Published online Sep 15, 2012. doi: 10.4239/wjd.v3.i9.161
Revised: August 29, 2012
Accepted: September 5, 2012
Published online: September 15, 2012
AIM: To investigate efficacy and safety of vildagliptin compared to other oral antidiabetics in clinical practice in Germany.
METHODS: In this prospective, open, observational study, patients with type 2 diabetes mellitus (T2DM) previously on oral monotherapy were selected by their treating physician to receive either vildagliptin add-on to metformin (cohort 1), vildagliptin + metformin single-pill combination (SPC) (cohort 2) or another dual combination therapy with oral antidiabetic drugs (OADs) (cohort 3). According to routine clinical practice, interim examinations occurred every 3 mo: at baseline, after approximately 3 mo and after approximately 6 mo. Parameters documented in the study included demographic and diagnostic data, history of T2DM, data on diabetes control, vital signs, relevant prior and concomitant medication and disease history. Efficacy was assessed by changes in HbA1c and fasting plasma glucose (FPG) 3 mo and 6 mo after initiation of dual combination therapy. Safety was assessed by adverse event reporting and measurement of specific laboratory values (serum creatinine, total bilirubin, alanine aminotransferase, aspartate aminotransferase, creatine kinase).
RESULTS: Between October 2009 and January 2011, a total of 3881 patients were enrolled in this study. Since 47 patients were withdrawn due to protocol violations, 3834 patients were included in the statistical analysis. There were no relevant differences between the three cohorts concerning age, body weight and body mass index. Average diabetes duration was approximately 6 years and mean HbA1c was between 7.6% and 7.9% at baseline. Antidiabetic treatment was recorded in 3648 patients. Patients were treated with vildagliptin add-on to metformin (n = 603), vildagliptin + metformin (SPC) (n = 2198), and other oral OADs including combinations of metformin with sulfonylurea (n = 370), with glitazones (n = 123), other dipeptidyl peptidase-4 inhibitors (n = 99). After 6 mo of treatment, the absolute decrease in HbA1c (mean ± SE) was significantly more pronounced in patients receiving vildagliptin add-on to metformin (-0.9% ± 0.04%) and vildagliptin + metformin (SPC) (-0.9% ± 0.03%) than in patients receiving other OADs (-0.6% ± 0.04%; P < 0.0001). In addition, significant cohort differences were observed for the improvement in FPG after 6 mo treatment (vildagliptin add-on to metformin: -291 mg/L ± 18.3 mg/L; vildagliptin +metformin (SPC): -305 mg/L ± 9.6 mg/L; other antidiabetic drugs: -209 mg/L ± 14.0 mg/L for (P < 0.0001). Moderate decreases in body weight (absolute difference between last control and baseline: mean ± SE) were observed for patients in all cohorts (vildagliptin add-on to metformin: -1.4 kg ± 0.17 kg; vildagliptin + metformin (SPC): -1.7 kg ± 0.09 kg; other OADs: -0.8 kg ± 0.13 kg). No significant differences in adverse events (AEs) and other safety measures were observed between the cohorts. When performing an additional analysis by age (patients < 65 years vs patients ≥ 65 years), there was no relevant difference in the most common AEs between the two age groups and the AE profile was similar to that of the overall patient population.
CONCLUSION: Clinical practice confirms that vildagliptin is an effective and well-tolerated treatment in combination with metformin in T2DM patients.
- Citation: Blüher M, Kurz I, Dannenmaier S, Dworak M. Efficacy and safety of vildagliptin in clinical practice-results of the PROVIL-study. World J Diabetes 2012; 3(9): 161-169
- URL: https://www.wjgnet.com/1948-9358/full/v3/i9/161.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i9.161
Type 2 diabetes mellitus (T2DM) is one of the most common non-communicable diseases worldwide and will be one of the most challenging health problems in the 21st century[1]. It is estimated that the world prevalence of diabetes among adults (aged 20-79 years) will be 7.7%, affecting 439 million adults, by 2030[2]. Thus, in addition to preventive measures such as lifestyle changes, effective and safe treatments are necessary to manage T2DM.
So far, metformin has been recommended by the American Diabetes Association[3] and is widely used as the first-line antidiabetic drug of choice[4]. However, progression of the underlying pathogenetic factors despite metformin treatment in T2DM patients frequently requires additional glucose lowering drugs[5]. Thus, the treatment of T2DM has moved towards combining metformin with other drugs with a different mechanism of action. Oral antidiabetic medications which can be used in combination with metformin or alone include dipeptidyl peptidase-4 (DPP-4) inhibitors, which act by improving α- and β-cell sensitivity to glucose via increasing concentrations of active GLP-1[6]. Vildagliptin is a DPP-4 inhibitor which has been shown to improve glycemic control (without the weight gain and hypoglycemia) in combination with metformin[7]. In an extensive clinical study program, vildagliptin has been shown to be an efficacious and safe treatment both as monotherapy and in combination with metformin[8-11]. When studied in comparison to the respective monotherapy treatments, combinations of vildagliptin and metformin provided superior efficacy while still showing a comparable overall tolerability profile and a low risk of hypoglycemia[12,13].
Evidence on the efficacy and safety of vildagliptin has been obtained from clinical studies, which were usually conducted in a restricted and highly regulated environment and may, thus, not necessarily reflect the everyday reality of diabetes management. Observational studies have been suggested as a tool complementing randomized controlled trials to investigate efficacy and safety of treatment strategies under conditions of clinical practice[14]. Observational studies are important for the detection of rare or late adverse effects of treatments or insights into the efficacy in daily medical practice[14,15].
