Published online Aug 15, 2016. doi: 10.4251/wjgo.v8.i8.635
Peer-review started: February 2, 2016
First decision: March 14, 2016
Revised: May 21, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: August 15, 2016
Processing time: 189 Days and 15.8 Hours
AIM: To estimate the levels of serum cytokines in chronic pancreatitis (CP) and pancreatic ductal adenocarcinoma (PDAC) patients in order to evaluate their usefulness as possible biomarkers.
METHODS: The study included 167 Caucasian patients: 74 with PDAC (28 men and 42 women, aged 30-88 years), 78 with CP (50 men and 21 women, aged 20-79 years) and 15 age-matched healthy controls hospitalized in the Department of Digestive Tract Diseases, Medical University of Lodz, Poland between 2006 and 2013. Serum MCP-1, transforming growth factor (TGF)-β1, HA and s-Fr were measured in patients with CP (n = 78), PDAC (n = 74) and healthy controls (n = 15) using ELISA (Corgenix United Kingdom Ltd R and D Systems). The severity of CP was assessed according to the Cambridge classification.
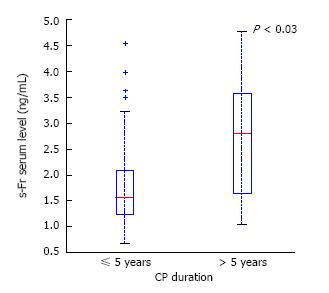
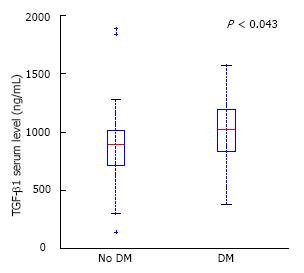
RESULTS: Both patients with CP and PDAC had a significantly higher mean TGF-β1 serum level (1066 ± 582 and 888 ± 356 vs 264 ± 93, P < 0.0001), mean s-Fr (2.42 ± 1.385 and 2.41 ± 1.275 vs 0.6 ± 0.370, P < 0.0001) and mean HA (199 ± 254 and 270 ± 358 vs 40 ± 26, P < 0.0001) compared to controls. There was no difference in mean MCP-1 between all the groups. There were no significant differences in any cytokine levels between the PC and PDAC groups. No significant differences between serum cytokines depending on age, gender or smoking status were found in CP patients. Mean s-Fr concentration was significantly higher in CP, lasting longer than 5 years compared to those with a shorter disease clinical course (2.639 ± 1.125 vs 1.870 ± 0.970, P < 0.03). There was no correlation between tumor size, localization or TNM classification and serum TGF-β1, MCP-1, s-Fr and HA levels in patients with PDAC. No significant differences between cytokines depending on diabetes presence in CP were found. Nevertheless, mean serum TGF-β1 concentration in PDAC patients was higher in those with diabetes compared to the remaining group (986 vs 839, P = 0.043).
CONCLUSION: Serum TGF-β1, s-Fr and HA may be considered additional diagnostic markers of CP and PDAC. TGF-β1 may be useful to predict endocrine insufficiency in PDAC.
Core tip: Fibrosis begins in an early stage of the disease in chronic pancreatitis and in pancreatic ductal adenocarcinoma. Cytokines such as transforming growth factor beta-1, hyaluronic acid, soluble fractalkine and monocyte chemoattractant protein-1 take part in connective tissue proliferation. It is essential to diagnose pancreatic diseases in the early stage in order to start an appropriate therapy. Cytokines can be useful clinical biomarkers.
- Citation: Kozak A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Małecka-Panas E, Gąsiorowska A. Utility of different serum fibrosis markers in diagnosing patients with chronic pancreatitis and pancreatic adenocarcinoma. World J Gastrointest Oncol 2016; 8(8): 635-641
- URL: https://www.wjgnet.com/1948-5204/full/v8/i8/635.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i8.635
Pancreatic fibrosis is the characteristic feature of chronic pancreatitis (CP) and pancreatic ductal adenocarcinoma (PDAC)[1]. The inflammation in CP leads to irreversible destruction of pancreatic parenchyma, interstitial fibrosis and the following loss of exocrine and endocrine tissue[2]. Consequently, CP significantly impairs quality of life[3]. The mechanisms which induce fibrosis in PDAC and CP are not completely elucidated but involve persistent activation of pancreatic stellate cells (PSC). The fibrosis process starts in the early stage of CP and progresses with the duration of the disease[4]. Accurate biological and functional markers of early stage CP are strongly needed for early treatment and prevention of complications. Furthermore, as patients with longstanding CP are at a markedly increased risk of developing pancreatic cancer, diagnosing CP at an early stage may result in cancer prevention[5]. According to Whitcomb et al[6], pancreatic fibrosis itself in chronic CP is a major risk factor for pancreatic cancer. Interstitial fibrosis markers could play a potential role as early stage CP diagnostic markers.
PDAC coexists with CP in 20%-35% of cases. In these circumstances, diagnostic tools such as US, EUS and CT are insufficient because their sensitivity and specificity is unsatisfactory[7]. Several early stage markers have been proposed in CP and PDAC[8]. Transforming growth factor beta-1 (TGF-β1) and extracellular matrix proteins, especially hyaluronic acid (HA) and pro-collagen III peptide (P-III-P), are suggested to play a pivotal role in early stage pancreatitis[7,9]. TGF-β1 expression was found in adjacent areas to pancreatic fibrosis in PSC and acinar cells. TGF-β1 is thought to regulate the synthesis of collagen in PSC[10,11]. TGF-β1 contributes to healing, acts as monocyte and fibroblast chemoattractant factors and increases synthesis and secretion of extracellular matrix[12]. In addition, TGF-β1 plays a role in wound healing, stimulating growth of connective tissue and scar formation[13,14]. Basically, the same process takes place in pancreatic tissue when areas of focal necrosis are replaced by fibrosis. High expression of TGF-β1 is observed in the fibrosis area in models of chronic pancreatitis in rats[15]. TGF-β1 up-regulates collagens at the same time as down-regulating collagenases and stromelysins in fibroblasts[16]. Inhibition of the TGF-β1 signaling pathway significantly reduced fibrosis in experimental pancreatitis models[17,18].
Soluble fractalkine (s-Fr) was also proposed as a marker of early stage pancreatic fibrosis. S-Fr activates multiple signaling cascades in PSCs. This cytokine directly induces PSC proliferation without increasing the release of inflammatory mediators[19]. S-Fr is secreted from PSC in response to various stimuli and contributes to the CP progression, also enhancing the migration of inflammatory cells, inducing vascular smooth muscle and endothelial cell proliferation[20].
Monocyte chemoattractant protein-1 (MCP-1) acts like a strong chemoattractant factor for monocytes, macrophages and lymphocytes. Recent studies show that blocking MCP-1 activity suppresses the progression of experimental CP induced with dibutyltin dichloride in rats[21]. Decreasing MCP-1 and TGF-β levels with anti-inflammatory drugs resulted in a reduced severity of CP, including the extent of inflammatory cell infiltration and stromal fibrosis in a caerulein-induced CP mouse model[22].
In addition to CP, pancreatic fibrosis also plays a crucial role in PDAC. Progressive fibrosis associated with PDAC may have diagnostic and prognostic relevance. Fibrotic degeneration is considered one of the major causes of chemotherapy resistance. New diagnostic markers are strongly needed in order to diagnose PDAC at an early stage.
Diabetes develops in more than half of patients with chronic pancreatitis[23]. Most patients with pancreatic tumors have impaired glucose tolerance[24]. Endocrine and exocrine insufficiency are both consequences of advanced fibrosis.
The aim of this study was to determine the potential diagnostic value of early stage fibrosis markers such as chemokines (s-Fr and MCP-1), cytokines (TGF-β1) and markers of extracellular matrix (HA) in patients with CP and PDAC. Moreover, the usefulness of these cytokines as possible biomarkers of endocrine pancreatic function in CP and PDAC was considered.
The study included 167 Caucasian patients: 74 with PDAC (28 men and 42 women, aged 30-88 years), 78 with CP (50 men and 21 women, aged 20-79 years) and 15 age-matched healthy controls hospitalized in the Department of Digestive Tract Diseases, Medical University of Lodz, Poland between 2006 and 2013. Written informed consent was obtained from all patients. The study protocol was approved by the ethics committee of Lodz Medical University.
The diagnosis of pancreatic tumors was established by ultrasound, computed tomography or endoscopic methods (EUS, ECPW). Histological samples were obtained by different methods: Endoscopic ultrasound-guided fine needle aspiration, brushing at ECPW or surgical sampling. The size of the tumor ranged from 1.5 to 6.0 cm in PDAC patients. At the time of diagnosis most tumors were over 3 cm in size (55.5%) and localized in the head of pancreas (86%). PDAC patients were classified according to the TNM classification: T1 = 21 patients (28%); T2 = 31 patients (42%); T3 = 10 patients (13%); T4 = 12 patients (16%); N0 = 55 patients (74%); N1 = 19 patients (26%); M0 = 47 patients (64%); and M1 = 27 patients (36%). Twenty-eight (38%) of PDAC patients had coexisting CP. Twenty-four PDAC patients (56%) had an average weight (BMI 18.5-24.99; mean 23.1 kg/m2), 11 patients were obese, 2 were overweight and 6 had a BMI under 18.5. Diabetes mellitus, if not diagnosed earlier, was detected with an abnormal glucose tolerance test (OGTT) in 24 PDAC patients.
The diagnosis of CP was determined by standard imaging criteria and clinical course. The diagnosis of CP was based on the following findings: Calcifications, pancreatic stones, dilatation, stenosis or cyst formation of the main pancreatic duct and its branches shown by ultrasound, computed tomography or endoscopic retrograde pancreatography. Most CP patients had a history of alcohol abuse (42 patients, 59.2%). CP patients were classified into two groups according to Cambridge classification[25]: 23 patients with mild CP (normal + equivocal + mild) and 43 with severe CP (moderate + marked). Among the studied CP patients, most (31 patients, 77%) had an average weight (BMI 18.5-24.99, mean 21.1 kg/m2), 4 were obese, none were overweight and 5 had a BMI under 18.5. Mean CP duration was 5.9 years (range 1-19). Diabetes mellitus coexisted in 30 patients (42.5%). All cases were DM type 2. We also evaluated the potential prognostic value of tested substances in PDAC, correlating survival with TGF-β1, MCP-1, s-Fr and HA serum levels.
Peripheral venous blood samples were obtained from all analyzed patients at the time of hospital admission. Serum TGF-β1, MCP-1, s-Fr and HA concentrations were determined with ELISA (Corgenix United Kingdom Ltd R and D Systems). Correlation of chemokines, cytokines and clinical data at the time of diagnosis in CP and PDAC patients was evaluated.
Statistical analysis and graphical data presentation were performed using PANDAS (Python Data Analysis Library) and Matplotlib in the IPython Notebook environment. The significance of differences between the groups was assessed using non-parametric methods (Mann-Whitney test). Two-sided P values were computed and a difference was considered statistically significant at P≤ 0.05.
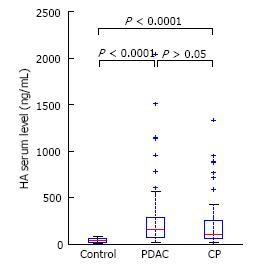
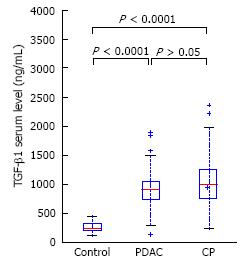
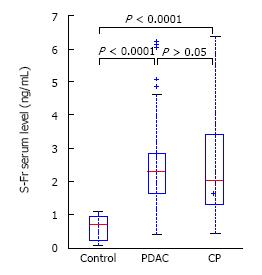
Patients with CP and PDAC had a significantly higher plasma HA level (199 ± 254 and 270 ± 358 vs 40 ± 26 ng/mL, P < 0.0001) (Figure 1) compared to the controls. In CP patients, the TGF-β1 level was 1066 ± 582 pg/mL and in PDAC patients, 888 ± 356 pg/mL, which was significantly higher than in healthy controls, 264 ± 93 pg/mL, P < 0.0001 (Figure 2). Our study showed a significant difference between s-Fr level in CP and PDAC patients compared to controls (2.42 ± 1.385 and 2.41 ± 1.275 vs 0.6 ± 0.370 ng/mL, P < 0.0001) (Figure 3).
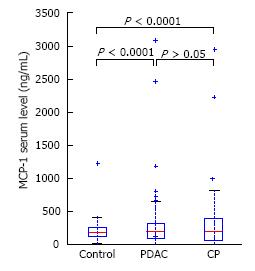
MCP-1 level did not significantly differ between CP (313 ± 453 pg/mL) and PDAC (318 ± 481 pg/mL) patients or controls (252 ± 288 pg/mL), (P = NS) (Figure 4).
There was no significant differences between s-Fr, HA, TGF-β1 and MCP-1 levels in CP and PDAC patients.
In CP patients, only the s-Fr level depended on the duration of the disease (Figure 5). In patients with a CP duration of more than 5 years, serum level of s-Fr was significantly higher compared to CP patients with a shorter disease clinical course (2.639 ± 1.125 vs 1.87 ± 0.970, P < 0.03).
In patients with PDAC and diabetes, serum level of TGF-β1 was significantly higher than in PDAC without endocrine insufficiency (986 ± 341 vs 844 ± 364, P < 0.05) (Figure 6).
No significant differences between serum level of TGF-β1, s-Fr, HA or MCP-1 depending on etiology, age, gender, endocrine insufficiency or Cambridge score in CP patients were found.
There was no correlation between tumor size, localization, TNM classification or coexisting CP and serum TGF-β1, MCP-1, s-Fr and HA levels in patients with PDAC. No tested substances in PDAC patients was related to survival.
The levels of tested substances also did not correlate with each other in both pancreatic diseases. The relationship of the levels of fibrosis markers and desmoplasia in PDAC should be examined in patients who undergo resection. However, in our study only 9 PDAC patients had surgery and statistical analysis requires a larger study group.
In this study, we showed that CP patients had elevated serum levels of HA, TGF-β1, s-Fr but not MCP-1 compared to the healthy controls. Similarly, Yasuda et al[26] showed elevation of TGF-β1 and s-Fr but not MCP-1 levels in patients with CP. In the Pedersen et al[27] study, serum MCP-1 levels were also not different in patients with CP and controls. However, in experimental rat CP models, serum and pancreatic MCP-1 concentration significantly increased with the progression of fibrosis[28]. It is known that migration of monocytes is one of the earliest events in the formation of pancreatic fibrosis. Thus, MCP-1 is considered to be a prefibrogenic factor in the progression of chronic pancreatitis and is thought to be a marker of early stage pancreatitis[29]. It may be assumed that the level of MCP-1 is normalized in patients with a longer disease clinical course.
Our results showed a significantly higher serum level of s-Fr in CP with a duration over 5 years compared to those with a shorter clinical course. Other studies confirmed that the s-Fr serum level increases with the progression of CP[30]. Ceyhan et al[30] assessed the s-Fr and its receptor (CX3CR1) levels in CP and normal pancreas by QRT-PCR, Western Blot and immunohistochemistry analyses. Their expression correlated with the severity of fibrosis and CP duration.
The observations of D’Hease et al[31] also suggest that s-Fr level correlates noticeably with severe fibrosis and the presence of pain in CP. Ceyhan et al[30] showed that fractalkine mRNA expression was associated with the severity of fibrosis in CP and CP duration.
Evaluation of serum in CP patients revealed significantly higher TGF-β1 levels compared to healthy controls. This corresponds with the findings of Detlefsen et al[32] who studied material obtained after surgical resection. They found TGF-β receptors expressed on most myofibroblasts in the pancreatic tissue from CP patients. Moreover, receptors were mainly expressed in the early to moderate stages of pancreatic fibrosis.
In cases with more advanced tissue damage, the expression of TGF-β receptor appeared to be less intense than during the early stages[32]. This finding may suggest that TGF-β1 should be taken into consideration as an early stage fibrosis marker.
Other authors suggested that the expression of TGF-β1 in CP tissue (detected by PCR assay and confirmed by Western blot assay) rises with the severity of CP assessed by the presence of exocrine and endocrine insufficiency, pain (scored 0-4) and complications associated with CP[26]. However, no correlation between CP advancement and TGF-β1 levels was found in our study.
We found that serum levels of HA, TGF-β1, s-Fr were elevated in PDAC patients compared to healthy controls. Similar results were obtained by Erkan et al[33] who described elevated levels of extracellular matrix proteins, laminin, procollagen III peptide (P-III-P) and HA in patients with PDAC. The levels of tested substances increased with the fibrosis advancement. In our study, we found that plasma TGF-β1 levels were elevated in PDAC patients with coexisting diabetes compared to patients without endocrine insufficiency. This finding corresponds with the findings that TGF-β signaling stimulation inhibits proliferation of the pancreatic β-cells in vitro[34]. Furthermore, overexpression of TGF-β1 in the pancreatic β-cells of transgenic mice (which express TGF-β-inducible early response gene on acinar cells) induces islet cell development from ductular-like structures[35]. We did not find any studies analyzing the correlation between TGF-β1 levels in pancreatic disorders and coexisting diabetes in humans in the current literature.
In our study, there was no significant difference between the concentration of MCP-1 in PDAC patients and healthy controls. Some authors have hypothesized that serum MCP-1 levels are elevated in obese PDAC patients. In our study, MCP-1 level was not significantly higher in PDAC patients with a BMI above 25 compared to those with a normal weight. Other findings show a correlation of high circulating MCP-1 levels in obese individuals in general[36]. This may be the reason why mostly cachectic or average weight PDAC and CP patients do not show elevated MCP-1 levels.
Other investigators have suggested that MCP-1 may be the differentiation marker for benign and malignant lesions (comparing PDAC and IPMN)[37]. However, our results showed no differences between MCP-1 levels in PDAC and CP patients. We were also looking for a correlation between various clinical features and levels of tested markers in CP patients. No significant differences between serum level of TGF-β1, s-Fr, HA and MCP-1 depending on etiology, age, gender, endocrine insufficiency or Cambridge score in CP patients were found. In our study, the level of s-Fr was not significantly higher in those with alcohol-related CP compared to CP of other etiologies. In the CP rats study, Uchida et al[38] proved a positive correlation of fractalkine level and alcohol intake. They used Wistar/Bonn Kobori (WBN/Kob) rats, widely accepted as a rodent model of CP.
We also looked for the correlation between various clinical features and levels of tested markers in CP patients. No correlation was found between tumor size, localization and serum TGF-β1, MCP-1, s-Fr and HA levels.
Our results reveal that the levels of the markers did not correlate with each other. Similar results were published by Ito[29] who showed that serum s-Fr did not correlate with MCP-1 or TGF-β1 in CP patients. Our results were confirmed in the Adrych et al[39] study in which no association between serum TGF-β1 and serum hyaluronic acid in CP patients was found. Our study confirmed that levels of fibrosis markers are elevated in CP and PDAC patients compared to healthy controls. However, tested substances do not distinguish CP from PDAC so they are not useful in a differential diagnosis. We did not find a correlation between marker levels and coexisting CP in PDAC patients.
Despite the considerable progress that has been made recently, the PDAC and CP prognosis is still poor and there are no specific treatments that can alter the course of these diseases.
The present study reveals that serum TGF-β1, s-Fr and HA may be considered additional diagnostic markers of CP and PDAC. This study showed a relationship between the duration of CP and serum concentration of s-Fr. Elevated s-Fr levels in CP patients with a longer disease clinical course suggest a correlation of the severity of CP and the advancement of fibrosis. Thus, s-Fr may be considered a biological marker of CP and may be useful to assess the progression of CP.
Our results confirm results from other studies, suggesting that s-Fr could be used as an early stage PDAC marker before the invasive stage begins[40]. Moreover, TGF-β1 level was elevated in patients with concomitant diabetes. Therefore, assessing TGF-β1 level may be useful to predict endocrine insufficiency in PDAC patients.
Late diagnosis of chronic pancreatitis or pancreatic adenocarcinoma limits treatment and results in a worse prognosis. Despite worldwide investigation, suitable diagnostic markers have not been proposed yet.
Different serum fibrosis markers are taken into consideration as diagnostic and prognostic markers.
This is the first study to evaluate different fibrosis markers in the serum of chronic pancreatitis (CP) and pancreatic ductal adenocarcinoma (PDAC) patients compared to healthy controls. Significantly higher s-Fr, HA and transforming growth factor-β1 serum levels compared to healthy controls suggest the potential utility of tested substances as diagnostic markers.
The presented results require further investigations in a larger study group.
Pancreatic fibrosis is the characteristic feature of chronic pancreatitis and pancreatic ductal adenocarcinoma. Revealing desmoplasia at an early stage can be a useful diagnostic tool.
This study presents a topic of interest in a diagnostic field. The methods and study population are adequate and the conclusions are reasonable and of possible practical use. This article presents an important issue and is the first study to evaluate different fibrosis markers in the serum of CP and PDAC patients compared to healthy controls.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ishiguro H S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Longnecker DS. Pathology and pathogenesis of diseases of the pancreas. Am J Pathol. 1982;107:99-121. [PubMed] |
| 2. | Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endosc. 2014;47:212-216. [PubMed] [DOI] [Full Text] |
| 3. | Mokrowiecka A. Pińkowski D, Małecka-Panas E. Assessment of quality of life in patients with chronic pancreatitis. Med Sci Monit. 2011;17:CR583-CR588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Emmrich J, Weber I, Sparmann G, Liebe S. Activation of pancreatic stellate cells in experimental chronic pancreatitis in rats. Gastroenterology. 2000;118:A 166. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 758] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 6. | Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315-G319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Motkowska M, Romatowski J, Januszko M, Piotrowska-Staworko G, Łaszewicz W. Endoscopic ultrasound elastography in digestive tract diseases. Przegląd Gastroenterologiczny. 2012;7:63-69. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Małecka-Panas E. Subclinical inflammation and endothelial dysfunction in patients with chronic pancreatitis and newly diagnosed pancreatic cancer. Dig Dis Sci. 2016;61:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Löhr JM. What are the useful biological and functional markers of early-stage chronic pancreatitis? J Gastroenterol. 2007;42 Suppl 17:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | di Mola FF, Friess H, Martignoni ME, Di Sebastiano P, Zimmermann A, Innocenti P, Graber H, Gold LI, Korc M, Büchler MW. Connective tissue growth factor is a regulator for fibrosis in human chronic pancreatitis. Ann Surg. 1999;230:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Makar AB, McMartin KE, Palese M, Tephly TR. Formate assay in body fluids: application in methanol poisoning. Biochem Med. 1975;13:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 99] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 513] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 659] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 14. | Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1176] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 15. | Yamamoto M, Otani M, Otsuki M. A new model of chronic pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G700-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064-14071. [PubMed] |
| 17. | Wildi S, Kleeff J, Mayerle J, Zimmermann A, Böttinger EP, Wakefield L, Büchler MW, Friess H, Korc M. Suppression of transforming growth factor beta signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut. 2007;56:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Zhu Y, Colak T, Shenoy M, Liu L, Mehta K, Pai R, Zou B, Xie XS, Pasricha PJ. Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis. Mol Pain. 2012;8:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Uchida M, Ito T, Nakamura T, Hijioka M, Igarashi H, Oono T, Kato M, Nakamura K, Suzuki K, Takayanagi R. Pancreatic stellate cells and CX3CR1: occurrence in normal pancreas and acute and chronic pancreatitis and effect of their activation by a CX3CR1 agonist. Pancreas. 2014;43:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res. 2010;85:825-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Zhao HF, Ito T, Gibo J, Kawabe K, Oono T, Kaku T, Arita Y, Zhao QW, Usui M, Egashira K. Anti-monocyte chemoattractant protein 1 gene therapy attenuates experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Gut. 2005;54:1759-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Bai H, Chen X, Zhang L, Dou X. The effect of sulindac, a non-steroidal anti-inflammatory drug, attenuates inflammation and fibrosis in a mouse model of chronic pancreatitis. BMC Gastroenterol. 2012;12:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Gasiorowska A, Czupryniak L, Małecka-Panas E, Wiśniewska-Jarosińska M, Drzewoski J. [Microvascular complications in pancreatic diabetes]. Pol Arch Med Wewn. 2001;105:469-474. [PubMed] |
| 24. | Matejak-Górska MA, Durlik M, Kałuża B, Milczarczyk A, Franek E. Preoperative glucose abnormalities in patients with pancreatic tumours. Prz Gastroenterol. 2014;9:105-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 457] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Yasuda M, Ito T, Oono T, Kawabe K, Kaku T, Igarashi H, Nakamura T, Takayanagi R. Fractalkine and TGF-beta1 levels reflect the severity of chronic pancreatitis in humans. World J Gastroenterol. 2008;14:6488-6495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Pedersen N, Larsen S, Seidelin JB, Nielsen OH. Alcohol modulates circulating levels of interleukin-6 and monocyte chemoattractant protein-1 in chronic pancreatitis. Scand J Gastroenterol. 2004;39:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Inoue M, Ino Y, Gibo J, Ito T, Hisano T, Arita Y, Nawata H. The role of monocyte chemoattractant protein-1 in experimental chronic pancreatitis model induced by dibutyltin dichloride in rats. Pancreas. 2002;25:e64-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Ito T. Can measurement of chemokines become useful biological and functional markers of early-stage chronic pancreatitis? J Gastroenterol. 2007;42 Suppl 17:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Ceyhan GO, Deucker S, Demir IE, Erkan M, Schmelz M, Bergmann F, Müller MW, Giese T, Büchler MW, Giese NA. Neural fractalkine expression is closely linked to pain and pancreatic neuritis in human chronic pancreatitis. Lab Invest. 2009;89:347-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | D’Haese JG, Demir IE, Kehl T, Winckler J, Giese NA, Bergmann F, Giese T, Büchler MW, Friess H, Hartel M. The impact of MFG-E8 in chronic pancreatitis: potential for future immunotherapy? BMC Gastroenterol. 2013;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod Pathol. 2006;19:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H, Kleeff J. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 34. | Suzuki T, Dai P, Hatakeyama T, Harada Y, Tanaka H, Yoshimura N, Takamatsu T. TGF-β Signaling Regulates Pancreatic β-Cell Proliferation through Control of Cell Cycle Regulator p27 Expression. Acta Histochem Cytochem. 2013;46:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Cook T, Urrutia R. TIEG proteins join the Smads as TGF-beta-regulated transcription factors that control pancreatic cell growth. Am J Physiol Gastrointest Liver Physiol. 2000;278:G513-G521. [PubMed] |
| 36. | Murdolo G, Hammarstedt A, Sandqvist M, Schmelz M, Herder C, Smith U, Jansson PA. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J Clin Endocrinol Metab. 2007;92:2688-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Sullivan J, Gong Q, Hyslop T, Lavu H, Chipitsyna G, Yeo CJ, Arafat HA. Serum monocyte chemoattractant protein-1 in pancreatic cancer. J Oncol. 2011;2011:518394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Uchida M. ERK pathway and sheddases play an essential role in ethanol-induced CX3CL1 release in pancreatic stellate cells. Lab Invest. 2013;93:41-53. [PubMed] |
| 39. | Adrych K. Increase in transforming growth factor β-1, laminin, and hyaluronic acid serum concentrations in advanced chronic pancreatitis. Polish Gastroenterology. 2010;17:98-102. |
| 40. | Celesti G, Di Caro G, Bianchi P, Grizzi F, Marchesi F, Basso G, Rahal D, Delconte G, Catalano M, Cappello P. Early expression of the fractalkine receptor CX3CR1 in pancreatic carcinogenesis. Br J Cancer. 2013;109:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |