Copyright
©The Author(s) 2015.
World J Diabetes. Aug 10, 2015; 6(9): 1092-1096
Published online Aug 10, 2015. doi: 10.4239/wjd.v6.i9.1092
Published online Aug 10, 2015. doi: 10.4239/wjd.v6.i9.1092
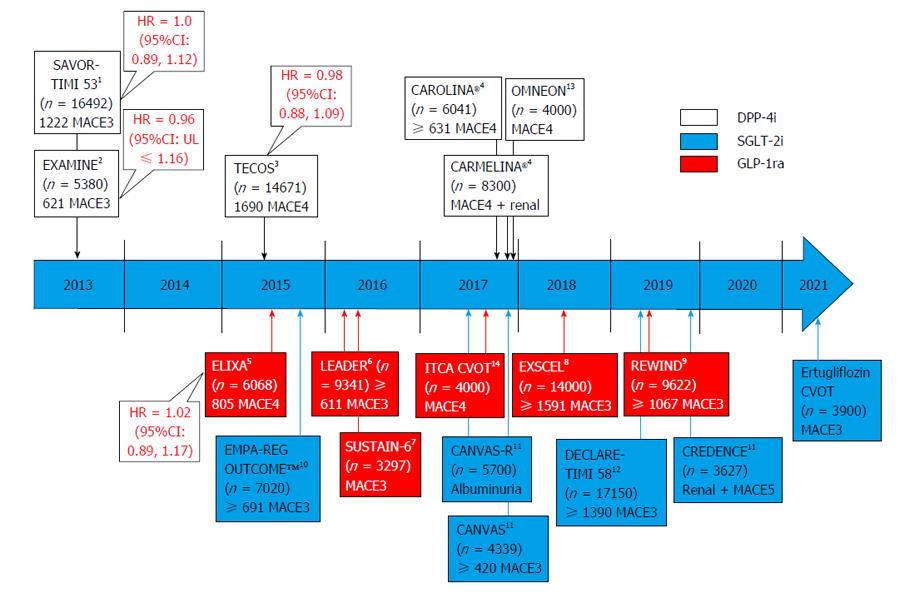
Figure 1 Anticipated ending of outcome trials in type 2 diabetes and their primary outcomes and patient/event numbers involving di-peptidyl peptidase-4 inhibitors, glucagon-like protein-1 receptor analogues and sodium glucose co-transporter-2 inhibitors.
Superscript note indicate study drug(s) in testing. All trials are placebo controlled except CAROLINA® that compared vs the sulfonylurea glimepiride. 1Saxagliptin, Astra Zeneca; 2Alogliptin, Takeda; 3Sitagliptin, Merck; 4Linagliptin, Boehringer Ingelheim/Eli Lilly; 5Lixisenatide, Sanofi Aventis; 6Liraglutide, Novo Nordisk; 7Semaglutide, Novo Nordisk; 8Exenatide, Astra Zeneca; 9Dulaglutide, Eli Lilly; 10Empagliflozin, Boehringer Ingelheim/Eli Lilly; 11Canagliflozin, J and J; 12Dapagliflozin, Astra Zeneca; 13Omarigliptin (once weekly tablet), Merck; 14ITCA 650 [once/twice yearly exenatide via subcutaneous mini-pump (Duros device)], Intarcia Therapeutics. DPP-4: Di-peptidyl peptidase-4; GLP-1: Glucagon-like protein-1; SGLT-2: Sodium glucose co-transporter-2; MACE3: Composite endpoint of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke; MACE4: MACE3 plus hospitalized unstable angina pectoris; MACE5: MACE4 plus hospitalized congestive heart failure.
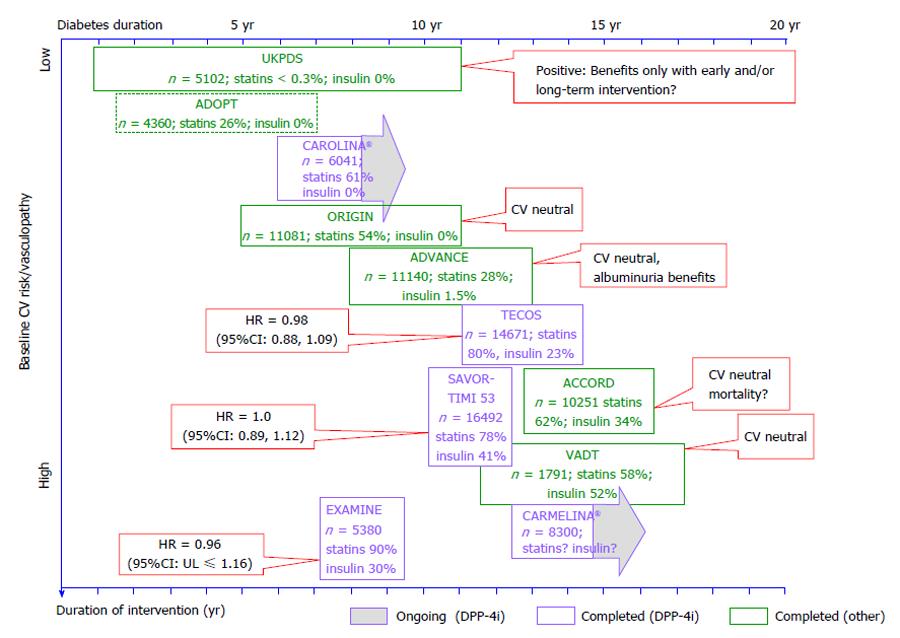
Figure 2 Selected outcome trials in type 2 diabetes with a focus on di-peptidyl peptidase-4 inhibitor studies, and their results, in the context of the duration of intervention and the study population’s diabetes duration and baseline cardiovascular risk.
CV: Cardiovascular; UKPDS: United Kingdom Prospective Diabetes Study; VADT: Veterans affairs diabetes trial.
- Citation: Johansen OE. Interpretation of cardiovascular outcome trials in type 2 diabetes needs a multiaxial approach. World J Diabetes 2015; 6(9): 1092-1096
- URL: https://www.wjgnet.com/1948-9358/full/v6/i9/1092.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i9.1092