To gain more information about the real-life situation in the treatment of type 2 diabetes with vildagliptin in Germany, we have performed this large observational study “Pill burden and compliance in type-2 diabetic patients treated with vildagliptin” (PROVIL). The aim of this study was to investigate the therapeutic efficacy, safety and the pill burden of a combination therapy of vildagliptin with metformin (vildagliptin add-on to metformin, GALVUS®, referred to as “vildagliptin add-on to metformin”) or a fixed combination therapy of vildagliptin and metformin [EUCREAS®, referred to as “vildagliptin + metformin single-pill combination (SPC)”] compared to other oral antidiabetic drugs (OADs) in routine medical practice.
The PROVIL study was conducted as open, observational multi-center study between October 2009 and January 2011 in practices of 867 general practitioners and internists in Germany. The study was registered in accordance with § 67 (6) German Drug Law (Arzneimittelgesetz, AMG) and conducted according to the applicable regulatory requirements and recommendations. As far as possible within the setting of an observational, non-interventional trial, this study was conducted in accordance with ICH-GCP. For all included patients written informed consent for documentation was obtained. The participating physicians received a compensation for the documentation of each patient in accordance with the official scale of physicians’ fees(Gebührenordnung für Ärzte, GOÄ). The study was approved by the Ethics committee at the University of Leipzig. Participation in this study did not affect individual treatment according to medical needs of the patients. The procedures and decisions of the physicians were not influenced and the frequency and scope of examinations was to be according to practice routine. Additional examinations exceeding the usual scope were not required.
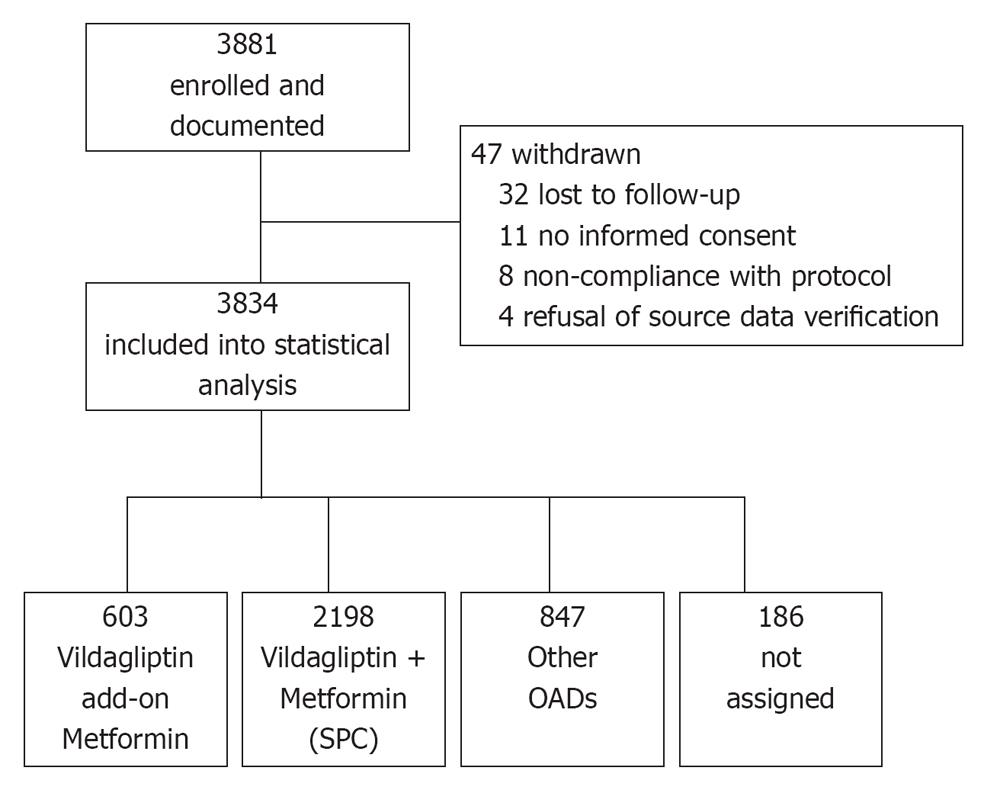
A total of 3881 patients were enrolled in 867 practices (Figure 1). Patients of either sex with T2DM who had the following criteria were included into this non-interventional study: patients who had received oral monotherapy, whose T2DM was considered inadequately controlled by this therapy by the physician and for whom the physician, thus, decided a therapy with vildagliptin add-on to metformin, vildagliptin + metformin SPC or another dual combination therapy with OADs. Since this was an observational study, all patients were treated based on routine clinical practice. No specific exclusion criteria did apply. To obtain a sufficient number of patients for the individual treatment cohorts, this study aimed to document patients on vildagliptin add-on to metformin (cohort 1) and vildagliptin + metformin SPC (cohort 2) vs other OADs (cohort 3).
The study duration was about 6 mo. According to routine practice, interim examinations were expected so that patients were evaluated three times: at baseline (first visit), after approximately 3 mo and after approximately 6 mo. Parameters documented in the study included demographic and diagnostic data, history of T2DM, data on diabetes control independent of this study and according to the summary of product characteristics (SmPC) and laboratory parameters [HbA1c, fasting plasma glucose (FPG), serum creatinine, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase], vital signs, relevant prior and concomitant medication and diseases were documented. After 3 and 6 mo weight, measurements on diabetes control and laboratory parameters independent of this study and according to the SmPC, as well as vital signs were repeated. In addition, changes in antidiabetic therapy, premature discontinuation, and occurrence of adverse events (AEs) were documented. After 6 mo, efficacy and tolerability of the oral dual antidiabetic combination therapy was assessed by the treating physician and continuation of oral dual antidiabetic therapy was recorded.
