Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.204
Revised: October 26, 2011
Accepted: October 31, 2011
Published online: November 15, 2011
AIM: To study the combinative effects of nanocerium and selenium in a murine model of diabetes.
METHODS: Cerium oxide (CeO2) nanoparticles (60 mg/kg per day) and sodium selenite (5 μmol/kg per day) alone or in combination, or the metal form of CeO2 (60 mg/kg) were administered for 2 wk by intraperitoneal injection to streptozotocin-induced diabetic rats. At the end of treatment blood was collected, liver tissue dissected and then oxidative stress markers, extent of energy depletion and lipid profile were evaluated.
RESULTS: Antioxidant enzymes and high density lipoprotein decreased whereas oxidative stress, adenosine diphosphate/adenosine triphospahte levels, cholesterol, triglyceride and low density lipoprotein increased on induction of diabetes. All were improved by a combination of nanocerium and sodium selenite. There was a relative amelioration by CeO2 nanoparticles or sodium selenite alone, but the metal form of CeO2 showed no significant improvement.
CONCLUSION: The combination of nanocerium and sodium selenite is more effective than either alone in improving diabetes-induced oxidative stress.
- Citation: Pourkhalili N, Hosseini A, Nili-Ahmadabadi A, Hassani S, Pakzad M, Baeeri M, Mohammadirad A, Abdollahi M. Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes 2011; 2(11): 204-210
- URL: https://www.wjgnet.com/1948-9358/full/v2/i11/204.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i11.204
Diabetes mellitus (DM) is an endocrine-metabolic disorder of increasing occurrence and clinical relevance, contributing to high morbidity and mortality rates. DM is increasing in the world due to population ageing, urbanization and obesity. With regard to the growing incidence, the study of the physiological roots of DM becomes important for the emergence of novel therapeutic procedures.
Increased oxidative stress is an important contributor to the development and progression of diabetes and its complications. Diabetes usually occurs with increased production of free radicals or impaired antioxidant defense[1]. Under diabetic conditions, glucose is prone to oxidation resulting in the generation of hydrogen peroxide and reactive intermediates such as the hydroxyl radical, the most reactive and toxic of free radicals[2].
The liver is the main organ involved in detoxifying free radicals and thus oxidative stress in liver happens in the early stages of diabetes. Strategies to reduce the formation of oxidative stress are important in the treatment of DM[3]. Cerium oxide (CeO2) nanoparticles were thought to increase antioxidant power due to their catalytic effect in stimulating superoxide dismutase (SOD) activity and detoxifying free radicals by remaining active in tissues for extended periods through moving spontaneously between the oxidized and reduced state[4].
Selenium is an essential trace element that possesses a potent antioxidant effect in dysfunctions seen in diabetes[5].
Thus, given this knowledge of the antioxidant potential of CeO2 nanoparticles and sodium selenite, and the pathophysiology of diabetes, the present study was aimed at evaluating the effects of these compounds, when used alone or in combination, on murine diabetes.
Adenosine diphosphate (ADP) sodium salt, adenosine triphospahte (ATP) disodium salt, Tris base, 1,1,3,3-tetraethoxypropane (MDA), 5,5’dithiobis-2-nitro benzoic acid (DTNB), methanol [high performance liquid chromatography (HPLC)-grade], trichloroacetic acid (TCA), potassium hydroxide, diethyl ether, tetrabutylammonium hydroxide, n-butanol, 2-thiobarbituric acid (TBA), KH2PO4 (analytical grade), 2,4,6-tripyridyl-s-triazine (TPTZ), sodium selenite, H2O2, phosphate buffer solution (PBS) from Merck (Tehran), 2,7-dichlorodihydrofluorescein diacetate from Sigma-Aldrich (Taufkirchen, Germany), CeO2 nanoparticles from Navarrean Nenoproducts Technology (Spain), streptozotocine (STZ) from Pharmacia and Upjohn (United States), SOD kit from Randox (United Kingdom) and a SUPELCOSIL™ LC-18-T HPLC column from SUPELCO (United Kingdom), commercial kits for cholesterol, triglyceride, low density lipoprotein (LDL), high density lipoprotein (HDL) from Parsazmoon (Tehran) and ketamine/xylazin from a local pharmacy were used in this study.
Male Wistar rats (180-200 g) were used. In vivo studies were performed according to ethical guidelines on the use of animals in research and the protocol was approved by the TUMS/PSRC review board. Experimental diabetes was induced in fasted rats by a single intraperitoneal (ip) injection of streptozotocin at a dose of 75 mg/kg in 0.1 mol/L citrate buffer at pH 4.5. Within 1 wk of injection, rats showing blood glucose values above 300 mg/dL were included in the study.
Animals were randomly divided into six groups with six rats in each group. Group 1 rats (normal control group) were injected intraperitoneally with normal saline (NS) for 2 wk. Group 2 rats (diabetes control group) received a single ip injection of STZ (75 mg/kg) and NS for 2 wk. Group 3 rats were injected with treatment STZ (75 mg/kg) and the metal form of CeO2 (60 mg/kg). In Group 4 CeO2 nanoparticles (60 mg/kg) were used instead of the metal form of CeO2. In Groups 5 and 6, a single dose of STZ (75 mg/kg) was used in addition to sodium selenite (5 μmol/kg per day) (Group 5) or a combination of sodium selenite (5 μmol/kg per day) and CeO2 nanoparticles (60 mg/kg) (Group 6).
