta Biomed. 2009;80:93-101. [PubMed] |
| 82. | Irwin N, Green BD, Gault VA, Greer B, Harriott P, Bailey CJ, Flatt PR, O’Harte FP. Degradation, insulin secretion, and antihyperglycemic actions of two palmitate-derivitized N-terminal pyroglutamyl analogues of glucose-dependent insulinotropic polypeptide. J Med Chem. 2005;48:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650-19655. [PubMed] |
| 84. | Steensgaard DB, Thomsen JK, Olsen HB, Knudsen LB. The molecular basis for the delayed absorption of the once daily human GLP-1 analogue, liraglutide. Diabetes. 2008;57:A164. |
| 85. | Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thøgersen H, Wilken M, Johansen NL. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007;50:6126-6132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 86. | Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 531] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 87. | Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK, Kinninger LA, Trautmann ME. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab. 2012;14:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbæk N, Holst J, Nauck M. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab. 2011;96:1695-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, Blonde L, Rosenstock J; Liraglutide Effect Action in Diabetes-6 Study Group. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300-1303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 90. | Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1097] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 91. | Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 92. | Eli Lilly Nederland B. V. Bydureon® (exenatide once-weekly) European SPC. [accessed 2014 Oct 7]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf. |
| 93. | Weise WJ, Sivanandy MS, Block CA, Comi RJ. Exenatide-associated ischemic renal failure. Diabetes Care. 2009;32:e22-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Malm-Erjefält M, Bjørnsdottir I, Vanggaard J, Helleberg H, Larsen U, Oosterhuis B, van Lier JJ, Zdravkovic M, Olsen AK. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab Dispos. 2010;38:1944-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 95. | Candeias E, Duarte AI, Carvalho C, Correia SC, Cardoso S, Santos RX, Plácido AI, Perry G, Moreira PI. The impairment of insulin signaling in Alzheimer’s disease. IUBMB Life. 2012;64:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Sebastião I, Candeias E, Santos MS, de Oliveira CR, Moreira PI, Duarte AI. Insulin as a Bridge between Type 2 Diabetes and Alzheimer Disease - How Anti-Diabetics Could be a Solution for Dementia. Front Endocrinol (Lausanne). 2014;5:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 97. | Shao Y, Yuan G, Feng Y, Zhang J, Guo X. Early liraglutide treatment is better in glucose control, β-cell function improvement and mass preservation in db/db mice. Peptides. 2014;52:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Yosida M, Dezaki K, Uchida K, Kodera S, Lam NV, Ito K, Rita RS, Yamada H, Shimomura K, Ishikawa SE. Involvement of cAMP/EPAC/TRPM2 activation in glucose- and incretin-induced insulin secretion. Diabetes. 2014;63:3394-3403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 99. | Davies MJ, Kela R, Khunti K. Liraglutide - overview of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab. 2011;13:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 472] [Cited by in RCA: 550] [Article Influence: 50.0] [Reference Citation Analysis (6)] |
| 101. | Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 737] [Cited by in RCA: 652] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 102. | Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simó R; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 621] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 103. | Liu J, Liu Y, Chen L, Wang Y, Li J. Glucagon-Like Peptide-1 Analog Liraglutide Protects against Diabetic Cardiomyopathy by the Inhibition of the Endoplasmic Reticulum Stress Pathway. J Diabetes Res. 2013;2013:630537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. 2014;16:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 105. | Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, Pocock S, Steinberg WM, Bergenstal RM, Mann JF. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823-30.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 106. | Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 453] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 107. | Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 108. | Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 679] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 109. | Ji W, Chen X, Lv J, Wang M, Ren S, Yuan B, Wang B, Chen L. Liraglutide Exerts Antidiabetic Effect via PTP1B and PI3K/Akt2 Signaling Pathway in Skeletal Muscle of KKAy Mice. Int J Endocrinol. 2014;2014:312452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 111. | Davidson JA, Brett J, Falahati A, Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocr Pract. 