Published online Aug 15, 2017. doi: 10.4251/wjgo.v9.i8.333
Peer-review started: February 7, 2017
First decision: March 28, 2017
Revised: May 10, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: August 15, 2017
Processing time: 191 Days and 1.6 Hours
To investigate the impact of histology on outcome in advanced oesophageal cancer treated with first-line fluoropyrimidine-based chemotherapy.
Individual patient data were pooled from three randomised phase III trials of fluoropyrimidine-based chemotherapy ± platinum/anthracycline in patients with advanced, untreated gastroesophageal adenocarcinoma or squamous cell carcinoma (SCC) randomised between 1994 and 2005. The primary endpoint was overall survival of oesophageal cancer patients according to histology. Secondary endpoints were response rates and a toxicity composite endpoint.
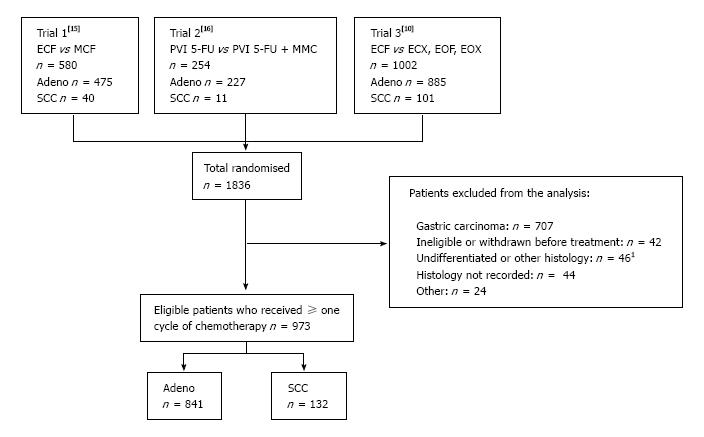
Of the total 1836 randomised patients, 973 patients (53%) were eligible (707 patients with gastric cancer were excluded), 841 (86%) had adenocarcinoma and 132 (14%) had SCC. There was no significant difference in survival between patients with adenocarcinoma and SCC, with median overall survivals of 9.5 mo vs 7.6 mo (HR = 0.85, 95%CI: 0.70-1.03, P = 0.09) and one-year survivals of 38.8% vs 28.2% respectively. The overall response rate to chemotherapy was 44% for adenocarcinoma vs 33% for SCC (P = 0.01). There was no difference in the frequency of the toxicity composite endpoint between the two groups.
There was no significant difference in survival between adenocarcinoma and SCC in patients with advanced oesophageal cancer treated with fluoropyrimidine-based chemotherapy despite a trend for worse survival and less chemo-sensitivity in SCC. Tolerance to treatment was similar in both groups. This analysis highlights the unmet need for SCC-specific studies in advanced oesophageal cancer and will aid in the design of future trials of targeted agents.
Core tip: There is a lack of published data on differential treatment response according to histology in oesophageal cancer. This paper shows improved response rates with first-line chemotherapy and a trend towards improved survival in adenocarcinoma compared to squamous cell carcinoma (SCC). It is increasingly recognised that these histological subtypes represent discrete disease entities with divergent treatment pathways in both the early stage and advanced settings. Novel treatments in SCC remain sparse and there are few dedicated trials in this subtype. This data highlights the poor outcomes seen with chemotherapy alone and the need for further research, particularly for SCC.
- Citation: Davidson M, Chau I, Cunningham D, Khabra K, Iveson T, Hickish T, Seymour M, Starling N. Impact of tumour histological subtype on chemotherapy outcome in advanced oesophageal cancer. World J Gastrointest Oncol 2017; 9(8): 333-340
- URL: https://www.wjgnet.com/1948-5204/full/v9/i8/333.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i8.333
Oesophageal cancer is the eighth most common malignancy worldwide with an estimated 456000 new cases and 400000 deaths worldwide in 2012, ranking it the sixth most common cause of cancer-related deaths[1]. Despite recent advances in genetic and molecular characterisation and the development of novel targeted agents survival rates for oesophageal carcinoma have changed little for many decades, and outcomes for advanced disease remain poor. Worldwide, squamous cell carcinoma (SCC) is the predominant histological subtype however in North America and Northern Europe the incidence of oesophageal adenocarcinoma has increased in the last 20 years whereas that of SCC has decreased[2,3]. This is likely to reflect the distinct aetiological factors implicated in the development of the two diseases. SCC is strongly correlated with excessive alcohol consumption, cigarette smoking and poor socioeconomic status whereas adenocarcinoma is associated with obesity and gastroesophageal reflux disease (GORD)[3-6]. Thus the rise in adenocarcinoma may be in part due to changing lifestyle factors in Western populations[3]. Genomic technology has been applied to both gastric and oesophageal cancer in an effort to improve understanding and stratification on a genetic and molecular level, with emerging differences in the genetic landscape between the two histological subtypes suggesting a need for more tailored therapeutic strategies[7,8]. Historically however treatment patterns for both subtypes have been similar, with many clinical trials evaluating chemotherapy conducted since the mid-1990s including patients with gastric, oesophageal, or oesophagogastric junction (OGJ) cancer, regardless of histology. Similarly, studies in early stage oesophageal cancer often include both histological subtypes, such as the recent CROSS trial evaluating neoadjuvant chemo-radiation. This identified disparities in outcome according to histology, with a statistically significant overall survival benefit seen only in the smaller SCC cohort[9].
In the advanced disease setting cisplatin/fluorouracil with the possible addition of a third drug - either epirubicin or a taxane - is commonly used as a first-line chemotherapy, and second-line agents include irinotecan, docetaxel and paclitaxel[10,11]. More recently treatment patterns have diverged, with the introduction of novel molecularly-targeted therapy for gastroesophageal adenocarcinomas. Notably effective therapies targeting HER2 (trastuzumab) and the vascular endothelial growth factor receptor 2 (ramucirumab) are applicable only to adenocarcinomas[12-14].
Three randomised phase III studies of fluoropyrimidine-based combination chemotherapy have been published in patients with advanced gastroesophageal cancer including oesophageal SCC and adenocarcinoma[10,15,16]. In multivariate Cox regression analysis histology was not identified as a variable impacting on survival, however patients with SCC accounted for less than 10% of the patients in each trial. Although SCC normally represent a small minority of patients enrolled on most clinical trials it is not clear what influence histologic subtype exerts on response rate or survival duration in patients treated with cytotoxic chemotherapy regimens for metastatic disease, and SCC has been associated with both worse, better or similar outcomes to adenocarcinoma[17-19]. The distinct epidemiological, genetic and molecular characteristics of SCC as compared to adenocarcinoma could potentially influence response to therapies administered in the advanced disease setting. In this pooled analysis of the three randomised phase III studies which included patients with both advanced oesophageal SCC and adenocarcinoma, we aimed to evaluate whether there was a differential treatment effect according to histology.
Between 1994 and 2005, 1836 patients were randomised predominantly from the United Kingdom in three multi-centre randomised controlled trials of fluoropyrimidine-based chemotherapy in patients with untreated locally advanced or metastatic carcinoma of oesophagus, OGJ, or stomach[10,15,16]. Similar eligibility criteria were applied in the three trials; patients had histologically confirmed inoperable adenocarcinoma, SCC or undifferentiated carcinoma of the oesophagus, OGJ or stomach, adequate haematological, renal and hepatic function and an Eastern Co-operative Oncology Group performance status (PS) of 0-2. Written informed consent was obtained from all patients and all three studies were approved by the Scientific and Research Ethics Committees of the participating institutions.
The first study randomised 580 patients between 1995 and 1998 to treatment with ECF [epirubicin 50 mg/m2 intravenously (IV) and cisplatin 60 mg/m2IV infusion with hydration on day 1 plus 5-FU 200 mg/m2 per day by protracted venous infusion (PVI)] or MCF [mitomycin C (MMC) 7 mg/m2 on day 1 every six weeks, cisplatin 50 mg/m2IV day 1 and PVI-5-FU 300 mg/m2 per day][15]. The second study randomised 254 patients between 1994 and 2001 to PVI 5-FU (300 mg/m2 per day) or the same dose of PVI 5-FU plus MMC (7 mg/m2 every six weeks)[16]. The third study conducted randomised 1002 patients between 2000 and 2005 to ECF, ECX (X denotes capecitabine given at a dose of 625 mg/m2 twice a day continuously), EOF (O denotes oxaliplatin 130 mg/m2 on day 1 every three weeks replacing cisplatin) and EOX[10].