For sample preparation, animals were anesthetized with an intramuscular (im) injection of ketamine (4 mg/100 g) and xylazine (1 mg/100 g) mixture. Blood was collected from the heart into a heparinized syringe. Blood samples were centrifuged at 1200 g for 10 min at 4°C and plasma was frozen at -80°C until use. Liver tissue was dissected and stored immediately on ice and then 100 mg of liver was homogenized in PBS (50 mmol/L, pH 7), then centrifuged at 30 000 g for 30 min at 4°C. The supernatant was collected and stored at -80°C. Extra liver tissues were frozen quickly in liquid nitrogen for further analysis.
At the end of treatment, blood glucose levels were measured using a glucometer and the animal’s weight was determined using an animal balance.
Total antioxidant capacity: Reduction of Fe3+ to Fe2+ by the biological sample is an indicator of antioxidant capacity. The complex between Fe2+ and TPTZ produces a blue color with a maximum absorbance at 593 nm as previously described[6].
Total thiol molecules: Total thiol group was determined using DTNB as the reagent. One hundred microliters of the sample was mixed with 0.1 mol/L 1500 μL phosphate buffer pH 7.4 and 400 μL of 2 mol/L DTNB. After incubation at 37°C for 30 min, absorbance of the samples was measured against a blank at 412 nm.
Lipid peroxidation: Thiobarbituric acid-reactive substances were measured using 1,1’,3,3’- tetraethoxypropane as a standard and from a standard curve of TBA adduct formation[6].
Catalase: The activity of catalase (CAT) was measured by observing the initial rate of hydrogen peroxide disappearance in a spectrophotometer at 240 nm. Results are reported as a constant rate per second per liter plasma and as micromoles of formaldehyde produced per mg of protein[7].
SOD stimulation: According to the kit protocol, xanthine and xanthine oxidase generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) and create a red formazon dye. The SOD activity is measured by the degree of inhibition of this reaction. There is 50% inhibition of the rate of reduction of INT by one unit of SOD. Data are shown as unit/mg of protein.
Reactive oxygen species: The activity of reactive oxygen species (ROS) was determined by use of DCF-DA, which is converted into highly fluorescent DCF by cellular peroxides. The sample was divided into two equal parts. In one fraction, 40 μL of 1.25 mmol/L DCF-DA in methanol was added for ROS determination. In the other fraction 40 μL of methanol was added, as a control for tissue auto-fluorescence. All samples were incubated for 15 min in a 37°C water-bath.. Fluorescence was measured at 488 nm excitation and 525 nm emission, using a fluorescence plate reader as described previously[8]. Results were expressed as nmol/min/mg of protein.
Cholesterol, triglyceride and lipoproteins: Aliquots of serum were taken for determination of total cholesterol by the enzymatic colorimetric assay method and triglycerides determined by enzymatic glycerol phosphate oxidase/peroxidase method, Autoanalyzer and Elitech kit were used. LDL was precipitated by adding phosphotungstic acid and magnesium ions to the serum. Centrifugation left only the HDL in the supernatant.
ADP/ATP: The frozen liver was removed and quickly homogenized (4°C) in 1 mL of ice-cold 6% TCA. The homogenate was centrifuged at 12 000 g for 10 min at 4°C and the supernatant was neutralized to a pH of 6.5 with 4 mol/L KOH. After filtering through a Millipore filter, the neutralized extract was used to determine ATP and ADP concentration (μg/mL per mg of tissue) by ion-pair HPLC. Standard curves were created using standard solutions of ATP and ADP and then samples were tested to measure energy changes as an ADP/ATP ratio[9].
Statistical analysis: All values were reported as a mean ± SE. Statistical analysis of data was carried out by analysis of variance followed by Newman-Keuls and Stat Direct version 2.7.7. It should be noted that, P values less than 0.05 were considered statistically significant.