Since only OADs were allowed in this trial, no daily blood glucose measurement was needed based on the SmPC. We did not include additional blood glucose measurements in this observational study setting to reflect real life clinical practice.
The data in all documentation forms were examined for their plausibility by the data management department. Additionally, for a defined percentage (2%, i.e., 28 centers in line with common practice in Germany[16]) randomly chosen study centers, the documentation forms were compared with the source documents during on-site monitoring.
According to the predefined statistical analysis plan, the statistical evaluation was carried out using basic descriptive statistical methods and was interpreted in an explorative way. The difference in AE incidences between the cohorts was tested by a Chi-square test and the changes in HbA1c and fasting blood glucose were tested by a Kruskal Wallis test. Insofar as statistical procedures were used their results are to be understood as being descriptive not confirmatory. The statistical evaluation was carried out using SAS® Version 9.2 for Windows, (SAS Institute, Cary, NC). Patients who discontinued treatment for any reason and for whom no further data were available after the baseline visit were not included in the analysis.
A total of 3881 patients were enrolled into this study. Of these, 3834 were included in the statistical analysis as 47 patients were withdrawn (Figure 1). About 2801 patients received vildagliptin either as vildagliptin add-on to metformin (n = 603) or vildagliptin + metformin (SPC) (n = 2198). 847 patients had received other OAD combination therapies. For 186 patients assignment to one of these three cohorts was not possible, due to inconsistent cohort information documented by the treating physician.
The most common daily dose in the vildagliptin add-on to metformin cohort was 100 mg (50 mg bid) vildagliptin and 2000 mg metformin (20.2% of patients), followed by 50 mg (50 mg qd) vildagliptin and 1000 mg metformin (15.6%) and 50 mg (50 mg qd) vildagliptin and 2000 mg metformin (13.1%). The vildagliptin + metformin (SPC) cohort daily dose at the initial visit was 50 mg/850 mg twice a day in 31.8% of the patients and 50 mg/1000 mg twice a day in 63.9% of patients.
There were no significant differences between the 3 treatment cohorts concerning age, body weight and BMI (Table 1). On average, patients had been diagnosed with T2DM for about 6 years and the mean HbA1c was between 7.6% and 7.9% at baseline.
| Vildagliptin add-on to metformin | Vildagliptin + metformin (SPC) | Other OADs | ||||
| n | mean ± SD or n (%) | n | mean ± SD or n (%) | n | mean ± SD or n (%) | |
| Sex | ||||||
| Male | 338 (56.1) | 1247 (56.7) | 436 (51.5) | |||
| Female | 263 (43.6) | 938 (42.7) | 404 (47.7) | |||
| Age (yr) | ||||||
| Total | 578 | 63.0 ± 11.1 | 2115 | 62.4 ± 10.6 | 819 | 63.2 ± 11.0 |
| Male | 324 | 62.1 ± 10.8 | 1200 | 61.4 ± 10.2 | 422 | 62.5 ± 11.0 |
| Female | 252 | 64.1 ± 11.5 | 902 | 63.7 ± 11.0 | 392 | 64.0 ± 11.0 |
| Weight (kg) | ||||||
| Total | 601 | 89.3 ± 16.8 | 2183 | 90.6 ± 17.5 | 836 | 87.9 ± 16.5 |
| Male | 337 | 93.7 ± 16.3 | 1239 | 95.1 ± 17.0 | 432 | 92.3 ± 15.6 |
| Female | 262 | 83.7 ± 15.7 | 931 | 84.6 ± 16.3 | 397 | 83.0 ± 16.0 |
| BMI (kg/m²) | ||||||
| Total | 601 | 30.6 ± 5.3 | 2181 | 31.1 ± 5.5 | 836 | 30.3 ± 5.2 |
| Male | 337 | 30.4 ± 5.0 | 1239 | 30.7 ± 5.1 | 432 | 29.8 ± 4.7 |
| Female | 262 | 30.9 ± 5.6 | 929 | 31.6 ± 6.0 | 397 | 30.8 ± 5.6 |
| Underweight/normal weight (BMI < 25) | 70 (11.6) | 174 (8.0) | 90 (10.8) | |||
| Overweight/obese (BMI ≥ 25) | 531 (88.4) | 2007 (92.0) | 746 (89.2) | |||
| Mean (Median) duration of type II diabetes mellitus (yr) | 556 | 6.2 ± 5.3 (median: 5.0) | 2010 | 6.2 ± 5.1 (median: 5.0) | 588 | 5.9 ± 5.2 (median: 4.5) |
| < 1 | 78 (14.0) | 235 (11.7) | 93 (15.8) | |||
| ≥ 1 and < 5 | 197 (35.4) | 771 (38.4) | 215 (36.6) | |||
| ≥ 5 | 281 (50.5) | 1004 (50.0) | 280 (47.6) | |||
| HbA1c (%) | 597 | 7.8 ± 1.2 | 2186 | 7.9 ± 1.3 | 832 | 7.6 ± 1.2 |
| < 6.5 | 43 (7.2) | 150 (6.9) | 91 (10.9) | |||
| ≥ 6.5 and < 7.5 | 222 (37.2) | 747 (34.2) | 357 (42.9) | |||
| ≥ 7.5 and < 10 | 300 (50.3) | 1119 (51.2) | 353 (42.4) | |||
| ≥ 10 | 32 (5.4) | 167 (7.6) | 31 (3.7) | |||
| Fasting plasma glucose (mg/dL) | 560 | 158.6 ± 47.2 | 2091 | 160.4 ± 49.0 | 797 | 151.3 ± 46.5 |
| Serum creatinine (μmol/L) | 552 | 82.4 ± 21.4 | 2053 | 82.2 ± 19.0 | 769 | 84.4 ± 22.8 |
In all three cohorts, the most common concomitant medication given at baseline in addition to any anti-diabetic medication was medication for the cardiovascular system (in 66.2% to 73.2%) followed by medication for the musculoskeletal system (in 23.1% to 24.6% of patients), for the alimentary tract and metabolism (in 20.3% to 25.6% of patients), for the blood and blood forming organs (in 19.2% to 21.2% of patients), and for the nervous system (in 19.0% to 22.9% of patients).
