Copyright
©The Author(s) 2025.
World J Diabetes. Jun 15, 2025; 16(6): 106720
Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.106720
Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.106720
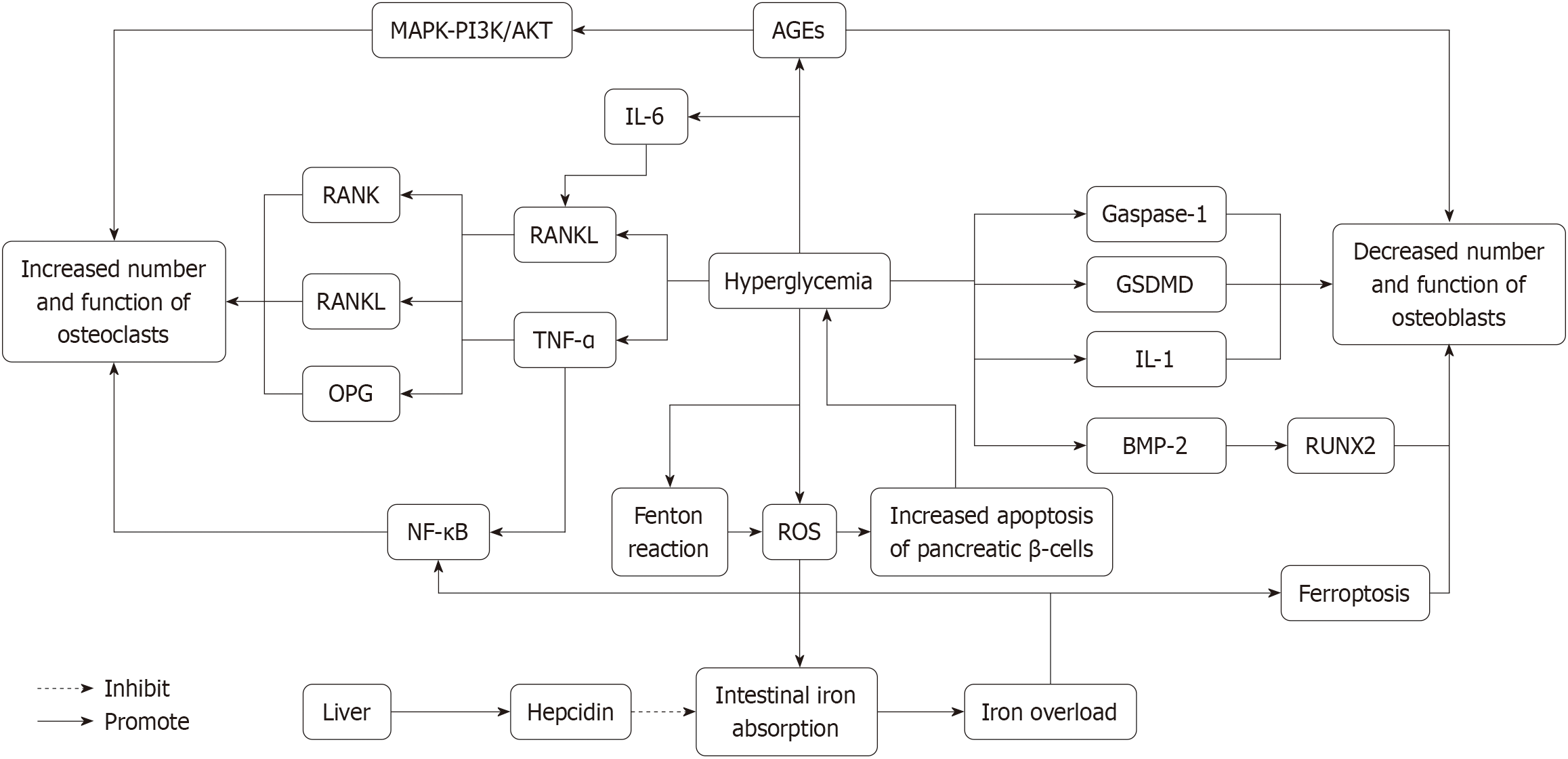
Figure 1 Mechanism diagram of high glucose-induced osteoporosis.
This schematic illustrates hyperglycemia-induced disruption of bone metabolism through advanced glycation end-products and reactive oxygen species signaling pathways. Hyperglycemia promotes the accumulation of advanced glycation end products (AGEs), which bind to receptor activator of nuclear factor-κB (RANK) and RANK ligand (RANKL) receptors, activating the bone resorption process. AGE-mediated signaling is mediated via the mitogen-activated protein kinase mitogen-activated protein kinase, phosphoinositide 3-kinase/protein kinase B, and nuclear factor kappa B pathways, which in turn upregulate pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin (IL)-6, promoting osteoclast differentiation and activation, thereby increasing their number and function. Osteoclast formation is regulated by the balance between RANKL and osteoprotegerin (OPG), with OPG acting as a decoy receptor to inhibit osteoclastogenesis. Meanwhile, hyperglycemia generates reactive oxygen species (ROS) through the Fenton reaction, leading to pancreatic β-cell apoptosis and causing iron overload by increasing intestinal iron absorption. The liver responds to iron overload by upregulating hepcidin, which exacerbates iron accumulation and further disrupts bone metabolism. Additionally, AGEs and ROS inhibit osteoblast function by activating molecules such as gasdermin D, Gaspase-1, IL-1, and bone morphogenetic protein-2, leading to reduced runt-related transcription factor 2 expression and impaired osteoblast activity, thereby hindering bone formation. ROS and iron accumulation also promote ferroptosis, further exacerbating osteoblast dysfunction, which results in an imbalance between bone resorption and formation, ultimately leading to osteoporosis and other bone-related complications associated with diabetes. AGEs: Advanced glycation end products; BMP-2: Bone morphogenetic protein-2; GSDMD: Gasdermin D, a protein associated with programmed necrosis, playing a key role in the release of inflammatory factors such as interleukin-1β; IL-1: Interleukin-1; MAPK: Mitogen-activated protein kinase; NF-κB: Nuclear factor kappa B; OPG: Osteoprotegerin; PI3K/AKT: Phosphoinositide 3-kinase/protein kinase b; RANK: Receptor activator of nuclear factor-κB; RANKL: Receptor activator of nuclear factor-κB ligand; ROS: Reactive oxygen species; RUNX2: Runt-related transcription factor 2, a key transcription factor for osteoblast differentiation, regulating the expression of bone formation-related genes; TNF-α: Tumor necrosis factor-α.
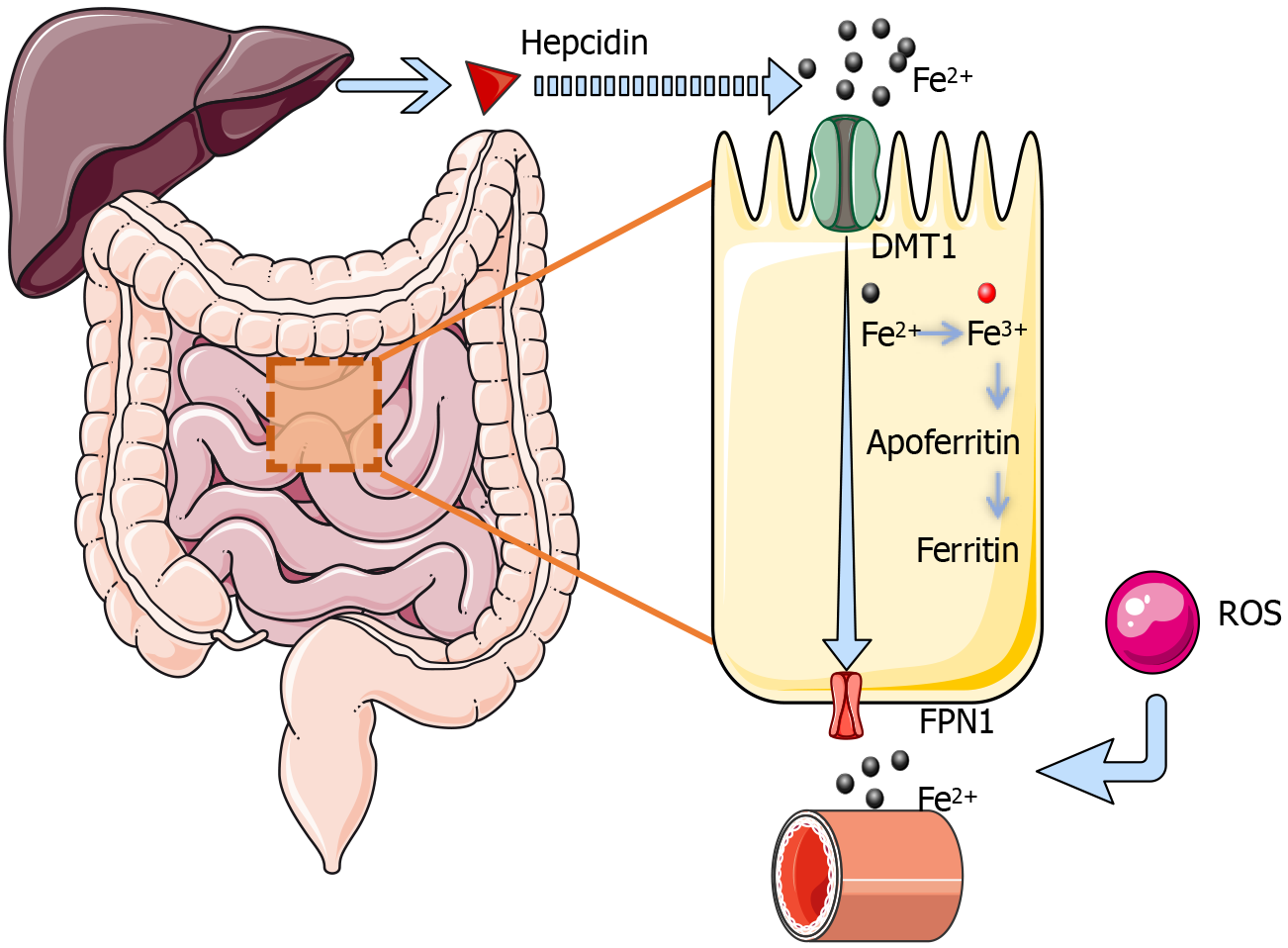
Figure 2 The process of iron absorption in the small intestine.
Iron is primarily absorbed in the upper small intestine through an energy-dependent active transport process involving two key steps: Uptake of iron from the intestinal lumen by mucosal cells and its subsequent transport into the plasma. This process requires the participation of multiple transport proteins. Divalent metal transporter 1, located on the apical membrane of mucosal cells, facilitates the H+-dependent active transport of Fe2+ into intestinal epithelial cells. On the basolateral membrane, FPN1 mediates the export of Fe2+ from the cells into the bloodstream, also via an H+-dependent mechanism. Any inorganic iron that remains within the mucosal cells without being exported can be oxidized to Fe3+ and stored temporarily as ferritin by binding to apoferritin. This stored iron is slowly released into the bloodstream over time. Iron that is not utilized is eventually lost with the natural turnover and shedding of mucosal cells, preventing excessive accumulation. This sophisticated balance ensures efficient iron absorption by the intestinal mucosa while protecting the body from iron overload. Under normal conditions, iron homeostasis is regulated by hepcidin, a liver-secreted hormone that modulates iron availability. Elevated ROS can further promote iron release and increase its bioavailability, potentially exacerbating iron overload. DMT1: Divalent metal transporter 1; FPN1: Ferroportin 1; ROS: Reactive oxygen species.
- Citation: Wang YB, Li ZP, Wang P, Wang RB, Ruan YH, Shi Z, Li HY, Sun JK, Mi Y, Li CJ, Zheng PY, Zhang CJ. Iron dysregulation, ferroptosis, and oxidative stress in diabetic osteoporosis: Mechanisms, bone metabolism disruption, and therapeutic strategies. World J Diabetes 2025; 16(6): 106720
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/106720.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.106720