2011;17:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Nandakoban H, Furlong TJ, Flack JR. Acute tubulointerstitial nephritis following treatment with exenatide. Diabet Med. 2013;30:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Kaakeh Y, Kanjee S, Boone K, Sutton J. Liraglutide-induced acute kidney injury. Pharmacotherapy. 2012;32:e7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 114. | Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 115. | Lee J, Hong SW, Rhee EJ, Lee WY. GLP-1 Receptor Agonist and Non-Alcoholic Fatty Liver Disease. Diabetes Metab J. 2012;36:262-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 116. | Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, Araki N, Tanaka K, Yamaguchi M, Matsuda Y. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res. 2015;45:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 117. | Armstrong M, Falahati A, Houlihan DD, Elbrand B, Schmidt WE, Gough S, Newsome PN. Effects of two years of liraglutide treatment on fatty liver disease in patients with type 2 diabetes: analysis of the liraglutide effect and action in diabetes-2 extension trial. Gut. 2010;59:A1-A2. [DOI] [Full Text] |
| 118. | Li Z, Ni CL, Yao Z, Chen LM, Niu WY. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism. 2014;63:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Rigato M, Fadini GP. Comparative effectiveness of liraglutide in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:107-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Zhang F, Tong Y, Su N, Li Y, Tang L, Huang L, Tong N. Weight loss effect of glucagon-like peptide-1 mimetics on obese/overweight adults without diabetes: A systematic review and meta-analysis of randomized controlled trials-1/:meta. J Diabetes. 2015;7:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Sjoholm A. Liraglutide therapy for type 2 diabetes: Overcoming unmet needs. Pharmaceuticals. 2010;3:764-781. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Kostev K, Rex J, Rockel T, Heilmaier C. Effects of selected antidiabetics on weight loss--a retrospective database analysis. Prim Care Diabetes. 2015;9:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221:T1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 124. | Holst JJ. Incretin hormones and the satiation signal. Int J Obes (Lond). 2013;37:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 125. | Baumgartner I, Pacheco-López G, Rüttimann EB, Arnold M, Asarian L, Langhans W, Geary N, Hillebrand JJ. Hepatic-portal vein infusions of glucagon-like peptide-1 reduce meal size and increase c-Fos expression in the nucleus tractus solitarii, area postrema and central nucleus of the amygdala in rats. J Neuroendocrinol. 2010;22:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 127. | Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69-72. [PubMed] |
| 128. | Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J. 2008;55:867-874. [PubMed] |
| 129. | Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453-14457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 130. | Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103-3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 131. | Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 132. | Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492-2500. [PubMed] |
| 133. | Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, Doran S, Jax T, Zdravkovic M, Chapman IM. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 134. | Trapp S, Hisadome K. Glucagon-like peptide 1 and the brain: central actions-central sources? Auton Neurosci. 2011;161:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Ng SY, Wilding JP. Liraglutide in the treatment of obesity. Expert Opin Biol Ther. 2014;14:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Wadden D, Cahill F, Amini P, Randell E, Vasdev S, Yi Y, Church J, Sun G. Circulating glucagon-like peptide-1 increases in response to short-term overfeeding in men. Nutr Metab (Lond). 2013;10:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 506] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 138. | Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012;59:889-894. [PubMed] |
| 139. | Studies found for: liraglutide and obesity in ClinicalTrials.gov. US National Institutes of Health. [accessed 2014 Oct 9]. Available from: http://clinicaltrials.gov/ct2/results?term=liraglutide and obesity&Search=Search. |
| 140. | Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner S. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36:843-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 473] [Article Influence: 33.8] [Reference Citation Analysis (1)] |