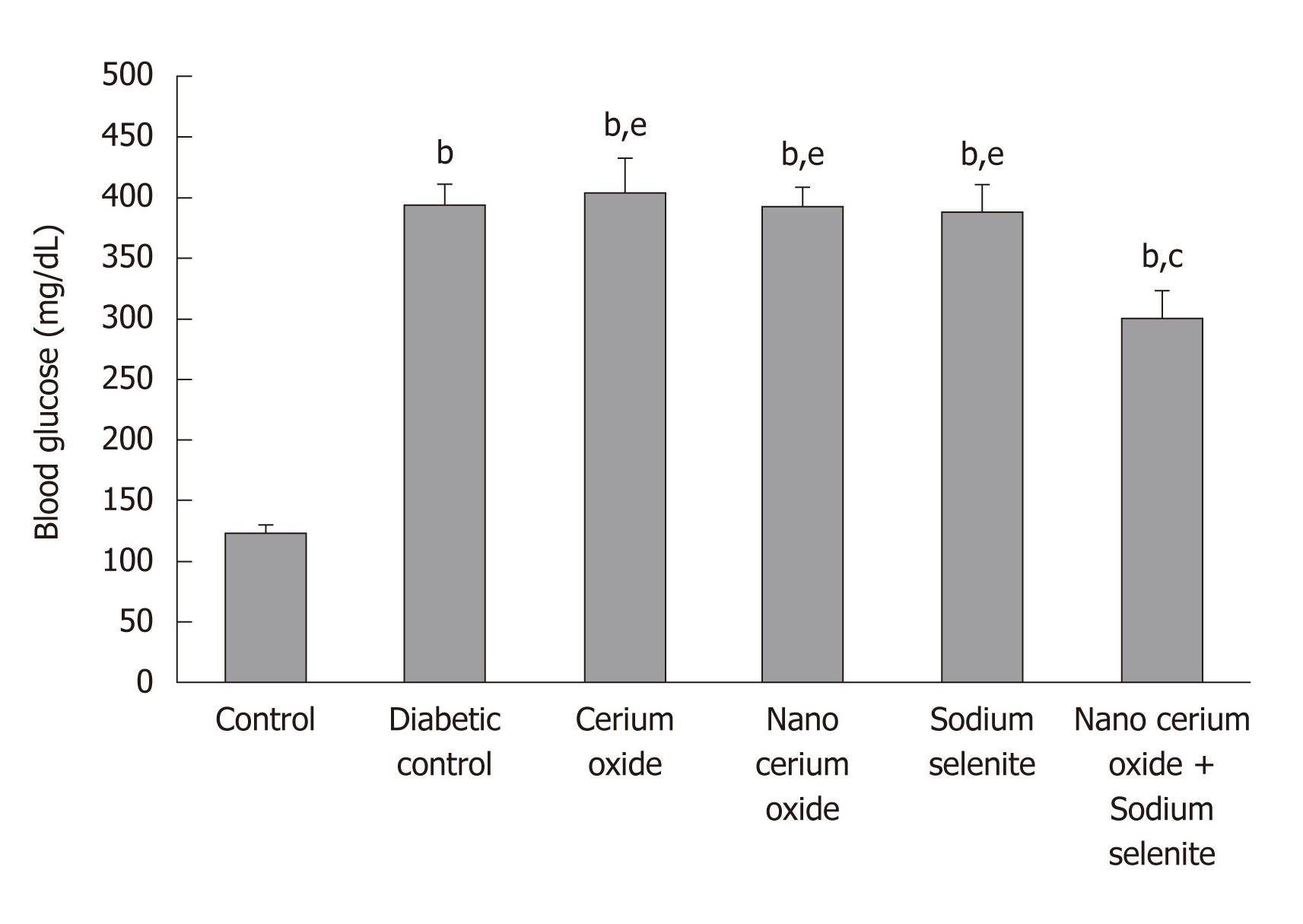
The weight of diabetic rats decreased significantly compared to control rats (P < 0.05). Blood glucose in diabetic rats significantly increased compared with control rats (P < 0.001). After use of sodium selenite and CeO2 nanoparticles combined and CeO2 nanoparticles alone, a significant increase in animal weight (P < 0.01 and P < 0.05, respectively) was observed compared to diabetic rats. Also, a significant decrease in blood glucose (P < 0.05) was shown only by the combination of sodium selenite and CeO2 nanoparticles, when compared to diabetic rats. No significant changes in animal weight were observed when using sodium selenite and the metal form of CeO2 (Table 1 and Figure 1).
| Animal groups | Body weight index | Liver weight index | ||
| Initial (g) | Final (g) | LW (g) | BW/LW | |
| Control | 187.25 ± 3.68 | 207.5 ± 3.22 | 7.54 ± 0.23 | 25.86 ± 1.18 |
| Diabetic control | 185.75 ± 3.94 | 172.6 ± 1.76a | 10.02 ± 0.30c | 16.5 ± 0.78c |
| Cerium oxide | 192.25 ± 6.14 | 185.6 ± 4.70f | 9.77 ± 0.42b | 19.59 ± 0.86b,f |
| Nanocerium oxide | 189.00 ± 3.71 | 202.2 ± 2.01d | 8.35 ± 0.12d | 24.15 ± 0.49e |
| Sodium selenite | 187.75 ± 5.80 | 193.3 ± 6.38 | 8.69 ± 0.44 | 22.31 ± 1.00d |
| Nanocerium oxide + Sodium selenite | 195.20 ± 3.00 | 214.8 ± 8.77e | 9.73 ± 0.38b | 24.11 ± 1.04e |
The liver weight/body weight index in diabetic rats significantly decreased (P < 0.001) compared with control animals. Administration of sodium selenite, CeO2 nanoparticles and a combination of sodium selenite and CeO2 nanoparticles led to an increase in this index (P < 0.05, P < 0.01, respectively) as compared to diabetic rats (Table 1).