The OAD therapy used during the study course is summarized in Table 2. Apart from vildagliptin add-on to metformin and vildagliptin + metformin (SPC), patients in this study received combinations of metformin with sulfonylurea (n = 370), with glitazones (n = 123) or other DPP-4 inhibitors (n = 99).
| Type of therapy | n (%) |
| Total number of patients | 3648 |
| Metformin + vildagliptin | 603 (16.5) |
| Metformin + vildagliptin (SPC) | 2198 (60.3) |
| Metformin + sulfonylureas | 370 (10.1) |
| Glibenclamide/metformin | 96 (2.6) |
| Glimepiride/metformin | 241 (6.6) |
| Gliquidone/metformin | 1 (< 0.1) |
| Nateglinide/metformin | 2 (0.1) |
| Repaglinide/metformin | 30 (0.8) |
| Metformin + glitazones | 123 (3.4) |
| Pioglitazone/metformin | 99 (2.7) |
| Rosiglitazone/metformin | 24 (0.7) |
| Metformin + other DPP-4 inhibitors | 99 (2.7) |
| Saxagliptin/metformin | 7 (0.2) |
| Sitagliptin/metformin | 92 (2.5) |
| Other1 | 255 (7.0) |
The efficacy of vildagliptin add-on to metformin, vildagliptin + metformin (SPC) and other antidiabetic drugs was assessed by changes in HbA1c and FPG.
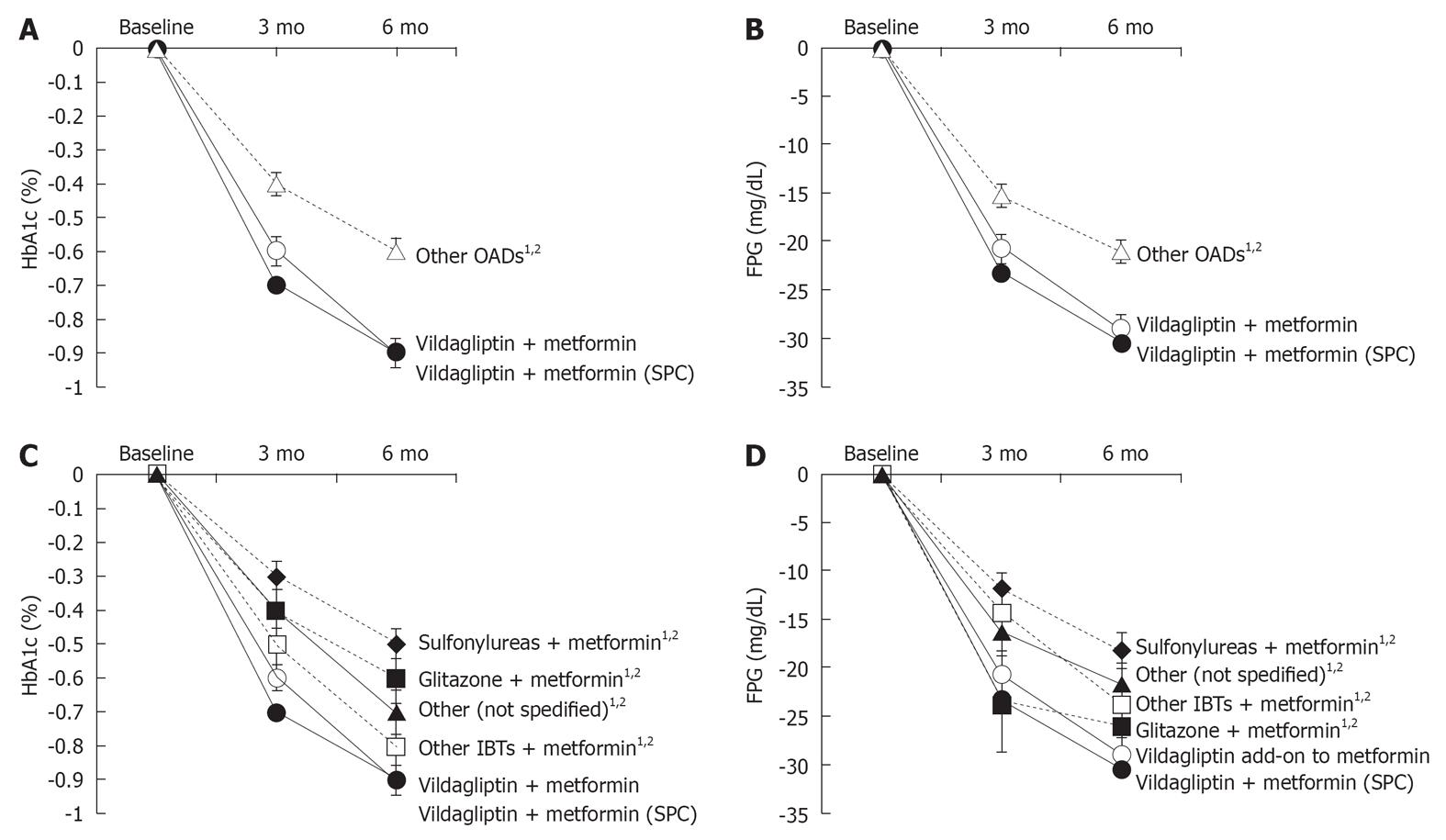
HbA1c values significantly decreased in all 3 treatment cohorts after 3 and 6 mo of treatment [mean ± SE after 6 mo: vildagliptin add-on to metformin -0.9% ± 0.04%; vildagliptin + metformin (SPC): -0.9% ± 0.03%; other OAD -0.6% ± 0.04% (Figure 2A)]. Using a Kruskal-Wallis test, pairwise cohort comparisons showed statistically significant differences comparing vildagliptin add-on to metformin and vildagliptin + metformin (SPC) to the other OADs cohort both at 3 mo and at 6 mo (all P < 0.0001).