A maximum of 8 cycles of chemotherapy (24 wk) with response assessment computed tomography (CT) scans at 12 and 24 wk was stipulated in the three study protocols. Overall survival (OS) was the primary outcome measure in these trials and toxicity data was recorded at each treatment visit every three weeks.
Only eligible patients with squamous carcinoma or OGJ adenocarcinoma who received at least one dose of chemotherapy were included in this analysis which was based on individual patient data from these trials.
OS was the primary endpoint of this pooled analysis and was calculated from the date of randomisation until death from any cause, or censored at the date of last follow-up for surviving patients according to the Kaplan-Meier method. Survival analyses were performed on the eligible population and compared between patients with SCC and adenocarcinoma using the log rank test. Multivariate survival analysis was performed using Cox proportional hazard model and stratified for treatment centres. The following factors were included: Histology, gender, primary site (oesophagus vs OGJ), liver or peritoneal metastases (presence vs absence), serum alkaline phosphatase (< 100 U/L vs ≥ 100 U/L) and performance status (0-1 vs 2) based on previously-identified prognostic factors in advanced OG cancer[20,21], as well as treatment arm and trial.
Objective response rates between SCC and adenocarcinoma were compared using χ2 test. A chemotherapy-specific toxicity composite endpoint (TCE) was constructed as a surrogate for undesirable cyctotoxic-related toxicities. TCE was defined as the first occurrence of grade 3 or 4 diarrhoea, neutropenia, febrile neutropenia, fever, infection, nausea and vomiting or grade ≥ 2 renal or neurotoxicity. TCE was compared between the two histological subtypes using χ2 test. Time to TCE was compared between SCC and adenocarcinoma using log rank test.
Two-sided P value of less than 0.05 were considered significant for the overall survival endpoint, and 95%CI quoted. Analyses were performed using SPSS package version 23 (SPSS Inc, Chicago, IL, United States).
Of the 1836 patients randomised to the three trials, 973 patients (53%) were eligible for this pooled analysis as indicated in Figure 1. Seven hundred and seven of the 1836 patients (39%) were excluded due to the primary tumour origin being gastric. Of the 973 eligible patients 841 (86%) had adenocarcinoma and 132 (14%) had SCC. Baseline patient characteristics are shown in Table 1. These were broadly balanced between the two histological sub-types except that predictably a greater proportion of adenocarcinoma occurred at the OGJ with metastases to the liver/peritoneum, and there were more males with adenocarcinoma.
| Adeno | SCC | Total | |
| No. of patients | 841 | 132 | 973 |
| Median age (range) | 62 (22-84) | 60 (37-77) | 61 (22-84) |
| Gender | |||
| Male | 730 (87) | 95 (72) | 825 (85) |
| Female | 111 (13) | 37 (28) | 148 (15) |
| Performance status1 | |||
| 0 | 223 (27) | 33 (25) | 256 (26) |
| 1 | 489 (58) | 75 (57) | 564 (58) |
| 2 | 127 (15) | 23 (18) | 150 (15) |
| Sub-site | |||
| Oesophagus | 438 (52) | 117 (89) | 555 (57) |
| OGJ | 403 (48) | 15 (11) | 418 (43) |
| Extent of disease2 | |||
| Locally advanced | 219 (26) | 36 (27) | 255 (26) |
| Metastatic | 622 (74) | 95 (72) | 717 (74) |
| Location of metastases | |||
| Liver | 340 (46) | 46 (35) | 386 (40) |
| Peritoneum | 41 (5) | 4 (3) | 45 (4.5) |
| Lung | 136 (16) | 18 (14) | 154 (16) |
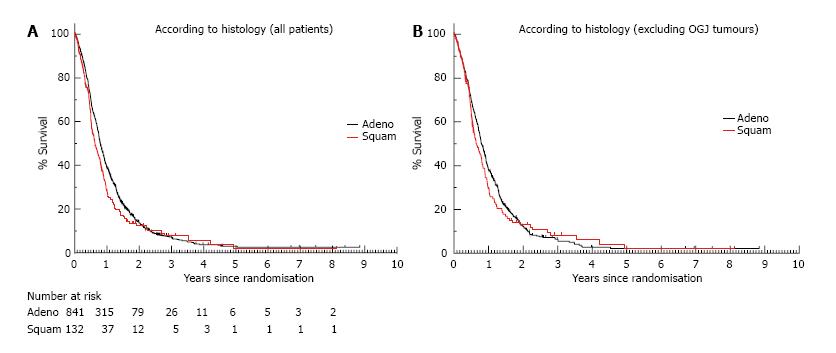