A significant increase in lipid peroxidation (LPO) (P < 0.001) and a decrease in total antioxidant capacity (TAC) and total thiol molecules (TTM) (P < 0.001 and P < 0.01, respectively) in the diabetic group were recorded in comparison to controls in both plasma and liver (Table 2). Administration of sodium selenite, CeO2 nanoparticles and a combination of sodium selenite and CeO2 nanoparticles decreased LPO (P < 0.05 in plasma and liver, P < 0.01 in plasma, P < 0.05 in liver, P < 0.001 in plasma and liver, respectively) as compared to the diabetic group. The metal form of CeO2 caused no significant changes. A significant increase of TAC (P < 0.001) was observed by the sodium selenite/CeO2 nanoparticle combination in plasma and liver. Use of sodium selenite and CeO2 nanoparticles in liver increased TAC (both P < 0.01) in comparison to the diabetic group. No significant changes were produced by the metal form of CeO2, sodium selenite, and CeO2 nanoparticles in plasma or by the metal form of CeO2 in liver. The level of TTM after use of sodium selenite and a combination of sodium selenite and CeO2 nanoparticles showed a significant increase (P < 0.05 and P < 0.01, respectively) compared to the diabetic group in both plasma and liver. No significant changes were detected by administration of CeO2 nanoparticles and the metal form of CeO2 (Table 2).
| Groups | LPO | TAC | TTM | |||
| Plasma (nmol/mL) | Liver (nmol/mg protein) | Plasma (μmol/mL) | Liver (nmol/mg protein) | Plasma (mmol/L) | Liver (nmol/mg protein) | |
| Control | 2.08 ± 0.36 | 2.16 ± 0.23 | 528.62 ± 7.45 | 5.47 ± 0.34 | 0.512 ± 0.026 | 10.02 ± 0.36 |
| Diabetic control | 6.97 ± 0.33b | 5.37 ± 0.34b | 224.20 ± 13.43b | 1.45 ± 0.19b | 0.376 ± 0.024a | 7.91 ± 0.30a |
| Cerium oxide | 6.85 ± 0.42b,h | 5.31 ± 0.31b,h | 231.43 ± 5.68b,h | 1.73 ± 0.23b,h | 0.360 ± 0.016a,g | 6.67 ± 0.40b,h |
| Nanocerium oxide | 4.92 ± 0.42b,d,f | 4.12 ± 0.22b,c,f | 272.33 ± 10.49b,h | 3.13 ± 0.29b,d,h | 0.379 ± 0.010a,f | 7.10 ± 0.25b,h |
| Sodium selenite | 5.14 ± 0.33b,c,g | 3.95 ± 0.22a,c,f | 257.46 ± 12.28b,h | 2.98 ± 0.17b,d,h | 0.480 ± 0.025c,f | 9.32 ± 0.32c |
| Nanocerium oxide + Sodium selenite | 3.19 ± 0.30e | 2.60 ± 0.26e | 493.46 ± 7.45e | 4.97 ± 0.28e | 0.500 ± 0.024d | 9.83 ± 0.30d |
In the diabetic group there was a significant decrease in CAT activity in both plasma and liver (P < 0.001) compared to control rats. However, administration of CeO2 nanoparticles, sodium selenite, and combination of sodium selenite and CeO2 nanoparticles caused a significant increase in this marker (P < 0.01, P < 0.05 and P < 0.001, respectively). No significant changes were observed after administration of the metal form of CeO2 (Table 3). SOD activity in both plasma and liver of diabetic rats significantly decreased as compared with controls (P < 0.01 and P < 0.001, respectively). After use of CeO2 nanoparticles, and a combination of sodium selenite and CeO2 nanoparticles, a significant increase in plasma SOD activity (P < 0.05) was observed compared to diabetic rats. No significant changes were observed when using sodium selenite and the metal form of CeO2. However, there was a significant increase in SOD activity in liver (P < 0.01) compared with diabetic rats only by combination of sodium selenite and CeO2 nanoparticles (Table 3).