FPG concentrations decreased significantly in all 3 cohorts after 3 and 6 mo of treatment compared to baseline [mean ± SE after 6 mo: vildagliptin add-on to metformin: -291 mg/L ± 18.3 mg/L; vildagliptin +metformin (SPC): -305 mg/L ± 9.6 mg/L; other OADs: -209 mg/L ± 14.0 mg/L (Figure 2B)]. Patients receiving vildagliptin add-on to metformin and vildagliptin + metformin (SPC) showed a significantly greater reduction in FPG both after 3 and 6 mo than patients receiving another dual combination therapy with OADs (all P < 0.0001).
The absolute changes in HbA1c between baseline at 6 mo were more pronounced for vildagliptin add-on to metformin and vildagliptin + metformin (SPC) than for the other OADs (Figure 2C). A Kruskal-Wallis test showed that the differences between vildagliptin add-on to metformin and metformin in combination with the respective other substance were statistically significant at 6 mo (sulfonylureas: P < 0.0001; glitazone: P < 0.0001; other incretin-based therapies: P = 0.0327; and other substances: P = 0.0020). Similar differences were observed for the comparison between vildagliptin + metformin (SPC) and metformin in combination with other substances (sulfonylureas: P < 0.0001; glitazone: P < 0.0001; other incretin-based therapies: P = 0.0046; and other substances: P < 0.0001).
Decrease in FPG was more pronounced in the cohorts treated with vildagliptin add-on to metformin and vildagliptin + metformin (SPC) compared to other OADs (Figure 2D). After 6 mo, statistically significant differences compared to metformin in combination with the respective other substances were seen for both vildagliptin add-on to metformin (P: sulfonylureas: P < 0.0001; glitazone: P = 0.0219; other incretin-based therapies: P = 0.0203; other: P = 0.0078; Kruskal-Wallis test) and vildagliptin + metformin (SPC) (sulfonylureas; P < 0.0001; glitazone: P = 0.0054; other incretin-based therapies: P = 0.0048; other: P = 0.0004; Kruskal-Wallis test).
In a subgroup analysis of patients with an HbA1c ≥ 6.5% at baseline, we found that in the vildagliptin add-on to metformin cohort 57.7% and in the vildagliptin+metformin (SPC) cohort 61.1% had an improvement at the last control visit, while only 45.3% of the patients in the other antidiabetics drugs cohort had an improvement at this time (Table 3). A total of 25.3% in the vildagliptin add-on to metformin cohort, 23.5% in the vildagliptin + metformin (SPC) and 19.8% in the other OAD cohort even reached an HbA1c of < 6.5% at the last control visit.
| Changes | Vildagliptin add-on to metformin | Vildagliptin + metformin (SPC) | Other OADs |
| Patients with an HbA1c-value of ≥ 6.5% at initial visit | n = 553 | n = 2033 | n = 741 |
| Patients with improvement in HbA1c at the last control visit compared to baseline | 319 (57.7) | 1242 (61.1) | 336 (45.3) |
| Of these, patients with HbA1c value of < 6.5% at the last control visit | 140 (25.3) | 477 (23.5) | 147 (19.8) |
In elderly patients (≥ 65 years), both HbA1c and FPG decreased compared to baseline in all three treatment cohorts. For HbA1c, absolute differences between last control and baseline were greater in the vildagliptin add-on to metformin (-0.7% ± 0.06%) and the vildagliptin + metformin (SPC) cohort (-0.8% ± 0.04%) than for other antidiabetic drugs (-0.5% ± 0.04%) in elderly patients. Similarly, the absolute changes in FPG (mean ± SE) were also greater in the vildagliptin add-on to metformin (-274 mg/L ± 26.5 mg/L) and the vildagliptin + metformin (SPC) cohort (-268 mg/L ± 14.3 mg/L) than in the other antidiabetics cohort (-157 mg/L ± 21.6 mg/L). SAEs and AEs did not differ from the younger population. There was only one reported mild hypoglycemic event in the vildagliptin/metformin SPC cohort.
Moderate decreases in body weight (absolute difference between last control and baseline: mean ± SE) were observed for patients in all cohorts [vildagliptin add-on to metformin: -1.4 kg ± 0.17 kg; median: -1.0 kg; vildagliptin + metformin (SPC): -1.7 kg ± 0.09 kg; median: -1.0 kg; other OADs: -0.8 kg ± 0.13 kg; median: 0.0 kg]. There was no difference in body weight fluctuations between younger and older patients across all cohorts.
A total of 50 patients (8.3%) in the vildagliptin add-on to metformin cohort reported 77 AEs, 209 patients (9.5%) in the vildagliptin + metformin (SPC) cohort experienced 336 AEs and 67 patients (7.9%) in the other antidiabetics cohort experienced 77 AEs. For the comparison of AE incidence between the 3 cohorts a χ2 test was performed: there was no statistically significant difference between the 3 cohorts (P: 0.3185) (Table 4). Only 3 cases of hypoglycemic events were reported: “hypoglycemia” in one patient in the vildagliptin + metformin (SPC) cohort and “blood glucose decreased” for one patient in the vildagliptin + metformin (SPC) cohort and one patient in the other antidiabetics cohort.
| Events | |||
| Vildagliptin add-on to metformin | Vildagliptin + metformin (SPC) | Other OADs | |
| Total adverse events | 77 (100.0) | 336 (100.0) | 77 (100.0) |
| Adverse events with suspected causal relationship1 | 34 (44.2) | 151 (44.9) | 31 (40.3) |
| Serious adverse events | 20 (26.0) | 118 (35.1) | 16 (20.8) |
| Serious adverse events with suspected causal relationship1 | 1 (1.3) | 22 (6.5) | 6 (7.8) |
| Most common adverse events (preferred terms) | |||
| Glycosylated haemoglobin increased | 18 (23.4) | 102 (30.4) | 26 (33.8) |
| Blood glucose increased | 11 (14.3) | 34 (10.1) | 6 (7.8) |
| Blood pressure increased | 5 (6.5) | 16 (4.8) | 9 (11.7) |
| Blood pressure systolic increased | 5 (6.5) | 16 (4.8) | 7 (9.1) |
| Treatment noncompliance | 4 (5.2) | 18 (5.4) | 1 (1.3) |
| Hypertension | 3 (3.9) | 13 (3.9) | 7 (9.1) |
| Selected hepatic adverse events (preferred terms) | |||
| Transaminases increased | 0 (0.0) | 2 (0.6) | 1 (1.3) |
| Alanine aminotransferase increased | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Aspartate aminotransferase increased | 1 (1.3) | 1 (0.3) | 0 (0.0) |
To assess hepatic safety of the treatments, we examined the time courses of specified liver laboratory parameters (total bilirubin, ALT, AST) and evaluated the reported hepatic AEs and SAEs. For total bilirubin, ALT, and AST no relevant changes during the study were seen. However, laboratory values were missing for a considerable percentage of patients (ranging from about 35% of patients to about 70% of patients depending on cohort and laboratory value). A total of 129 of 3834 patients (3.4%) discontinued therapy: 31 of 603 patients (5.1%) in the vildagliptin add-on to metformin cohort, 73 of 2198 patients (3.3%) in the vildagliptin + metformin (SPC) cohort and 21 of 847 patients in the other oral antidiabetics cohort (2.5%). In the vildagliptin add-on to metformin and vildagliptin + metformin (SPC) cohort, the most frequent reason for discontinuation was inadequate blood sugar control (45.2% and 34.2% of patients, respectively), followed by change of therapy in the vildagliptin add-on to metformin cohort (38.7% of patients) and by AE in the vildagliptin + metformin (SPC) cohort (31.5%). In the other OADs cohort, the most frequent reason for discontinuation was change of therapy (57.1% of patients) followed by inadequate blood sugar control (38.1% of patients).
During the observation period, 4 patients died: 1 patient in the vildagliptin add-on to metformin cohort (event: lung neoplasm malignant; not related to treatment) and 3 patients in the vildagliptin + metformin (SPC) cohort (events: convulsion and brain neoplasm, not related to treatment; cardiac failure, bile duct cancer and cardiac arrest, not related to treatment; and death, relationship to treatment not assessable).
When performing an additional analysis by age (patients younger than 65 years vs patients aged 65 years and older), there was no relevant difference in the most common AEs between the two age groups and the AE profile was similar to that of the overall population.
The present study was conducted to provide real-life data regarding the safety and efficacy of vildagliptin compared to other OADs in combination with metformin in the treatment of T2DM. Vildagliptin in a free or fixed combination with metformin decreased HbA1c and FPG concentrations to a greater extent than other OAD-metformin combinations after 3 and 6 mo of therapy without increasing any AEs or safety parameters. The results support previous observations from randomized clinical trials (RCTs) and provide important information about the use of vildagliptin and other antidiabetic agents in clinical practice.
About 3881 patients with T2DM were enrolled into this study, without triaging the patients by other inclusion and exclusion criteria, thus reflecting a heterogeneous patient population as observed in routine clinical practice. The observed reductions in HbA1c and FPG with vildagliptine are comparable with data from RCTs[7] and support previous evidence that vildagliptin is effective and well-tolerated in combination with metformin in T2DM patients.
The decrease in HbA1c at 6 mo compared to baseline (-0.9% ± 0.03%) was slightly less in magnitude than that reported from a large randomized, double-blind, active-controlled study (-1.1% ± 0.1%) employing similar doses of vildagliptin and metformin[8]. Apart from the different study design, patients in this RCT had higher baseline HbA1c values (8.4% ± 1.0%) than patients in the present study population [vildagliptin + metformin: 7.8% ± 1.2%; vildagliptin + metformin (SPC): 7.9% ± 1.3%]. Also, treatment compliance is maximized in clinical trials, since patients have to follow strict treatment protocols with frequent follow-up visits and additional patient support[17,18] providing another explanation for lower efficacy outcomes between RCTs and observational studies. Also, the observed superior reduction in HbA1c and FPG with vildagliptin when compared to other oral antidiabetic agents is in agreement with recently published observational and clinical data[7,19].
The overall safety and tolerability of vildagliptin and the other antidiabetic agents was assessed by AE monitoring and specific laboratory parameters. Especially hepatic safety has been an area of concern in DDP4-inhibitors[11]. To assess hepatic safety in the present study, the time course of specified liver laboratory parameters was examined (total bilirubin, ALT, AST). No relevant differences in hepatic safety parameters were observed during the study. However, a limitation is that laboratory values were not available for a considerable percentage of patients due to the non-interventional nature of this study.
The present safety data are consistent with the results from a meta-analysis of phase II and III clinical studies which indicated that vildagliptin was not associated with increased risk of hepatic events or hepatic enzyme elevations indicative of drug-induced liver injury[10]. Similar results were also seen in a pooled analysis of clinical trials[20].
Hypoglycemia is often the limiting factor in the glycemic management of diabetes. Reported rates of severe hypoglycemic events in clinical studies vary between 0.4% (ADVANCE)[21] and 3.1% per year (ACCORD)[22]. In UKPDS the rates of hypoglycemic episodes per year varied between 0.7% and 2.0% for major hypoglycemic episodes and between 7.9% and 25.5% for any hypoglycemic episode with the highest incidence in patients treated with sulfonylureas and insulin[23]. Especially in patients who receive sulfonylureas, the incidence of hypoglycemic events increases significantly when compared to incretin-based therapies[24]. Hypoglycemia can be considered a serious patient safety event with severe health complications, including dizziness, disorientation, slurred speech, convulsions, and death[25,26]. Unfortunately, the reporting rate of hypoglycemic events in clinical practice appears to be a major problem[27-29]. In the present observational study with 3834 analyzed patients only 3 hypoglycemic events were reported. Since 10.1% of the patients in this study received sulfonylureas (Table 2), the occurrence of a higher number of hypoglycemic events should have been expected in this study. A potential reason for this may be that many patients in routine practice are unaware of symptoms of hypoglycemia. Especially older patients over 65 years of age do not fully recognize the symptoms of hypoglycemia[27], an age group that displays 42% of the current study population. Also, recent data suggest that despite the risks of untreated hypoglycemia, nearly a third of patients with T2DM acknowledge that they do not routinely discuss the condition with their physician[29]. The high risk of hypoglycemia in T2DM patients and the low awareness of such events requires anti hyperglycemic treatments with a low risk of hypoglycemic events. Therefore, the selection of antidiabetic agents by physicians should consider also other factors beside blood sugar lowering such as weight gain as well as the potential to induce severe or frequent hypoglycemic events-especially in patients at a high risk, such as elderly or renally impaired patients[30].
In general, the majority of the evidence on the efficacy and safety of antidiabetic therapies stems from RCTs, which are generally recognized as “gold standard” for data evaluation. RCTs are fundamentally important in establishing the efficacy of new agents under optimal controlled conditions in carefully selected patients; however they are less informative in determining the effectiveness of a therapy under real-life conditions[31,32]. Since RCTs do not include many practical treatment issues encountered by the clinician in daily practice and the selected participants may not be representative for patients seen in the real-world clinical environment[31], the results may have limited applicability to patients in everyday reality of diabetes management. However, these limitations of RCTs are often ignored[14]. Therefore, observational studies can serve as an important addition to the clinician’s resources by complementing RCT data with information on treatment safety, efficacy, and treatment compliance in patients under real-life conditions[31,32]. Especially the larger sample size, the broad representation of many heterogeneous patients and the detection of rare or late adverse effects represent advantages of observational studies[14,15].
In summary, the data from the current observational study show that vildagliptin in combination with metformin is a safe and effective antidiabetic treatment for T2DM patients.
As other studies, also observational studies have inherent limitations[32]. First, observational studies have the risk of main selection bias. There were no strictly defined in- and exclusion criteria beside the contraindications mentioned in the SmPC of the respective medications. Therefore, confounding variables such as co-morbid diseases, treatment compliance and lifestyle interventions could affect the results. Also, subjects in the OAD cohort may have used varying doses of their oral antidiabetic agents which could affect treatment efficacy. Treatment compliance could have also been a variable between the different cohorts with impact on efficacy, whereas treatment compliance is maximized in clinical trials, since patients have to follow strict treatment protocols. However, it could be assumed that this compliance issue was similar among the study cohort. Furthermore, the low reporting rate of hypoglycemic events in this observational trial could be improved by better patient education, to make patients aware of the potential implications of hypoglycemic events. Another often discussed weakness of an observational study and in fact of every non-randomized study is that there may be a selection bias because the treating physician chooses which patients will be treated with which medication. A major strength of the PROVIL study is the determination of safety and efficacy parameters in T2DM patients under real-life conditions. Therapy was administered in routine practice and in accordance with the German SmPC. This allowed us to collect data in a real-life situation which provides information on typical patient characteristics and current treatment approaches and to obtain information on what is achieved in daily medical practice in the management of diabetes[14,15].
In conclusion, the present data suggest that vildagliptin in combination with metformin is a safe and effective antidiabetic treatment in daily medical practice by significantly reducing HbA1c and FPG without an increased incidence of AEs.
The authors acknowledge the cooperation of the patients, investigators and staff at all participating sites for this study. They wish to thank Franziska Pirkl, PhD, who provided medical writing services on behalf of Kantar Health GmbH.
Type 2 diabetes mellitus (T2DM) is one of the most common diseases worldwide and will be one of the most challenging health problems in the 21st century. In previous clinical trials it was shown that the dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin with and without metfomin can significantly reduce HbA1c without significant hypoglycemic events and weight gain. However, evidence on the efficacy and safety of vildagliptin has been obtained from clinical studies, which were usually conducted in a restricted and highly regulated environment and may, thus, not necessarily reflect the everyday reality of diabetes management. Observational studies have been suggested as a tool complementing randomized controlled trials to investigate efficacy and safety of treatment strategies under conditions of clinical practice.
DPP-4 inhibitors are an established treatment for T2DM. Numerous clinical studies showed that DPP-4 inhibitors are efficacious in treating hyperglycemia and well tolerated without hypoglycemia and weight gain. However, data from real clinical practice are lacking.