| 141. | Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, Trautmann M. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 142. | van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim Biophys Acta. 2009;1792:470-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 143. | Bordier L, Doucet J, Boudet J, Bauduceau B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab. 2014;40:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 144. | Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, Oliveira CR, Moreira PI. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation. Brain Res. 2012;1441:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 145. | Hussain S, Mansouri S, Sjöholm Å, Patrone C, Darsalia V. Evidence for cortical neuronal loss in male type 2 diabetic Goto-Kakizaki rats. J Alzheimers Dis. 2014;41:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 146. | Wang L, Zhai YQ, Xu LL, Qiao C, Sun XL, Ding JH, Lu M, Hu G. Metabolic inflammation exacerbates dopaminergic neuronal degeneration in response to acute MPTP challenge in type 2 diabetes mice. Exp Neurol. 2014;251:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 147. | Samarghandian S, Azimi-Nezhad M, Samini F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed Res Int. 2014;2014:920857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 148. | Wang JQ, Yin J, Song YF, Zhang L, Ren YX, Wang DG, Gao LP, Jing YH. Brain aging and AD-like pathology in streptozotocin-induced diabetic rats. J Diabetes Res. 2014;2014:796840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 149. | Moreira PI. High-sugar diets, type 2 diabetes and Alzheimer’s disease. Curr Opin Clin Nutr Metab Care. 2013;16:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 150. | Carvalho C, Correia SC, Santos MS, Baldeiras I, Oliveira CR, Seica R, Moreira PI. Vascular, oxidative, and synaptosomal abnormalities during aging and the progression of type 2 diabetes. Curr Neurovasc Res. 2014;11:330-339. [PubMed] |
| 151. | De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 450] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 152. | Srodulski S, Sharma S, Bachstetter AB, Brelsfoard JM, Pascual C, Xie XS, Saatman KE, Van Eldik LJ, Despa F. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 153. | Lin F, Jia J, Qin W. Enhancement of β-amyloid oligomer accumulation after intracerebroventricular injection of streptozotocin, which involves central insulin signaling in a transgenic mouse model. Neuroreport. 2014;25:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F, Gong CX. Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm. 2015;122:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 155. | Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 156. | Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084-7092. [PubMed] |
| 157. | Leal MC, Fernandez Gamba A, Morelli L, Castaño EM. [Cerebral proteolysis of amiloid-b peptide: relevance of insulin-degrading enzyme in Alzheimer’s disease]. Medicina (B Aires). 2009;69:466-472. [PubMed] |
| 158. | Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440-448. [PubMed] |
| 159. | Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301-308. [PubMed] |
| 160. | Cardoso SM. The mitochondrial cascade hypothesis for Parkinson’s disease. Curr Pharm Des. 2011;17:3390-3397. [PubMed] |
| 161. | Santos RX, Correia SC, Cardoso S, Carvalho C, Candeias E, Placido AI, Duarte AI, Santos MS, Moreira PI. Role of Mitochondria and Oxidative stress in Parkinson’s disease. Role of oxidative stress in chronic diseases. Florida, USA: CRC Press Taylor and Francis Group 2014; 521-548. |
| 162. | Santiago JA, Potashkin JA. System-based approaches to decode the molecular links in Parkinson’s disease and diabetes. Neurobiol Dis. 2014;72 Pt A:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 163. | Numao A, Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Clinical correlates of serum insulin-like growth factor-1 in patients with Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Parkinsonism Relat Disord. 2014;20:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 164. | Picillo M, Erro R, Santangelo G, Pivonello R, Longo K, Pivonello C, Vitale C, Amboni M, Moccia M, Colao A. Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naïve Parkinson’s disease. J Neurol. 2013;260:1724-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Morris JK, Zhang H, Gupte AA, Bomhoff GL, Stanford JA, Geiger PC. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2008;1240:185-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 166. | Moroo I, Yamada T, Makino H, Tooyama I, McGeer PL, McGeer EG, Hirayama K. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol. 1994;87:343-348. [PubMed] |
| 167. | Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, Lee PP, Brooks WM, Gerhardt GA, Geiger PC, Stanford JA. Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol. 2011;231:171-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 168. | Kao SY. Rescue of alpha-synuclein cytotoxicity by insulin-like growth factors. Biochem Biophys Res Commun. 2009;385:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 169. | Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke. 2008;3:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 170. | Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169-178. [PubMed] |