At the time of the data cut-off, 863 of the 973 patients (88%) had died and the median follow-up for surviving patients was 19 mo. The median survival for the whole cohort of 973 eligible patients was 9.4 mo (95%CI: 8.82-9.99). One year survival was 37.3% (95%CI: 37.27-37.33) and 2 year survival was 13.5% (95%CI: 13.48-13.52). There was no significant difference in survival between patients with adenocarcinoma and SCC, with median OS of 9.5 mo vs 7.6 mo (HR = 0.85, 95%CI: 0.70-1.03, P = 0.09), although the curves did appear to separate between 6 mo to 2 years suggestive of a poorer survival for SCC during this period (Figure 2A). One and two year survival figures for adenocarcinoma were 38.8% (95%CI: 38.77-38.83) and 13.6% (95%CI: 13.57-13.63) respectively and for SCC were 28.2% (95%CI: 28.12-28.28) and 12.3% (95%CI: 12.24-12.36). When considering “true” oesophageal cancer patients only and excluding those with junctional tumours, there was again no significant difference in survival between patients with adenocarcinoma (n = 438) and SCC (n = 117), with median OS of 9.5 mo vs 7.7 mo (HR = 0.91, 95%CI: 0.73-1.13, P = 0.38) (Figure 2B). In multivariate analysis, previously identified known prognostic factors of performance status, liver/peritoneal metastases and alkaline phosphatase were all significant. Histology and site of primary tumour were not shown to be significant prognostic factors. For effect of treatment received there was a significant association of treatment within trial 2[16]-which did not incorporate a platinum component into either treatment arm-with poorer outcome (Table 2).
| Factors | Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Overall survival | |||||||
| Histological arm | Adenocarcinoma (r) | 1.000 | |||||
| SCC | 1.196 | 0.972-1.471 | 0.089 | ||||
| Sex | Female (r) | 1.000 | |||||
| Male | 0.983 | 0.807-1.196 | 0.983 | ||||
| Subsite | Oesophagus (r) | 1.000 | |||||
| OGJ | 0.988 | 0.853-1.145 | 0.876 | ||||
| Liver mets | No (r) | 1.000 | 1.000 | ||||
| Yes | 1.671 | 1.433-1.948 | < 0.001 | 1.581 | 1.341-1.863 | < 0.001 | |
| Peritoneal mets | No (r) | 1.000 | 1.000 | ||||
| Yes | 2.290 | 1.583-3.314 | < 0.01 | 2.190 | 1.503-3.191 | < 0.001 | |
| ALP | < 100 U/I (r) | 1.000 | 1.000 | ||||
| ≥ 100 U/I | 1.608 | 1.357-1.908 | < 0.001 | 1.287 | 1.072-1.544 | 0.007 | |
| Performance score | 0-1 (r) | 1.000 | 1.000 | ||||
| 2-3 | 2.140 | 1.754-2.611 | < 0.001 | 1.703 | 1.374-2.110 | < 0.001 | |
| Treatment arm | EOX (r) | 1.000 | |||||
| EOF | 1.122 | 0.848-1.484 | 0.420 | ||||
| ECX | 1.139 | 0.862-1.505 | 0.361 | ||||
| ECF | 1.175 | 0.916-1.506 | 0.204 | ||||
| MCF | 1.176 | 0.870-1.589 | 0.291 | ||||
| PVI 5FU + MMC | 2.107 | 1.461-3.040 | < 0.001 | ||||
| PVI 5FU | 2.132 | 1.481-3.067 | < 0.001 | ||||
| Overall | < 0.001 | ||||||
| Study | Trial 3[10] (r) | 1.000 | 1.000 | ||||
| Trial 1[15] | 0.993 | 0.804-1.228 | 0.951 | 1.034 | 0.830-1.288 | 0.763 | |
| Trial 2[16] | 1.850 | 1.432-2.390 | < 0.001 | 1.736 | 1.326-2.271 | < 0.001 | |
| Overall | < 0.001 | < 0.001 | |||||
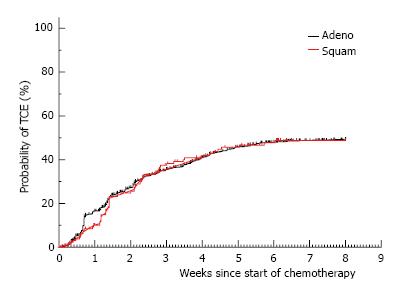
The objective response rate to chemotherapy (Table 3) was significantly higher for patients with adenocarcinoma compared to SCC (44% vs 33%, P = 0.01). A greater proportion of patients with SCC compared to adenocarcinoma progressed during chemotherapy (29% vs 19%, P = 0.01) and the proportion of patients with stable disease was similar for both histological subtypes. There was no difference in the proportion of patients experiencing the toxicity composite endpoint (TCE) for adenocarcinoma as compared to SCC (45% vs 44%, P = 0.77) (Table 3). Similarly there was no difference in the time to development of TCE (Figure 3) between the histological subtypes (HR = 0.98, 95%CI: 0.74-1.29, P = 0.9).
| Adeno | SCC | |
| No. of patients | 841 | 132 |
| Complete response | 48 (6) | 7 (5) |
| Partial response | 323 (38) | 36 (27) |
| Stable disease | 224 (30) | 35 (27) |
| Progressive disease | 157 (19) | 38 (29) |
| Objective response rate (95%CI) | 371 (44) 41%-48% | 43 (33) 25%-41% P = 0.01 |
| Toxicity composite endpoint (95%CI) | 381 (45) 42%-49% | 58 (44) 35%-53% P = 0.77 |
This study represents the largest pooled analysis of differential chemotherapy effects in patients with advanced oesophageal adenocarcinoma and SCC undergoing fluoropyrimidine-based chemotherapy in randomised phase III controlled trials with mature survival data. All three analysed trials incorporated a fluoropyrimidine in each treatment arm, and two of the trials included a platinum agent in each arm. In this pooled analysis there was no significant difference in overall survival between patients with adenocarcinoma compared to those with SCC with median overall survivals of 9.5 mo vs 7.6 mo (HR = 0.85, P = 0.09) respectively. A possible limitation of interpretation of this data is the imbalanced distribution of histological subtype between oesophageal and OGJ cancers. As expected, the proportion of SCC histology was higher in the oesophageal only group as compared to the total cohort of oesophageal and OGJ patients (21% vs 14%). A further analysis excluding OGJ patients however also did not show a significant difference in median OS between adenocarcinoma and SCC (9.5 mo vs 7.7 mo, HR = 0.91, P = 0.38). Histology and site of primary tumour were not shown to be predictors of survival in multivariate analysis, consistent with previously reported prognostic variables in oesophageal and gastric cancer based on smaller analyses[20,22,23]. The survival curves did appear to separate between 6 mo and 2 years, with SCC patients appearing to have worse survival during this period, but the curves then overlapped from two years onwards. Lack of a statistically significant difference in survival in the presence of a trend could reflect that this pooled analysis remains underpowered. Although the potential for heterogeneity may confound interpretation of data from pooled analyses, the eligibility criteria for these three trials were similar, individual patient data were used to strengthen the analysis, treatment arms and trials were incorporated in the multivariate analysis and survival outcomes from ECF, evaluated in the two largest trials[10,15], were consistent. Inclusion of patients with advanced SCC in these studies was controversial in terms of potentially creating a heterogeneous study population however based on the current analysis survival outcomes with standard chemotherapy are not significantly different with SCC compared to adenocarcinoma, although there may be a trend towards worse survival. The only differential treatment effect noted was a significant difference in objective response rates between adenocarcinoma and SCC (44% vs 33% respectively). A greater proportion of SCC patients also progressed during treatment (29% vs 19%), suggesting that oesophageal SCC may be less chemo-sensitive than adenocarcinoma.
There was no difference in time to development of TCE or of the proportion of patients with TCE between the two histological sub-types. A difference might have been expected given the association of co-morbid conditions with SCC. However, within clinical trials there may be selection bias favouring inclusion of fitter patients (patients with a performance status of 2 comprised only 15% of the pooled patient population in this analysis). Although this does potentially limit extrapolation of the results of this analysis to patients with SCC in the general population this would apply to most randomised controlled trials in this disease.
Application of genomic technology is revealing increasing differences between the histological subtypes of oesophageal cancer on a genetic and molecular level. In an analysis performed by the Cancer Genome Atlas, four gastric cancer subtypes have been proposed: Tumours positive for Epstein-Barr virus (EBV), microsatellite unstable tumours (MSI), genomically stable (GS) tumours and tumours with chromosomal instability (CIN)[24]. Each subtype was found throughout the stomach, but CIN tumours showed elevated frequency in the OGJ and cardia. In CIN tumours genomic amplifications of receptor tyrosine kinases such as VEGFA and cell cycle mediators such as CCND1 and CDK6 with potentially relevant clinical implications were found with increased frequency. Specific to oesophageal adenocarcinomas, a sequencing study of 149 tumours by a United States group published in Nature Medicine in 2013 confirmed recurrent mutations in known cancer-driving genes including TP53, CDKN2A, SMAD4, ARID1A and PIK3CA[25]. Similarly, a number of recent studies have applied NGS to the study of oesophageal SCC, demonstrating recurrent mutations in known oncogenic drivers including TP53, NOTCH1, PIK3CA and FAT1, as well as amplifications in CCND1 and CDKN2A[26]. The cell cycle regulation pathway is one of the most consistently altered in oesophageal SCC, where mutations are observed at a high frequency and are associated with poor prognosis and metastasis[27,28]. A recent study has compared the genomic profiles of 71 SCC and 231 oesophageal adenocarcinomas, focusing on the identification of therapeutically relevant genomic alterations in both groups[8]. Similarly high frequencies of clinically relevant genomic alterations were found in both histological subtypes; however the profiles of genomic alterations in the two diseases differed substantially. KRAS and HER2 were more frequently altered in adenocarcinoma, while MTOR pathway genes (PIK3CA, PTEN) and NOTCH1 were more frequently altered in SCC. Exploitation of the molecular differences between the two histological sub-types may help direct optimal application of targeted therapies in this disease.
Although our data is historical, the chemotherapy landscape for oesophageal cancers has not changed significantly in the intervening years. Targeted treatments for oesophageal adenocarcinomas are now in routine clinical use and starting to provide tangible improvement to patient outcomes, however there remains a relative lack of both applied research and effective treatments for advanced SCC. Given small patient numbers and apparently declining incidence, further randomised SCC-specific phase III trials of systemic therapy in advanced oesophageal cancer in Western populations will be challenging. Future improvements in outcome are likely to come from smaller studies investigating cohorts of patients enriched for discrete genetic aberrations, or from the use of combination immunotherapeutic approaches. Optimising the design of such studies using appropriate chemotherapies as either comparators or backbones to newer investigative agents requires an understanding of differential effectiveness and toxicity of standard chemotherapy regimes. This analysis demonstrated no significant difference in survival or tolerance to chemotherapy between patients with adenocarcinoma or SCC. Given the poor outcomes seen with chemotherapy it reinforces the need for SCC-specific trials in advanced oesophageal cancer.
The two main histological subtypes of oesophageal cancer, adenocarcinoma and squamous cell carcinoma (SCC), are increasingly regarded as discrete disease entities with divergent treatment pathways. This is reflected in recently-updated international clinical practice guidelines from both the National Comprehensive Cancer Institute and European Society of Medical Oncology, which recommend differing treatment approaches in early stage and, to a lesser extent, late stage disease dependent on histology.
Although the chemotherapy landscape for advanced oesophageal cancer has not changed in recent years, improved understanding of the molecular and genomic underpinnings of the disease have led to tangible improvements in outcome, with effective biological targeted agents such as trastuzumab and ramucirumab making a tangible difference to patient outcomes. The clinical application of such targeted agents has so far however been limited to the adenocarcinoma subtype. Emerging data on the use of immunotherapy suggests that it will also play a role in this condition. Recent preliminary data from trials of immunotherapy agents such as the KEYNOTE 028 study evaluating use of the anti-PD1 agent pembrolizumab in advanced oesophageal cancer have reported promising signal in both adenocarcinoma and SCC patients, and studies of immunotherapy in both histological subtypes are ongoing. Although SCC remains a significant health problem on a global scale, incidence in Western populations is declining and further large scale randomised trials restricted to this subtype are unlikely.