This is the first observational multi-center study that examines safety and efficacy of vildagliptin in combination with metformin in real life clinical practice in Germany.
This study confirms that vildagliptin in combination with metformin is a safe and efficacious antidiabetic treatment in daily medical practice by significantly reducing HbA1c and fasting plasma glucose without an increased incidence of adverse events.
HbA1c: Glycated hemoglobin is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. It is formed in a non-enzymatic glycation pathway by hemoglobin’s exposure to plasma glucose. The 2010 American Diabetes Association Standards of Medical Care in Diabetes added the A1c ≥ 48 mmol/mol (≥ 6.5%) as another criterion for the diagnosis of diabetes.
This is a very nice report of an observational study with vildagliptin in combination with metformin for the management of T2DM.
P-Reviewers Volker V, Joshua N S- Editor Wen LL L- Editor A E- Editor Zheng XM
| 1. | International Diabetes Federation. IDF Diabetes Atlas (2009). Available from: http://www.idf.org/diabetesatlas/5e/what-is-diabetes. [Cited in This Article: ] |
| 2. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [PubMed] [Cited in This Article: ] |
| 3. | American Diabetes Association. Standards of Medical Care in Diabetes-2011. Diabetes Care. 2011;34:S11-S61. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1833] [Cited by in F6Publishing: 1858] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 4. | Consoli A, Gomis R, Halimi S, Home PD, Mehnert H, Strojek K, Van Gaal LF. Initiating oral glucose-lowering therapy with metformin in type 2 diabetic patients: an evidence-based strategy to reduce the burden of late-developing diabetes complications. Diabetes Metab. 2004;30:509-516. [PubMed] [Cited in This Article: ] |
| 5. | Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713. [PubMed] [Cited in This Article: ] |
| 6. | Halimi S, Raccah D, Schweizer A, Dejager S. Role of vildagliptin in managing type 2 diabetes mellitus in the elderly. Curr Med Res Opin. 2010;26:1647-1656. [PubMed] [Cited in This Article: ] |
| 7. | Ahrén B, Foley JE, Bosi E. Clinical evidence and mechanistic basis for vildagliptin's action when added to metformin. Diabetes Obes Metab. 2011;13:193-203. [PubMed] [Cited in This Article: ] |
| 8. | Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895. [PubMed] [Cited in This Article: ] |
| 9. | Schweizer A, Dejager S, Foley JE, Kothny W. Assessing the general safety and tolerability of vildagliptin: value of pooled analyses from a large safety database versus evaluation of individual studies. Vasc Health Risk Manag. 2011;7:49-57. [PubMed] [Cited in This Article: ] |
| 10. | Ligueros-Saylan M, Foley JE, Schweizer A, Couturier A, Kothny W. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes Metab. 2010;12:495-509. [PubMed] [Cited in This Article: ] |
| 11. | Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2010;70:2089-2112. [PubMed] [Cited in This Article: ] |
| 12. | Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:506-515. [PubMed] [Cited in This Article: ] |
| 13. | Halimi S, Schweizer A, Minic B, Foley J, Dejager S. Combination treatment in the management of type 2 diabetes: focus on vildagliptin and metformin as a single tablet. Vasc Health Risk Manag. 2008;4:481-492. [PubMed] [Cited in This Article: ] |
| 14. | Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215-1218. [PubMed] [Cited in This Article: ] |
| 15. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [PubMed] [Cited in This Article: ] |
| 16. | Theobald K, Capan M, Herbold M, Schinzel S, Hundt F. Quality assurance in non-interventional studies. Ger Med Sci. 2009;7:Doc29 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2778825/. [Cited in This Article: ] |
| 17. | Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563-2571. [PubMed] [Cited in This Article: ] |
| 18. | Gold DT, McClung B. Approaches to patient education: emphasizing the long-term value of compliance and persistence. Am J Med. 2006;119:S32-S37. [PubMed] [Cited in This Article: ] |
| 19. | Pscherer S, Kostev K, Rockel T, Dworak M. HbA1c reduction in type 2 diabetes patients in clinical practice: comparison between vildagliptin and other DPP-4 inhibitors. Perfusion. 2011;24:206-211. [Cited in This Article: ] |
| 20. | Schweizer A, Dejager S, Foley JE, Shao Q, Kothny W. Clinical experience with vildagliptin in the management of type 2 diabetes in a patient population ≥75 years: a pooled analysis from a database of clinical trials. Diabetes Obes Metab. 2011;13:55-64. [PubMed] [Cited in This Article: ] |
| 21. | Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [PubMed] [Cited in This Article: ] |
| 22. | Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [PubMed] [Cited in This Article: ] |
| 23. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] [Cited in This Article: ] |
| 24. | Matthews DR, Dejager S, Ahren B, Fonseca V, Ferrannini E, Couturier A, Foley JE, Zinman B. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12:780-789. [PubMed] [Cited in This Article: ] |
| 25. | Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902-1912. [PubMed] [Cited in This Article: ] |
| 26. | Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389-1394. [PubMed] [Cited in This Article: ] |
| 27. | Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32:1513-1517. [PubMed] [Cited in This Article: ] |
| 28. | Brierley EJ, Broughton DL, James OF, Alberti KG. Reduced awareness of hypoglycaemia in the elderly despite an intact counter-regulatory response. QJM. 1995;88:439-445. [PubMed] [Cited in This Article: ] |
| 29. | Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141. [PubMed] [Cited in This Article: ] |
| 30. | Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, Fink JC. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1121-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 31. | Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009;122:114-120. [PubMed] [Cited in This Article: ] |
| 32. | Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887-1892. [PubMed] [Cited in This Article: ] |