| Groups | CAT activity | SOD activity | ||
| Plasma (U/mL) | Liver (U/mg protein) | Plasma (U/mL) | Liver (U/mg protein) | |
| Control | 13.74 ± 0.26 | 76.25 ± 1.39 | 7.81 ± 3.98 | 16.22 ± 0.56 |
| Diabetic control | 5.39 ± 0.70c | 51.26 ± 1.67c | 6.30 ± 2.33b | 11.78 ± 0.91c |
| Cerium oxide | 6.50 ± 0.74c,i | 52.20 ± 2.70c,i | 6.18 ± 3.13b,g | 10.02 ± 0.57c,i |
| Nanocerium oxide | 9.05 ± 0.51c,e,h | 66.38 ± 3.81e | 7.45 ± 1.99d | 12.37 ± 0.54b,g |
| Sodium selenite | 8.31 ± 0.73c,d,i | 63.09 ± 2.13b,d,g | 6.50 ± 1.51a | 11.78 ± 0.56c,h |
| Nanocerium oxide + Sodium selenite | 12.86 ± 0.44f | 72.75 ± 1.74f | 7.68 ± 2.55d | 15.55 ± 0.78e |
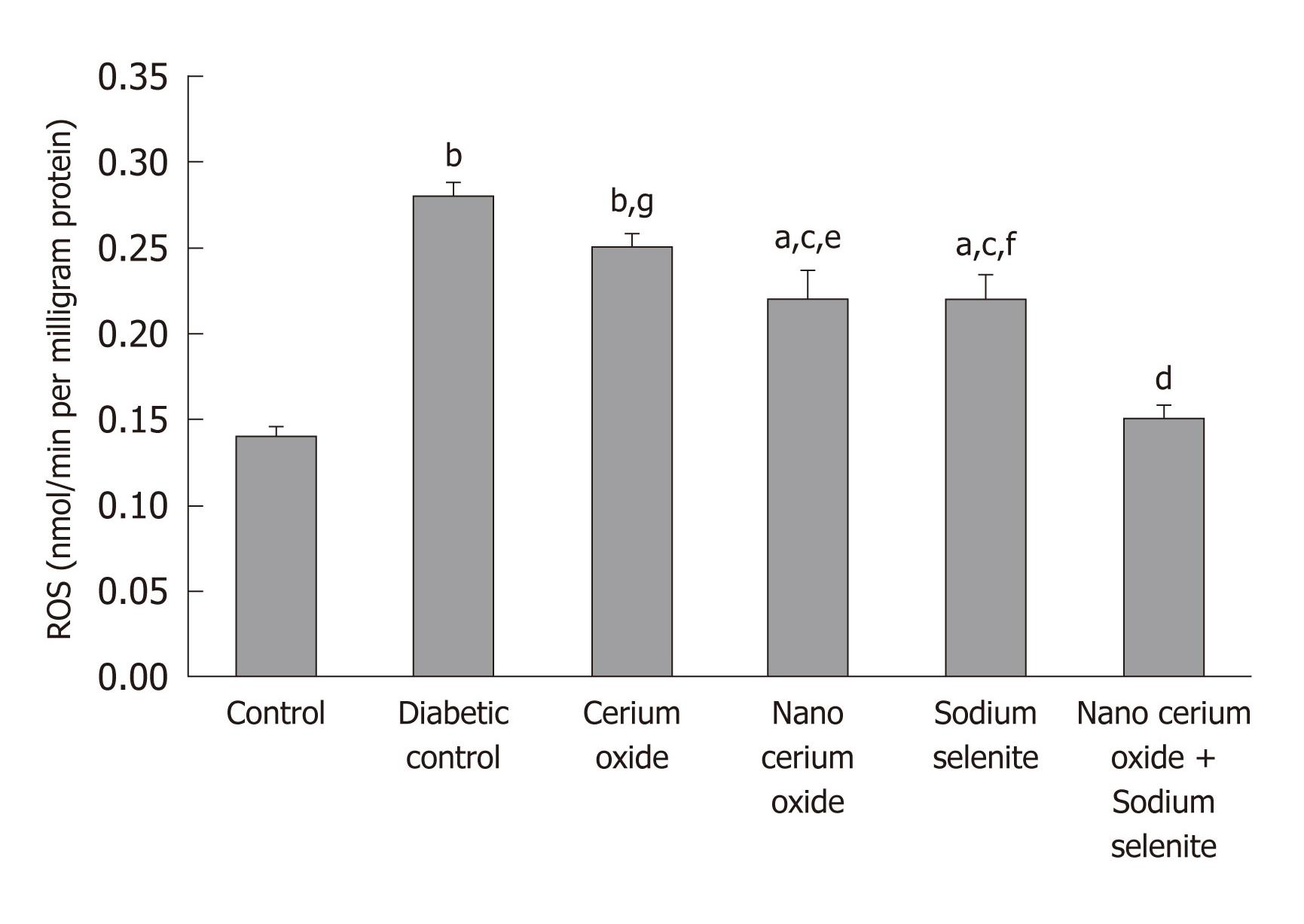
In the diabetic rats, hepatic ROS significantly increased (P < 0.001), but returned to almost normal, after use of sodium selenite, CeO2 nanoparticles and a combination of sodium selenite and CeO2 nanoparticles (P < 0.05 and P < 0.001, respectively). No significant changes were observed after administration of the metal form of CeO2 (Figure 2).
Total plasma cholesterol, triglycerides and LDL were significantly elevated (P < 0.01, P < 0.001 and P < 0.01, respectively) in diabetic rats as compared to the control group. Similarly, HDL was significantly reduced (P < 0.01) in diabetic rats as compared with the control group (Table 4). No significant changes were observed in plasma cholesterol by use of different treatments. Triglycerides were reduced (P < 0.05 and P < 0.01, respectively) by use of CeO2 nanoparticles, sodium selenite and a combination of sodium selenite and CeO2 nanoparticles as compared to the diabetic group but there was no significant change when using the metal form of CeO2. Administration of sodium selenite and a combination of sodium selenite and CeO2 nanoparticles significantly reduced (P < 0.05) plasma LDL compared to the diabetic group. The nano and metal forms of of CeO2 did not cause significant change.
| Animal Groups | Cholestrol (mg/dL) | Triglyceride (mg/dL) | HDL (mg/dL) | LDL (mg/dL) |
| Control | 69.41 ± 7.02 | 70.66 ± 6.66 | 41.28 ± 2.27 | 36.37 ± 1.91 |
| Diabetic control | 110.20 ± 5.09b | 166.09 ± 8.53c | 30.14 ± 1.83b | 51.42 ± 2.80b |
| Cerium oxide | 109.98 ± 5.53c | 166.05 ± 10.03c,f | 29.00 ± 1.95c,f | 60.14 ± 1.62c,g |
| Nanocerium oxide | 107.02 ± 1.48b | 123.37 ± 8.72b,d | 38.42 ± 1.46d | 49.00 ± 2.80a |
| Sodium selenite | 102.22 ± 6.77b | 123.25 ± 6.43b,d | 38.00 ± 1.43d | 40.66 ± 2.66d |
| Nanocerium oxide + Sodium selenite | 106.15 ± 5.79b | 113.10 ± 8.17a,e | 39.60 ± 2.13d | 39.14 ± 2.79d |
Plasma HDL was significantly improved (P < 0.05) by all treatments except for the metal form of CeO2 (Table 4).
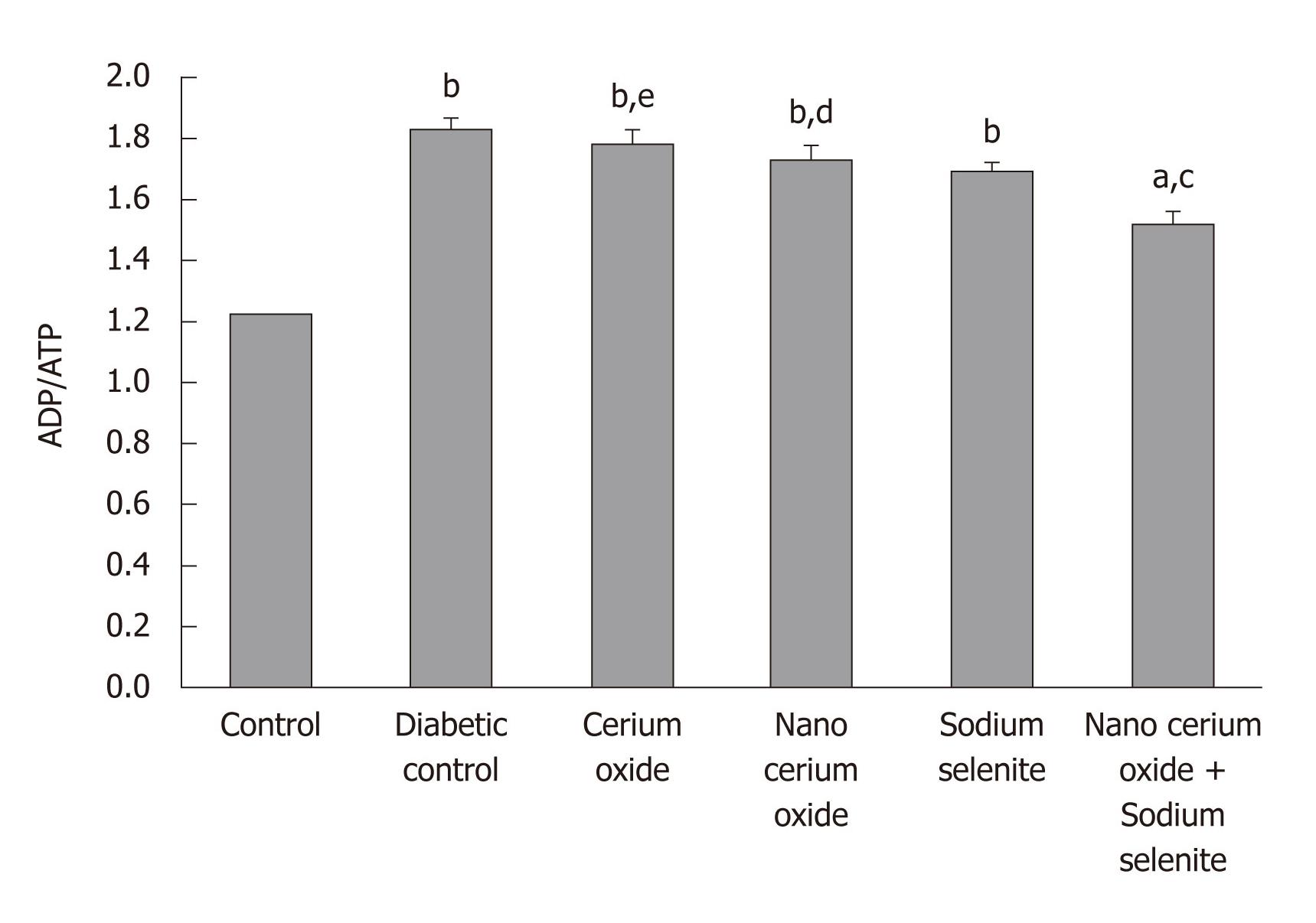
As observed in Figure 2, a significant increase in the liver ADP/ATP ratio in diabetic rats is evident in comparison to the control group (P < 0.001). The administration of a combination of sodium selenite and CeO2 nanoparticles reduced the ADP/ATP ratio in comparison to the diabetic group (P < 0.01); sodium selenite, CeO2 nanoparticles, or metal form of CeO2 used separately caused no significant change (Figure 3).
The present study indicates a significant improvement in biomarkers of diabetes including oxidative stress, energy compensation (ADP/ATP) and lipid profile by using a combination of sodium selenite and CeO2 nanoparticles. It is mentioned that a relative improvement in these biomarkers was shown by CeO2 nanoparticles and sodium selenite used separately, but no significant changes were found when the metal form of CeO2 was used. This is the first report on the benefit of a CeO2 nanoparticles/sodium selenite combination in diabetes treatment. The unique structure of CeO2 nanoparticles supports a potential role as a biological free radical scavenger or antioxidant. This metal oxide is characterized by monodisperse particles that are single crystals with few twin boundaries[10] and expanded lattice parameter[11] making this compound nonstoichiometric. Moreover, the cerium atom is characterized by dual oxidation state potential and oxygen vacancies[12]. This property is responsible for the free radical scavenging activity of CeO2 that makes it beneficial in diabetes. Other than the antioxidant effect of CeO2, it has many sites for catalysis that makes it more active and resident in a living cell for an extended period of time. In addition, CeO2 nanoparticles stimulate SOD activity[13,14].
On the other hand, selenium is an essential trace element possessing cardioprotective, antiproliferative and chemopreventive effects[14-16]. It is also a potent antioxidant for dysfunctions seen in diabetes[5] and colitis[17].
In fact, a clear link between oxidative stress and diabetes exists where liver is the main organ involved because the liver is rich in mitochondria to perform metabolic functions. Liver plays an important role in glucose metabolism, and in a chronic hyperglycemic state, liver oxidative stress is considered a relevant process. Oxidative stress induced by hyperglycemia leads to liver cell damage because liver is subject to ROS-mediated injury in diabetes. In the present study, a significant increase of oxidative stress biomarkers, ROS and a reduction in antioxidant enzymes in plasma and liver was observed and this has support from previous reports, as mentioned above.
On the other hand, ROS plays an important role in the regulation of hepatic glucose production. The anti-diabetic drugs which act through inhibition of hepatic gluconeogenesis produce concurrent antioxidant effects beneficial in the treatment of diabetes[18]. Thus, the antioxidant potential of CeO2 nanoparticles and selenium might be a mechanism for their glucose lowering effect and inhibition of glycogenolysis.
In diabetes, increased ROS coupled with depolarization of the inner mitochondrial membrane reduces ATP. Considering the large number of mitochondria present in liver, reduction in the ATP of liver in diabetes conditions seems rational[19] and explains the present reduction of liver ADP/ATP in diabetic rats.
The present findings also showed a significant increase in triglycerides, LDL, cholesterol and a reduction in the HDL of diabetic animals which were expected. One of the most important complications of diabetes is atherosclerosis and coronary heart disease, the result of abnormal lipid metabolism, hyperglycemia may promote LPO of LDL resulting in the generation of free radicals. The lipid profile of diabetes is characterized by low levels of HDL, elevated LDL and TG levels[20].
In conclusion, the combination of CeO2 nanoparticles/sodium selenite shows the best anti-oxidative effects beneficial in experimental diabetes. Therefore, this combination should be followed for further tests and clinical trials.
Diabetes mellitus (DM) is an endocrine-metabolic disorder of increasing occurrence and clinical relevance, contributing to high morbidity and mortality rates. DM is increasing in the world by population ageing, urbanization and obesity. The study of the physiological routes of DM is important for the development of novel therapeutic procedures for this increasingly common disease.
Increased oxidative stress is an important contributor to the development and progression of diabetes and its complications. Strategies to reduce the formation of oxidative stress are important in the treatment of DM. It seems that cerium oxide (CeO2) nanoparticles and sodium selenite, as two powerful antioxidants, are suitable for this purpose. The present study was aimed to evaluate the effects of these compounds on murine diabetes when used alone or in combination.
The present study indicates a significant improvement in biomarkers of diabetes including oxidative stress, energy compensation [adenosine diphosphate/adenosine triphospahte (ADP/ATP)] and lipid profile by using a combination of sodium selenite and CeO2 nanoparticles. It is mentioned that a relative improvement in these biomarkers was shown by CeO2 nanoparticles and sodium selenite when used alone but no significant change was found when the metal form of CeO2 was used. This is the first report on the benefit of a CeO2 nanoparticles/sodium selenite combination in diabetes.
DM is increasing in the world due to population ageing, urbanization and obesity and causes high morbidity and mortality rates. We suggest a combination of nanocerium and sodium selenite for the treatment of diabetes and its complications.
CeO2 nanoparticle - a powerful antioxidant with free radical scavenging properties. Selenium - an essential trace element that possesses a potent antioxidant effect.