| 171. | Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 172. | Sander D, Kearney MT. Reducing the risk of stroke in type 2 diabetes: pathophysiological and therapeutic perspectives. J Neurol. 2009;256:1603-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 173. | Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1829] [Cited by in RCA: 1803] [Article Influence: 106.1] [Reference Citation Analysis (0)] |
| 174. | Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 175. | Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:384017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 176. | McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem. 2011;96:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 177. | De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106:1971-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 502] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 178. | Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 179. | Gerozissis K. Brain insulin and feeding: a bi-directional communication. Eur J Pharmacol. 2004;490:59-70. [PubMed] |
| 180. | Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J Neuroimmunol. 2014;273:8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 181. | Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 182. | Steculorum SM, Solas M, Brüning JC. The paradox of neuronal insulin action and resistance in the development of aging-associated diseases. Alzheimers Dement. 2014;10:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 183. | Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J Clin Invest. 2012;122:1339-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 184. | Correia SC, Santos RX, Santos MS, Casadesus G, Lamanna JC, Perry G, Smith MA, Moreira PI. Mitochondrial abnormalities in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. Curr Alzheimer Res. 2013;10:406-419. [PubMed] |
| 185. | Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI. Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes. 2012;61:1234-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 186. | Moreira T, Cebers G, Pickering C, Ostenson CG, Efendic S, Liljequist S. Diabetic Goto-Kakizaki rats display pronounced hyperglycemia and longer-lasting cognitive impairments following ischemia induced by cortical compression. Neuroscience. 2007;144:1169-1185. [PubMed] |
| 187. | Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59-65. [PubMed] |
| 188. | Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232-2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 447] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 189. | Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 190. | Moreira PI, Santos MS, Sena C, Seiça R, Oliveira CR. Insulin protects against amyloid beta-peptide toxicity in brain mitochondria of diabetic rats. Neurobiol Dis. 2005;18:628-637. [PubMed] |
| 191. | Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68:632-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 192. | Duarte AI, Petit GH, Ranganathan S, Li JY, Oliveira CR, Brundin P, Björkqvist M, Rego AC. IGF-1 protects against diabetic features in an in vivo model of Huntington’s disease. Exp Neurol. 2011;231:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 193. | Lopes C, Ribeiro M, Duarte AI, Humbert S, Saudou F, Pereira de Almeida L, Hayden M, Rego AC. IGF-1 intranasal administration rescues Huntington’s disease phenotypes in YAC128 mice. Mol Neurobiol. 2014;49:1126-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 194. | Ribeiro M, Rosenstock TR, Oliveira AM, Oliveira CR, Rego AC. Insulin and IGF-1 improve mitochondrial function in a PI-3K/Akt-dependent manner and reduce mitochondrial generation of reactive oxygen species in Huntington’s disease knock-in striatal cells. Free Radic Biol Med. 2014;74:129-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 195. | Cardoso S, Santos RX, Correia SC, Carvalho C, Santos MS, Baldeiras I, Oliveira CR, Moreira PI. Insulin-induced recurrent hypoglycemia exacerbates diabetic brain mitochondrial dysfunction and oxidative imbalance. Neurobiol Dis. 2013;49:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 196. | de la Monte SM. Relationships between diabetes and cognitive impairment. Endocrinol Metab Clin North Am. 2014;43:245-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 197. | Correia S, Carvalho C, Santos MS, Proença T, Nunes E, Duarte AI, Monteiro P, Seiça R, Oliveira CR, Moreira PI. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008;4:358-364. [PubMed] |
| 198. | Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 199. | Moreira PI. Metformin in the diabetic brain: friend or foe? Ann Transl Med. 2014;2:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 200. | Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011;118:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 201. | Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 202. | Sakr HF. Effect of sitagliptin on the working memory and reference memory in type 2 diabetic Sprague-Dawley rats: possible role of adiponectin receptors 1. J Physiol Pharmacol. 2013;64:613-623. [PubMed] |
| 203. | Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol. 2013;65:1773-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 204. | Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 378] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 205. | Hölscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2014;221:T31-T41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 206. | Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Plácido A, Santos MS, Oliveira CR, Moreira PI. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta. 2013;1832:527-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 207. | Hamilton A, Patterson S, Porter D, Gault VA, Holscher C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res. 2011;89:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 208. | Agrawal R, Zhuang Y, Cummings BP, Stanhope KL, Graham JL, Havel PJ, Gomez-Pinilla F. Deterioration of plasticity and metabolic homeostasis in the brain of the UCD-T2DM rat model of naturally occurring type-2 diabetes. Biochim Biophys Acta. 2014;1842:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 209. | Cummings BP, Stanhope KL, Graham JL, Baskin DG, Griffen SC, Nilsson C, Sams A, Knudsen LB, Raun K, Havel PJ. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59:2653-2661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 210. | Hölscher C. The role of GLP-1 in neuronal activity and neurodegeneration. Vitam Horm. 2010;84:331-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 211. | During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173-1179. [PubMed] |
| 212. | Parthsarathy V, Hölscher C. The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. Eur J Pharmacol. 2013;700:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 213. | McClean PL, Gault VA, Harriott P, Hölscher C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer’s disease. Eur J Pharmacol. 2010;630:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 214. | Han WN, Hölscher C, Yuan L, Yang W, Wang XH, Wu MN, Qi JS. Liraglutide protects against amyloid-β protein-induced impairment of spatial learning and memory in rats. Neurobiol Aging. 2013;34:576-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 215. | McClean PL, Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76 Pt A:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 216. | Long-Smith CM, Manning S, McClean PL, Coakley MF, O’Halloran DJ, Holscher C, O’Neill C. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 217. | McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6587-6594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 537] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 218. | Parthsarathy V, Hölscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One. 2013;8:e58784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 219. | Yang Y, Zhang J, Ma D, Zhang M, Hu S, Shao S, Gong CX. Subcutaneous administration of liraglutide ameliorates Alzheimer-associated tau hyperphosphorylation in rats with type 2 diabetes. J Alzheimers Dis. 2013;37:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 220. | University of Aarhus. Identifying potential effects of liraglutide on degenerative changes. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: http://clinicaltrials.gov/ct2/show/NCT01469351?term=NCT01469351&rank=1 NLM Identifier: NCT01469351. |
| 221. | Imperial College London. Evaluating liraglutide in Alzheimer’s disease (ELAD). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: http://clinicaltrials.gov/ct2/show/NCT01843075?term=NCT01843075&rank=1 NLM Identifier: NCT01843075. |
| 222. | National Institute on Aging (NIA). A pilot clinical trial of exendin-4 in Alzheimer’s Disease. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: http://clinicaltrials.gov/ct2/show/NCT01255163?term=NCT01255163&rank=1 NLM Identifier: NCT01255163. |
| 223. | Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Rönnholm H. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86:326-338. [PubMed] |
| 224. | Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation. 2008;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 225. | Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106:1285-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 226. | Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730-2736. [PubMed] |
| 227. | Sato K, Kameda M, Yasuhara T, Agari T, Baba T, Wang F, Shinko A, Wakamori T, Toyoshima A, Takeuchi H. Neuroprotective effects of liraglutide for stroke model of rats. Int J Mol Sci. 2013;14:21513-21524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 228. | Wang MD, Huang Y, Zhang GP, Mao L, Xia YP, Mei YW, Hu B. Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway. Neuroscience. 2012;226:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 229. | Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK, Cho JH, Kim YM. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res. 2011;89:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 230. | Sharma MK, Jalewa J, Hölscher C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J Neurochem. 2014;128:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |