Copyright
©The Author(s) 2016.
World J Diabetes. Sep 15, 2016; 7(17): 412-422
Published online Sep 15, 2016. doi: 10.4239/wjd.v7.i17.412
Published online Sep 15, 2016. doi: 10.4239/wjd.v7.i17.412
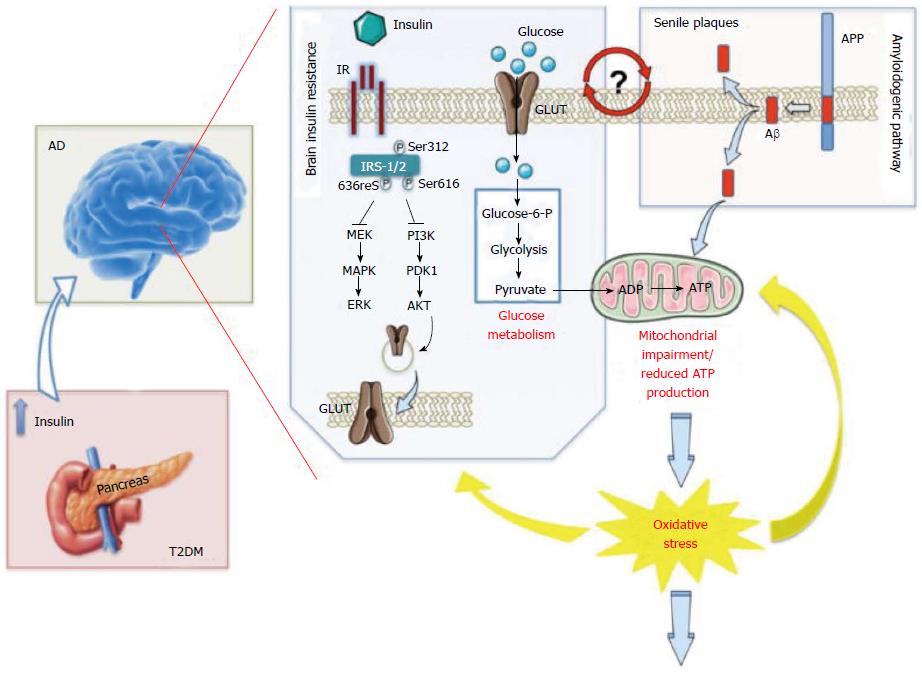
Figure 1 Increased oxidative stress level as a central event driving insulin resistance in Alzheimer’ disease brain[43].
Persistently high levels of circulating insulin [as observed in the first phase of type 2 diabetes mellitus (T2DM)] may exert a negative influence on memory and other cognitive functions by down regulation of insulin receptors (IR) at the blood brain barrier and consequent reduced insulin transport into the brain [as observed in Alzheimer’s disease (AD)], thus leading to insulin resistance. From a molecular point of view, the lack of interaction between insulin and IR is associated with an increase of the inhibitory phosphorylation on insulin receptor substrate-1/2 (IRS1/2) on Ser312, 616 and 636, which, in turn, negatively impacts on the two main arms of insulin-mediated signaling cascade: The PI3K and the MAPK pathways, both involved in the maintenance of synaptic plasticity and cell stress response. Furthermore, turning off insulin signaling results in impaired glucose transport (reduced translocation of the glucose transporter at the plasma membrane) and metabolism thus promoting an alteration of mitochondrial processes involved in energy production. In turn, impairment of mitochondria functions leads to a vicious circle in which reduced energy production is associated with an increase of reactive oxygen and nitrogen species (ROS and RNS) responsible for the oxidative/nitrosative damage of mitochondria as well as other cellular components. In addition, increased Aβ production and accumulation, which represents a key feature of AD pathology, also promotes mitochondrial impairment. Moreover, insulin resistance-associated impairments in glucose uptake and utilization are associated with increased endoplasmic reticulum (ER) stress, which deregulate lipid metabolism, causing accumulation of toxic lipids in the brain. All these events contribute to the increased oxidative stress levels responsible of neurodegeneration observed in AD brain. Although insulin resistance and Aβ production can be considered leading causes of the rise of oxidative stress, this latter, in turn, promotes IRS-1/2 Ser-312, -616 and -636 phosphorylation as well as the oxidative damage of protein involved in glycolysis, the Krebs cycle and ATP synthesis that are crucial events in the reduction of glucose metabolism and thus insulin resistance. Finally, because insulin resistance is associated with increased Aβ production and Aβ production is postulated to be responsible for the onset of insulin resistance, it remains to be clarified whether insulin resistance is a cause, consequence, or compensatory response to Aβ-induced neurodegeneration. ADP: Adenosine diphosphate; APP: β-Amyloid precursor protein; ATP: Adenosine triphosphate; AKT: Akt also known as protein kinase B (PKB); ERK: Extracellular signal-regulated kinase; GLUT: Glucose transporter; MAPK: Mitogen-activated protein kinase; MEK: MAPK/Erk kinase; PDK1: 3-phosphoinositide-dependent protein kinase 1; PI3K: Phosphoinositide 3 kinase.
- Citation: Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World J Diabetes 2016; 7(17): 412-422
- URL: https://www.wjgnet.com/1948-9358/full/v7/i17/412.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i17.412