There is a lack of randomised data on differential chemotherapy response according to histology in oesophageal cancer. This paper shows that adenocarcinomas had a significantly higher response rate to first line fluoropyrimidine-based chemotherapy than SCC. Although there was also a trend towards improved survival outcomes this did not reach statistical significance. This data confirms the generally poor outcomes seen with chemotherapy in advanced oesophageal cancer and suggests that oesophageal SCC may be a less chemotherapy-sensitive disease than adenocarcinoma.
Given the now established role of targeted agents in the management of advanced oesophageal adenocarcinoma and an emerging potential role for immunotherapeutic approaches it is possible that treatment pathways for the two subtypes will further diverge. Improvements in outcome are likely to come from smaller studies investigating targeted agents or combination immunotherapeutic approaches. Optimising the design of such studies using appropriate chemotherapies as either comparators or backbones to newer investigative agents requires knowledge of the differential effectiveness and toxicity of chemotherapy.
The study is interesting and relevant.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Becker KF, Marin JJG S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. In: Cancer IAfRo, editor. |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1961] [Article Influence: 163.4] [Reference Citation Analysis (5)] |
| 3. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 4. | Pandeya N, Williams G, Green AC, Webb PM, Whiteman DC; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136:1215-1224, e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Freedman ND, Abnet CC, Caporaso NE, Fraumeni JF Jr, Murphy G, Hartge P, Hollenbeck AR, Park Y, Shiels MS, Silverman DT. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol. 2016;45:846-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, Rotterdam H, West AB, Dubrow R, Stanford JL. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277-1284. [PubMed] |
| 7. | Agrawal N, Jiao Y, Bettegowda C, Hutfless SM, Wang Y, David S, Cheng Y, Twaddell WS, Latt NL, Shin EJ. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012;2:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 8. | Wang K, Johnson A, Ali SM, Klempner SJ, Bekaii-Saab T, Vacirca JL, Khaira D, Yelensky R, Chmielecki J, Elvin JA. Comprehensive Genomic Profiling of Advanced Esophageal Squamous Cell Carcinomas and Esophageal Adenocarcinomas Reveals Similarities and Differences. Oncologist. 2015;20:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, van Laarhoven HW, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1823] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 10. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1691] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 11. | Janowitz T, Thuss-Patience P, Marshall A, Kang JH, Connell C, Cook N, Dunn J, Park SH, Ford H. Chemotherapy vs supportive care alone for relapsed gastric, gastroesophageal junction, and oesophageal adenocarcinoma: a meta-analysis of patient-level data. Br J Cancer. 2016;114:381-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5317] [Article Influence: 354.5] [Reference Citation Analysis (3)] |
| 13. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1572] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 14. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1766] [Article Influence: 160.5] [Reference Citation Analysis (0)] |
| 15. | Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996-2004. [PubMed] |
| 16. | Tebbutt NC, Norman A, Cunningham D, Iveson T, Seymour M, Hickish T, Harper P, Maisey N, Mochlinski K, Prior Y. A multicentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Ann Oncol. 2002;13:1568-1575. [PubMed] |
| 17. | Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360-367; discussion 368-369. [PubMed] |
| 18. | Lund O, Hasenkam JM, Aagaard MT, Kimose HH. Time-related changes in characteristics of prognostic significance in carcinomas of the oesophagus and cardia. Br J Surg. 1989;76:1301-1307. [PubMed] |
| 19. | Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 398] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Chau I, Ashley S, Cunningham D. Validation of the Royal Marsden hospital prognostic index in advanced esophagogastric cancer using individual patient data from the REAL 2 study. J Clin Oncol. 2009;27:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Chau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T, Nicolson M, Harper P, Seymour M, Hickish T. The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma--individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol. 2009;20:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Polee MB, Hop WC, Kok TC, Eskens FA, van der Burg ME, Splinter TA, Siersema PD, Tilanus HW, Stoter G, van der Gaast A. Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer. 2003;89:2045-2050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4848] [Article Influence: 440.7] [Reference Citation Analysis (2)] |
| 25. | Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 604] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 26. | Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 854] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 27. | Chen J, Kwong DL, Cao T, Hu Q, Zhang L, Ming X, Chen J, Fu L, Guan X. Esophageal squamous cell carcinoma (ESCC): advance in genomics and molecular genetics. Dis Esophagus. 2015;28:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Zhou Y, Cheng C, Cui H, Cheng L, Kong P, Wang J, Li Y, Chen W, Song B. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet. 2015;96:597-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |