Copyright
©2010 Baishideng Publishing Group Co.
World J Diabetes. Sep 15, 2010; 1(4): 101-108
Published online Sep 15, 2010. doi: 10.4239/wjd.v1.i4.101
Published online Sep 15, 2010. doi: 10.4239/wjd.v1.i4.101
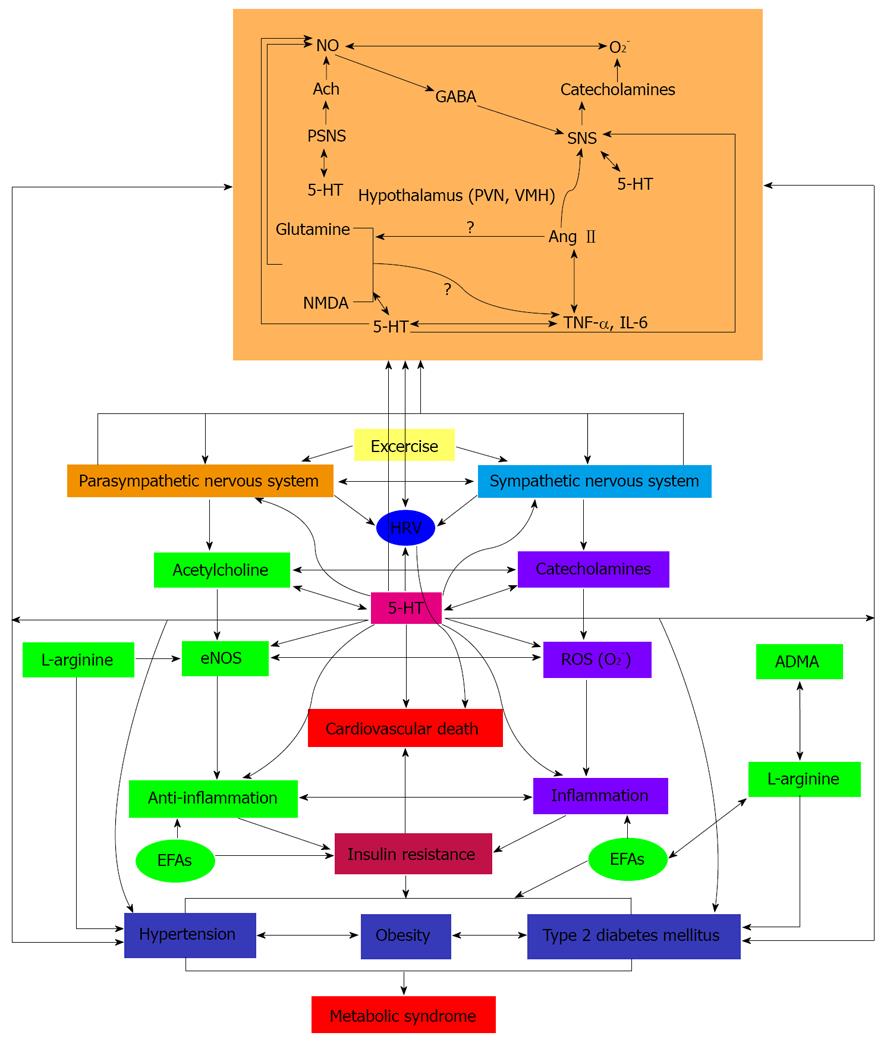
Figure 1 Scheme showing the interaction(s) among hypothalamic monoamines, autonomic nervous system, heart rate variability, inflammation, nitric oxide, essential fatty acids, dietary factors and type 2 diabetes mellitus and sudden cardiovascular death.
Fasting, hypoglycemia and streptozotocin-induced diabetes alter brain tryptophan and serotonin metabolism. Serum norepinephrine and epinephrine increased following injection of insulin in both the ventromedial and lateral hypothalamus. In diabetes, an increase in norepinephrine level in the anterior and the medial-basal hypothalamus and a concomitant rise in dopamine content in the hypothalamus was reported. Serotonin levels were low in the hypothalamus in prediabetic animals. The decrease in serotonin level and its metabolites in the hypothalamus gradually decreased with the duration of diabetes. Brain insulin receptors and brain monoamines and acetylcholine interact with each other. In diabetics, the responses of the monoamines in the hypothalamus are inadequate to plasma and brain insulin levels. The serotonin content was higher in the hypothalamus while in pons medulla oblongata, serotonin and dopamine and the metabolites 5-hydroxy-3-indole acetic acid and DOPAC were significantly reduced whereas norepinephrine was markedly increased with a reduction in serotonin content in the hippocampus. In diabetes, the distal projections of serotonin were normal accompanied by hyperinnervation of the hypothalamus but the shorter collaterals were lost in the pons medulla oblongata due to lack/deficiency of insulin, changes that could result in an inappropriate response of hypothalamic monoamines to insulin. Lower Heart rate variability (HRV) occurs in young adults with metabolic syndrome much before the development of type 2 diabetes mellitus that suggests lower vagal activity and an increase in sympathetic predominance in type 2 diabetes and the critically ill that increases the cardiovascular risk. Hence, intensive insulin therapy may lead to alterations in the brain and peripheral monoamines, serotonin and acetylcholine levels that could cause sporadic autonomic dysregulation and excessive serotonin inhibition that may precipitate pronounced bradycardia and apnea and death. EFAs: essential fatty acids; eNO: endothelial nitric oxide; ADMA: asymmetrical dimethylarginine.
- Citation: Das UN. Hypothesis: Intensive insulin therapy-induced mortality is due to excessive serotonin autoinhibition and autonomic dysregulation. World J Diabetes 2010; 1(4): 101-108
- URL: https://www.wjgnet.com/1948-9358/full/v1/i4/101.htm
- DOI: https://dx.doi.org/10.4239/wjd.v1.i4.101