The major finding of the study is that a combination of sodium selenite and CeO2 nanoparticles indicates a significant improvement in biomarkers of diabetes including oxidative stress, energy compensation (ADP/ATP) and lipid profile. In fact, this combination shows the most beneficial anti-oxidative effects in experimental diabetes. Therefore, this combination should be followed for further tests and clinical trials.
Peer reviewer: Khaled Abdul-Aziz Ahmed, Dr., Department of Medical Sciences, Ibb University, PO Box 70627, Ibb, Yemen
S- Editor Wu X L- Editor Hughes D E- Editor Zheng XM
| 1. | Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 489] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 2. | Kajbaf F, Mojtahedzadeh M, Abdollahi M. Mechanisms underlying stress-induced hyperglycemia in critically ill patients. Therapy. 2007;4:97-106. [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Di Naso FC, Simões Dias A, Porawski M, Marroni NA. Exogenous superoxide dismutase: action on liver oxidative stress in animals with streptozotocin-induced diabetes. Exp Diabetes Res. 2011;2011:754132. [PubMed] [Cited in This Article: ] |
| 4. | Andresscu EC, Leiter JC, Leiter JC. Method of neuroprotection from oxidant injury using metal oxide nanoparticles. United States patentUS 2010/0098768 A1. 2010;Apr 22. [Cited in This Article: ] |
| 5. | Ayaz M, Celik HA, Aydin HH, Turan B. Sodium selenite protects against diabetes-induced alterations in the antioxidant defense system of the liver. Diabetes Metab Res Rev. 2006;22:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Astaneie F, Afshari M, Mojtahedi A, Mostafalou S, Zamani MJ, Larijani B, Abdollahi M. Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch Med Res. 2005;36:376-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Ranjbar A, Ghahremani MH, Sharifzadeh M, Golestani A, Ghazi-Khansari M, Baeeri M, Abdollahi M. Protection by pentoxifylline of malathion-induced toxic stress and mitochondrial damage in rat brain. Hum Exp Toxicol. 2010;29:851-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Momtaz S, Lall N, Hussein A, Ostad SN, Abdollahi M. Investigation of the possible biological activities of a poisonous South African plant; Hyaenanche globosa (Euphorbiaceae). Pharmacogn Mag. 2010;6:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Hosseini A, Sharifzadeh M, Rezayat SM, Hassanzadeh G, Hassani S, Baeeri M, Shetab-Bushehri V, Kuznetsov DA, Abdollahi M. Benefit of magnesium-25 carrying porphyrin-fullerene nanoparticles in experimental diabetic neuropathy. Int J Nanomedicine. 2010;5:517-523. [PubMed] [Cited in This Article: ] |
| 10. | Zhang F, Chan SW, Spanier JF, Apak F, Jin Q, Robinson R, Herman IP. Cerium oxide nanoparticles: size-selective formation and structure analysis. Phys Lett. 2002;80:127-129. [Cited in This Article: ] |
| 11. | Perebeinos V, Chan SW, Zhang F. Madelung-Model prediction for the lattice constant scaling with the sizes of ionic nanocrystals of CeO2 and BaTiQ3. Solid State Commun. 2002;123:295-297. [DOI] [Cited in This Article: ] |
| 12. | Robinson RD, Spanier JE, Zhang F, Chan SW, Herman IP. Visible thermal emission from sub-band-gap laser excited cerium dioxide particles. J Appl Phys. 2002;92:1936-1941. [DOI] [Cited in This Article: ] |
| 13. | Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb). 2007;10:1056-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 823] [Cited by in F6Publishing: 721] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 14. | Celik HA, Aydin HH, Deveci R, Terzioglu E, Karacali S, Saydam G, Akarca U, Batur Y. Biochemical and morphological characteristics of selenite-induced apoptosis in human hepatoma Hep G2 cells. Biol Trace Elem Res. 2004;99:27-40. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Venardos K, Harrison G, Headrick J, Perkins A. Effects of dietary selenium on glutathione peroxidase and thioredoxin reductase activity and recovery from cardiac ischemia-reperfusion. J Trace Elem Med Biol. 2004;18:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Abdollahi M, Rahmat-Jirdeh N, Soltaninejad K. Protection by selenium of lead-acetate-induced alterations on rat submandibular gland function. Hum Exp Toxicol. 2001;20:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods. 2011;21:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Hoseini S, Esmaily H, Mohammadirad A, Abdollahi M. Effects of Sildenafil a Phosphodiesterase 5 Inhibitor on Rat Liver Cell Key Enzymes of Gluconeogenesis and Glycogenolysis. Int J Pharmacol. 2006;2:280-285. [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Rahimi R, Abdollahi M. A review on the mechanisms involved in hyperglycemia induced by organophosphorus pesticides. Pestic Biochem Physiol. 2007;88:115-121. [DOI] [Cited in This Article: ] |
| 20. | Vosough-Ghanbari S, Rahimi R, Kharabaf S, Zeinali S, Mohammadirad A, Amini S, Yasa N, Salehnia A, Toliat T, Nikfar S. Effects of Satureja khuzestanica on Serum Glucose, Lipids and Markers of Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Double-Blind Randomized Controlled Trial. Evid Based Complement Alternat Med. 2010;7:465-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (1)] |