Copyright
©2010 Baishideng.
World J Diabetes. May 15, 2010; 1(2): 27-35
Published online May 15, 2010. doi: 10.4239/wjd.v1.i2.27
Published online May 15, 2010. doi: 10.4239/wjd.v1.i2.27
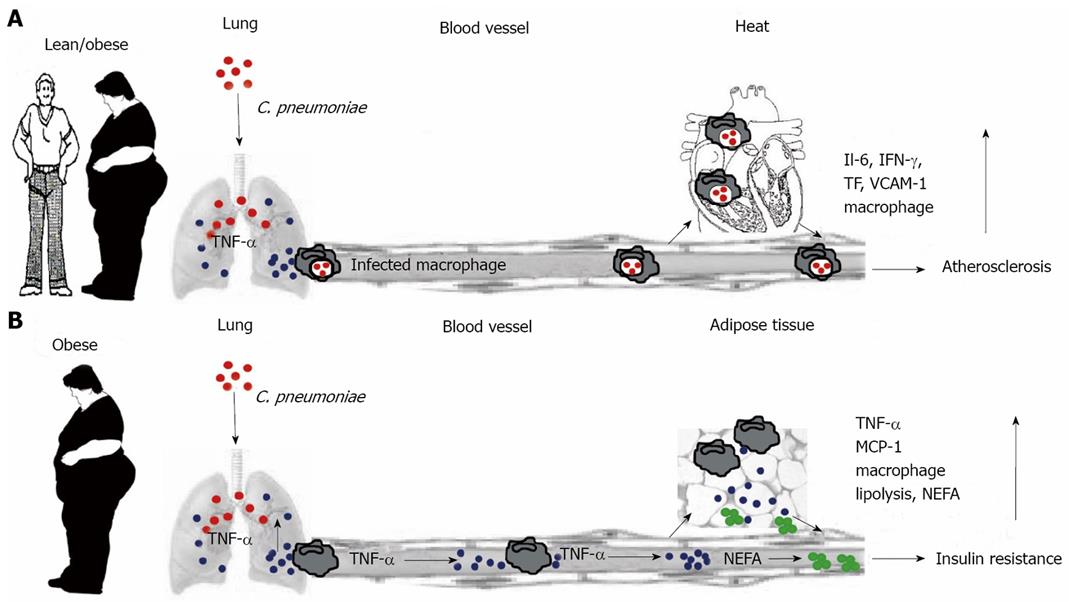
Figure 1 Schematic representation of C.
pneumoniae-mediated acceleration of inflammatory diseases associated with metabolic syndrome. A: Current concept of the influence of C. pneumoniae on atherosclerosis. After respiratory infection, C. pneumoniae (red) is endocytosed by alveolar macrophages of infected lean or obese individuals. Infected macrophages re-enter circulation and traffic to secondary organs including arterial endothelium. Disseminated C. pneumoniae organisms infect other cell types, leading to up-regulation of pro-inflammatory molecules such as IL-6, IFN-γ, tissue factor and vascular-cell-adhesion molecule 1. These cytokines, in particular IFN-γ, retard chlamydial replication and induce persistent infection. In arterial endothelium, infected macrophages and smooth muscle cells transform into foam cells, affecting atheroma biology and leading to plaque destabilization, thrombus formation or myocardial infarction[85]. B: Concept of C. pneumoniae-induced exacerbation of insulin resistance developed by Wang et al[84]. C. pneumoniae infection results in lung colonization, thereby increasing TNF-α release (blue). Circulating TNF-α induces insulin resistance by inhibiting the function of insulin receptor substrate-1 in peripheral tissues and further exacerbates insulin resistance by promoting lipolysis and increased NEFA (green) production in adipose tissue. TNF-α and NEFA promote further macrophage infiltration and excess production of pro-inflammatory molecules in adipose tissue. In contrast to the atherosclerosis concept, the perpetuation of adipose tissue inflammation is driven by circulating pro-inflammatory cytokines rather than by in situ production stimulated by infected macrophages. Sustained by continuous high-fat nutrition, the inflammatory condition of adipose tissue maintains the vicious cycle of insulin resistance in the absence of C. pneumoniae organisms.
- Citation: Wang CM, Kaltenboeck B. Exacerbation of chronic inflammatory diseases by infectious agents: Fact or fiction? World J Diabetes 2010; 1(2): 27-35
- URL: https://www.wjgnet.com/1948-9358/full/v1/i2/27.htm
- DOI: https://dx.doi.org/10.4239/wjd.v1.i2.27