Published online Dec 15, 2016. doi: 10.4251/wjgo.v8.i12.826
Peer-review started: April 6, 2016
First decision: June 7, 2016
Revised: September 19, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: December 15, 2016
Processing time: 249 Days and 13.6 Hours
To review the evidence on the association between specific colon adenoma features and the risk of future colonic neoplasia [adenomas and colorectal cancer (CRC)].
We performed a literature search using the National Library of Medicine through PubMed from 1/1/2003 to 5/30/2015. Specific Medical Subject Headings terms (colon, colon polyps, adenomatous polyps, epidemiology, natural history, growth, cancer screening, colonoscopy, CRC) were used in conjunction with subject headings/key words (surveillance, adenoma surveillance, polypectomy surveillance, and serrated adenoma). We defined non-advanced adenomas as 1-2 adenomas each < 10 mm in size and advanced adenomas as any adenoma ≥ 10 mm size or with > 25% villous histology or high-grade dysplasia. A combined endpoint of advanced neoplasia included advanced adenomas and invasive CRC.
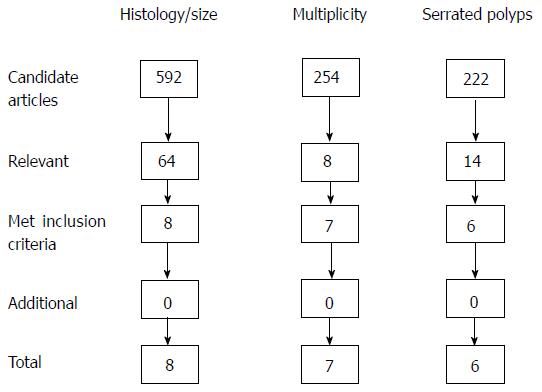
Our search strategy identified 592 candidate articles of which 8 met inclusion criteria and were relevant for assessment of histology (low grade vs high grade dysplasia, villous features) and adenoma size. Six of these studies met the accepted quality indicator threshold for overall adenoma detection rate > 25% among study patients. We found 254 articles of which 7 met inclusion criteria for the evaluation of multiple adenomas. Lastly, our search revealed 222 candidate articles of which 6 met inclusion criteria for evaluation of serrated polyps. Our review found that villous features, high grade dysplasia, larger adenoma size, and having ≥ 3 adenomas at baseline are associated with an increased risk of future colonic neoplasia in some but not all studies. Serrated polyps in the proximal colon are associated with an increased risk of future colonic neoplasia, comparable to having a baseline advanced adenoma.
Data on adenoma features and risk of future adenomas and CRC are compelling yet modest in absolute effect size. Future research should refine this risk stratification.
Core tip: The data on adenoma size, adenoma multiplicity and serrated polyps in terms of risk for future adenomas and colorectal cancer are compelling, however, the absolute effect size is relatively modest. Current guideline recommendations to perform colonoscopy surveillance at 3-5 years after baseline adenomas and serrated polyps appear appropriately tailored to the risk of future neoplasia.
- Citation: Calderwood AH, Lasser KE, Roy HK. Colon adenoma features and their impact on risk of future advanced adenomas and colorectal cancer. World J Gastrointest Oncol 2016; 8(12): 826-834
- URL: https://www.wjgnet.com/1948-5204/full/v8/i12/826.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i12.826
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death among men and women in the United States[1]. The lifetime probability of developing CRC is approximately 5%, with 90 percent of cases occurring after age 50. In 2016, an estimated 134500 people will be diagnosed with CRC and 49200 will die of the disease[1].
The vast majority of CRCs arise from a histologically-specific type of colon polyp, the adenoma, which forms as a result of sporadic mutation in the adenomatous polyposis coli pathway or DNA mismatch repair and by definition contains low-grade dysplasia. Over many years, a minority of adenomas may grow in size and progress from low-grade dysplasia to high-grade dysplasia, to carcinoma-in-situ to invasive carcinoma. More recently, serrated adenomas (named for the “sawtooth” pattern in the crypts) have been identified as accounting for approximately 20%-30% of CRCs. In this review, we will use the term “adenoma” to describe adenomatous and serrated colon polyps and “serrated polyps” to specify polyps with serrated histology.
Colonoscopy is the most widely used modality for CRC screening[2,3]. Advantages of colonoscopy include the ability of endoscopists both to identify and remove adenomas, which decreases the risk of subsequent CRC[4]. By definition, “screening colonoscopy” occurs in patients without a history of adenomas and “surveillance colonoscopy” occurs at set intervals (usually 3-5 years) in patients with a history of adenomas to survey for new adenomas[5]. It is important to understand the existing evidence upon which surveillance colonoscopy recommendations are made to help inform shared decision making with patients who have co-morbid conditions or limited life expectancy[6]. This review will focus on the association between specific adenoma features and future colonic neoplasia.
We searched the National Library of Medicine through PubMed for articles from 1/1/2003 to 5/30/2015. We did not search prior to 2003 because of technological advances in colonoscopy optics in 2002, which dramatically improved the diagnostic accuracy of colonoscopy; data prior to 2003 were not considered relevant to the current risk estimates of CRC after colonoscopy. We used the following filters: English language, human, age > 18, clinical trial, multicenter, prospective observational, meta-analysis. Specific MESH terms were used: Colon, colon polyps, adenomatous polyps, epidemiology, natural history, growth, cancer screening, colonoscopy, colorectal cancer. The MESH terms were used in conjunction with subject headings/key words: Surveillance, adenoma surveillance, polypectomy surveillance, and serrated adenoma. We excluded reviews, guidelines, editorials, case-control, cross-sectional and case series or reports.
We excluded studies of patients with inflammatory bowel disease, personal history of CRC, or family history of genetic CRC syndromes. We reviewed all abstracts for relevance. Full articles of the relevant abstracts were then reviewed with the quality of evidence graded by all three authors using the American Heart Association Evidence-Based Scoring System for Level of Evidence as follows: A: Data derived from multiple randomized clinical trials (RCTs); B: Data derived from a single randomized trial or nonrandomized studies; C: Consensus opinion of experts. The bibliographies of all included articles were also evaluated for additional articles by a single author [histology and size (AHC), multiple adenomas and serrated polyps (HKR)] then reviewed by all three authors for consensus.
We defined non-advanced adenomas as 1-2 adenomas each less than 10 mm in size[5]. We defined advanced adenomas as any adenoma ≥ 10 mm size or with > 25% villous histology or high-grade dysplasia[5]. A combined endpoint of advanced neoplasia included advanced adenomas and invasive CRC.
Our search strategy identified 592 candidate articles (Figure 1), of which 64 were relevant. We excluded 56 based on study design or absence of relevant primary outcome or predictors, leaving 8 studies (Table 1). Six of these studies met the accepted quality indicator threshold for overall adenoma detection rate (ADR) > 25% among study patients[7], including one study that explicitly described that ADR was > 25% for each individual endoscopist in study[8]. ADR was only 22% in the study by Bonithon-Kopp et al[9] and the meta analysis by Saini et al[10] did not present information on ADR.
| Ref. | Sample size | Median follow-up, yr | Predictor | Primary outcome | Absolute risk of outcome (%) | RR1 [95%CI] |
| RCT | ||||||
| Laiyemo et al[11] | 1905 | 4 | Villous | ACN | 9 (7-11) vs 5 (4-6) | 2.3 [1.5-3.4] |
| Bonithon-Kopp et al[9] | 552 | 3 | HGD | ACN | 9.8 vs 5.5 | 1.9 [1.0-3.6] |
| Villous | 10.3 vs. 6.8 | 1.7 [0.8-3.7] | ||||
| Pooled analysis | ||||||
| Martínez et al[14] | 8 studies | 3.9 | HGD | ACN | 16.0 (13.2-18.7) vs 10.6 (9.8-11.3) | 1.1 [0.8-1.4] |
| 9167 | Villous | 16.8 (15.1-18.5) vs 9.7 (9.0-10.4) | 1.3 [1.1-1.5] | |||
| Meta-analysis | ||||||
| Saini et al[10] | 5 studies | 3 | HGD | ACN | 4% risk difference (0-8) | 1.8 [1.1-3.2] |
| Villous | 2% risk difference (-1 to 4) | 1.3 [1.0-1.7] | ||||
| Prospective | ||||||
| Bertario et al[15] | 1086 | 10.5 | HGD | CRC | 2.8 SIR (0.3-10.2) vs 0.52 | Not available |
| Tubulovillous Villous | Any adenoma | Not available | 1.3 [1.0-1.6] | |||
| 1.8 [1.2-2.6] | ||||||
| 2Lieberman et al[12] | 1193 | 5.5 | No adenomas | ACN | 2.4 | Ref |
| HGD | 17 | 6.8 [2.6-18.1] | ||||
| Villous | 16 | 6.1 [2.5-14.7] | ||||
| Chung et al[8] | 3808 | 4.5 | Villous | ACN | Not available | 1.5 [0.7- 3.0] |
| Registry | ||||||
| Van Heijningen et al[13] | 2990 | 2 | HGD | AA | 13 | 1.2 [0.8-1.8] |
| Villous | 8 | 2.0 [1.2-3.2] | ||||
| HGD | ACN | 11 | Not available | |||
| Villous | 17 | |||||
A small to moderate association between adenoma histology and risk of future advanced adenomas and CRC with variable significance was found among the 8 studies. Four studies[11-14], including a pooling project of 8 prospective RCTs (evidence level A), found that villous histology was a significant risk factor for future advanced neoplasia (adjusted OR = 1.3; 95%CI: 1.1-1.5)[14]. Of note, the relative risk (RR) in Lieberman’s study (6.1; 95%CI: 2.5-14.7) is higher compared to the other studies because the comparator was subjects without any neoplasia, in contrast to subjects with adenomas without villous histology used in the other studies. In addition, Lieberman studied a Veteran’s Affairs (VA) population who are known to have higher rate of baseline adenomas compared to non-VA patients[12]. A prospective cohort study of 1086 patients with a median of 10.5 years of follow-up found that villous histology within an adenoma increased the relative risk of any future adenoma (1.8; 95%CI: 1.2-2.6) (evidence level B)[15]. A primary RCT[9], a meta analysis of 5 studies[10], and a prospective cohort study[8] found no association of villous histology with future neoplasia (evidence level B).
Similarly, histological findings of high-grade dysplasia had a small and variable association with risk of advanced neoplasia. The meta-analysis by Saini et al[10] found an increased RR of 1.8 (95%CI: 1.1-3.2)[10], whereas the primary RCT[9], pooling project[14], and prospective registry study[13] found no association (evidence level A). A prospective cohort study found that compared to an external control population, patients with high-grade dysplasia at baseline had an elevated SIR for CRC of 2.8 (95%CI: 0.3-10.2) compared to the reference group without high grade dysplasia (SIR 0.52; 95%CI: 0.3-0.95)[15]. In Lieberman’s prospective study of 1193 VA patients, he found a RR of advanced neoplasia of 6.8 (95%CI: 2.6-18.1) compared to those with no neoplasia at baseline[12].
In summary, villous histology within an adenoma may have a small association with future advanced neoplasia, however this was not seen uniformly across all studies. Compared to having no adenomas at baseline, adenomas with high-grade dysplasia are associated with an increased risk of future advanced neoplasia; however, compared to having adenomas that do not contain high-grade dysplasia, the association with future advanced neoplasia is small and variable depending on the study.
We used the same search strategy for histology to evaluate the impact of adenoma size on risk of future colonic neoplasia, finding the same 8 studies (Table 2)[8-15]. Larger adenoma size at baseline increased the risk of future advanced neoplasia. In Martinez’s pooling project of 8 prospective RCTs, the risk of advanced neoplasia increased for each increase in size category (evidence level A). When adenomas < 5 mm were considered the reference group, those with adenomas 10-19 mm and adenomas ≥ 20 mm had a RR of 2.3 (95%CI: 1.8-2.8) and 3.0 (95%CI: 2.2-4.0), respectively[14]. Similarly, four other prospective studies found that adenomas ≥ 10 mm imparted an increased RR of future advanced neoplasia ranging from 1.7 (95%CI: 1.2-2.3) to 3.0 (95%CI: 1.8-5.1) and 6.4 (95%CI: 2.7-14.9) (level of evidence B)[8,12,13]. On the other hand, Saini’s meta-analysis of 5 studies and a primary RCT, the European Fiber-Calcium Intervention trial (in which 552 patients with resected adenomas randomized to calcium and soluble fiber underwent surveillance colonoscopy at 3 years) failed to show any association between adenoma size and future advanced neoplasia (evidence level B)[9,10]. A prospective study by Bertario of 1086 patients did not show an association between polyp size ≥ 10 mm and SIR of advanced neoplasia (evidence level B)[15].
| Ref. | Sample size | Median follow-up, yr | Predictor | Primary outcome | Absolute risk of outcome (%) | RR [95%CI] |
| RCT | ||||||
| Laiyemo et al[11] | 1905 | 4 | ≥ 10 mm | ACN | 9 (7-11) vs 5 (4-6) | 0.9 [0.6-1.4] |
| Bonithon-Kopp et al[9] | 552 | 3 | ≥ 10 mm | ACN | 7.1 vs 7.8 | 1.1 [0.5-2.1] |
| Pooled analysis | ||||||
| Martínez et al[14] | 8 studies | 3.9 | < 5 mm | ACN | 8.7 (7.7-9.7) | Ref |
| 9167 | 10-19 mm | 15.9 (14.5-17.4) | 2.3 [1.8-2.8] | |||
| ≥ 20 mm | 19.3 (16.4-22.3) | 3.0 [2.2-4.0] | ||||
| Meta-analysis | ||||||
| Saini I et al[10] | 5 studies | 3 | ≥ 10 mm | ACN | 2% risk difference (-2 to 6) | 1.4 [0.9-2.3] |
| Prospective | ||||||
| Bertario et al[15] | 1086 | 10.5 | ≥ 20 mm | Any adenoma | Not available | 1.5 [1.1-2.1] |
| CRC | SIR | Not available | ||||
| Baseline | 0.52 [0.3-0.9] | |||||
| < 10 mm | 0.33 [0.1-0.9] | |||||
| ≥ 10 mm | 0.82 [0.3-1.8] | |||||
| Lieberman et al[12] | 1193 | 5.5 | ≥ 10 mm | ACN | 15.5 vs 2.4 | 6.4 [2.7-14.9] |
| Chung et al[8] | 3808 | 4.5 | ≥ 10 mm | ACN | Not available | 3.0 [1.8-5.1] |
| Registry | ||||||
| Van Heijningen et al[13] | 2990 | 2 | ≥ 10 mm | AA | 8 vs 4 | 1.7 [1.2-2.3] |
In summary, adenoma size ≥ 10 mm appears to be associated with future advanced neoplasia and the magnitude of risk increases for larger adenomas ≥ 20 mm in size.
Our search strategy revealed 254 articles of which 7 met inclusion criteria (Figure 1). Van Heijningen et al[13] noted that among 2990 consecutive colonoscopies in the Netherlands, there was an increased risk of advanced adenomas on surveillance exams depending on number of adenomas at initial screening colonoscopy (Table 3)[13]. Using participants with one adenoma as the reference group, those with 2, 3, 4 and ≥ 5 adenomas at baseline colonoscopy had 1.6 (95%CI: 1.1-2.4), 2.1 (95%CI: 1.3-3.4), 2.0 (95%CI: 0.9-4.6) and 3.3 (95%CI: 1.7-6.6) times the relative risk of future advanced adenomas, respectively (evidence level B)[13]. Lieberman et al[12] evaluated 1193 Veterans undergoing surveillance colonoscopy 5 years after baseline colonoscopy. Compared to those who were neoplasia-free at baseline, patients with 1-2 small adenomas and ≥ 3 adenomas had a RR of advanced adenoma at follow-up of 1.9 (95%CI: 0.83-4.4) and 5.0 (95%CI: 2.1-12.0), respectively, the latter of which was comparable to the risk of having a single advanced adenoma at baseline (evidence level A).
| Ref. | Sample size | Median follow-up, yr | Predictor | Primary outcome | Absolute risk of outcome (%) | RR [95%CI] |
| RCT | ||||||
| Laiyemo et al[11] | 1905 | 4 | ≥ 3 adenomas | ACN | 10 (7-14) vs 6 (5-7) | 1.5 [1.0-2.2] |
| Bonithon-Kopp et al[9] | 552 | 3 | ≥ 3 adenomas | ACN | 18.1 vs 5.0 | 2.7 [1.2-6.4] |
| Meta-analysis | ||||||
| Saini I et al[10] | 5 studies | 3 | ≥ 3 adenomas | ACN | 5% risk difference (1-10) | 2.5 [1.1-6.0] |
| Prospective | ||||||
| 1Lieberman et al[12] | 1193 | 5.5 | 1-2 | ACN | 4.6 | 1.9 [0.8-4.4] |
| ≥ 3 | 11.9 | 5.0 [2.1-12.0] | ||||
| Chung et al[8] | 3808 | 4.5 | ≥ 3 adenomas | ACN | Not available | 3.1 [1.5-6.6] |
| Registry | ||||||
| Van Heijningen et al[13] | 2990 | 2 | 1 | AA | 4 | Ref |
| 2 | 7 | 1.6 [1.1-2.4] | ||||
| 3 | 8 | 2.1 [1.3-3.4] | ||||
| 4 | 12 | 2.0 [0.9-4.6] | ||||
| ≥ 5 | 18 | 3.3 [1.7-6.6] | ||||
| Ng et al[18] | 4989 | 2 | AA | Not available | Adjusted OR | |
| 1 | 3.6 [2.6-5.0] | |||||
| 2 | 7.1 [4.9-10.4] | |||||
| 3 | 13.7 [0.9-4] | |||||
Bonithon-Kopp et al[9] found that in the European Fiber-Calcium Intervention trial, patients with ≥ 3 adenomas had a HR of 5.5 (95%CI: 2.4-12.6) of developing advanced adenomas at three year colonoscopy but only if one of the adenomas was proximal - if all the adenomas were distal, there was no increase in risk of advanced adenomas (0.83; 95%CI: 0.18-3.9) (evidence level A)[9]. Analysis of 4 year surveillance colonoscopy data from the Polyp Prevention Trial (n = 1905) found that compared to having one non-advanced adenoma, individuals with 2 or ≥ 3 adenomas had a RR for advanced neoplasia of 1.38 (95%CI: 0.92-2.1) and 1.84 (95%CI: 1.2-2.8), respectively[11]. Finally, a meta-analysis of older literature found that those with ≥ 3 adenomas at index colonoscopy were more likely to have recurrent advanced adenomas than were patients with 1 to 2 adenomas (RR = 2.5; 95%CI: 1.1-6.0) (evidence level B)[10]. In summary, these data suggest that adenoma number may confer a risk of future neoplasia comparable to adenoma size and as discussed below may be further influenced by the quality of the performance of colonoscopy.
Our search revealed 222 candidate articles of which 6 met inclusion criteria (Figure 1). Four were mainly cross-sectional studies, which evaluated the presence of concurrent adenomas and two evaluated the correlation with future neoplasia (Table 4). In a study of 10199 subjects, having a large serrated polyp ≥ 1 cm (LSP) was associated with an increased odds of concurrent advanced neoplasia (adjusted OR = 4.0; 95%CI: 2.8-5.7) and CRC (adjusted OR = 3.3; 95%CI: 2.2-5.0) compared to those who were neoplasia-free (evidence level B)[16]. Álvarez et al[17] reported that in 5059 patients randomized to undergo screening colonoscopy vs stool test, LSPs were associated with concurrent proximal (OR = 4.2; 95%CI: 1.7-10.2) and distal (OR = 2.6; 95%CI: 1.5-4.6) advanced neoplasia. Several other studies corroborate the relationship between proximal LSPs and concurrent advanced adenomas[18,19].
| Ref. | Sample size | Median follow-up, yr | Predictor | Primary outcome | Absolute risk | Risk [95%CI] |
| RCT | ||||||
| Holme et al[20] | 100210 | 10.9 | ≥ 10 mm serrated polyp | Future CRC | 3.4 vs 1.4 cases/1000 patient years | HR 3.3 [1.3-8.6] |
| Registry | ||||||
| Álvarez et al[17] | 5059 | None | Proximal l ≥ 10 mm | ACN | Not available | 4.2 [1.7-10.2] |
| Distal l ≥ 10 mm | 2.6 [1.5-4.6] | |||||
| Proximal HP | 1.6 [1.3-2.3] | |||||
| Hiraoka et al[16] | 10199 | None | ≥ 10 mm serrated polyps | ACN | 4.0 [2.8-5.7] | |
| CRC | Not available | 3.3 [2.2-5.0] | ||||
| Proximal CRC | 4.8 [2.5-8.4] | |||||
| Hazewinkel et al[19] | 1426 | None | Proximal SP | ACN | Not available | 2.4 [1.6-3.8] |
| Proximal HP | 2.0 [1.1-3.4] | |||||
| Prox SSA/P | 3.0 [1.5-6.2] | |||||
| ≥ 10 SP | 4.0 [1.9-8.6] | |||||
| ≥ 10 mm HP | 3.2 [1.1-9.1] | |||||
| ≥ 10 mm SSA/P | 5.0 [1.7-14.9] | |||||
| Ng et al[18] | 4989 | None | SSA | ACN | Not available | 4.5 [2.4-8.5] |
| Proximal SP | 2.2 [1.4-3.6] | |||||
| ≥ 10 mmSP | 59.3 [18.9-186.2] | |||||
| ≥ 3 SP | 4.9 [1.2-19.2] | |||||
| ≥ 3 non-advanced adenomas | 3.6 [2.6-5.0] | |||||
| Schreiner et al[21] | 3121 | None | Proximal SP | AA | 17.3 vs 10.0 | 1.9 [1.3-2.7] |
| ≥ 1 cm SP | 27.3 vs 10.3 | 3.4 [1.7-6.7] | ||||
| 1371 | 5.5 | Proximal SP | Future | |||
| without adenomas | AA | 5.1 vs 2.7 | 3.1 [1.6-6.2] | |||
| Proximal SP | 7.9 vs 6.3 | 1.2 [0.5-3.8] | ||||
| with nonadvanced adenoma | 28.9 vs 14.7 | 2.3 [1.0-5.0] | ||||
| Proximal SP | ||||||
| with advanced adenoma | ||||||
With regard to future lesions, a secondary analysis of a large randomized flexible sigmoidoscopy study from Norway that included a median follow-up of 10.9 years provides some insights (evidence level A)[20]. Having a LSP was associated with an increased risk of future CRC (adjusted OR = 3.3; 95%CI: 1.3-8.6), comparable to having a baseline advanced adenoma. Interestingly, none of the other serrated polyps left in situ developed CRC in that tumor, suggesting the serrated polyps might be a marker of field carcinogenesis rather than a precursor lesion[20]. Schreiner et al[21] found that patients with proximal non-dysplastic serrated polyps followed for median of 5.5 years had an increased odds for future adenomas of 3.1 (95%CI: 1.6-6.2) compared to those who were polyp free at baseline (evidence level B). Thus, while it is clear that certain serrated polyps can progress to CRC, those that are right sided and/or ≥ 1 cm are associated with future neoplasia and are a marker for concurrent adenomas and need to be considered as equivalent to an adenoma from a surveillance perspective.
Our review found that specific histologic features of adenomas (i.e., high grade dysplasia and villous features) are associated with a small risk of future advanced adenomas though data was inconsistent across studies (level B evidence). In particular, villous features did not confer a consistent or significant association, suggesting it may not be an important risk factor for future advanced adenomas. Data was even more inconsistent for adenoma size, although the linear association between size and risk is compelling (level B evidence). Size itself is challenging to determine reliably because of the lack of a standardized method for estimating adenoma size and the inter-observer variability among size estimation endoscopically as well as differences in estimations between endoscopic and pathology measurements[22,23]. Use of an open biopsy forceps as a reference standard for measurement during colonoscopy was accurate to the millimeter only 37% of the time[24]. The variability in estimating size of adenomas is concerning given that a 1 mm difference in size can change surveillance by 2 years. In a prospective study using size on pathology as gold standard, endoscopists mis-sized polyps 63% of the time, leading to inappropriate surveillance intervals 35% of the time[25]. Relying on pathology reports for size estimates is challenging, given that polyps are often removed piecemeal and can be fragmented during retrieval. Thus, the accuracy of size estimates for determining surveillance intervals should be viewed cautiously.
Having ≥ 3 adenomas at baseline is associated with an increased risk of future colonic neoplasia (level B evidence), particularly if at least one adenoma is located in the proximal colon, although more supporting data is needed. The findings of our study echo those of the seminal prospective randomized National Polyp Study[26], in which multiple adenomas (≥ 3; OR = 6.9; 95%CI: 2.6-18.3) and large adenomas (OR = 2.2; 95%CI: 0.6-7.8) were associated with future advanced adenomas at surveillance. In that study, however only multiplicity was a significant risk factor (P < 0.001). The risk conferred by villous features or high grade dysplasia at baseline was not included.
Current United States and European guidelines recommend repeat colonoscopy in 3 years for patients with ≥ 3 adenomas or any adenoma ≥ 10 mm size or with high grade dysplasia or villous features compared to 5-10 years for those with 1-2 small adenomas (Table 5)[5,27]. The British guidelines do not take into account advanced histology and recommend earlier follow-up at 1 year for those with at least 5 small adenomas or 3 adenomas if one is ≥ 10 mm in size[28]. Current level B evidence demonstrates a higher risk of future colonic neoplasia based on having a large serrated polyp (OR ranging from 3.3-4.2) and supports earlier surveillance at 3 years as recommended by guidelines in this group[5,28]. The recommendations for surveillance of serrated polyps are identical to adenomas[5]. While surveillance guidelines may be based primarily on adenoma features and risk of future neoplasia, they may also be influenced by national economics and local culture around population-based screening and surveillance, which can vary by country and continent.
| Organization and yearof guidelines | Recommendations for surveillance of adenomas | |
| Baseline finding | Timing of next exam, yr | |
| USMSTF on CRC[5], 2012 | 1-2 small adenomas | 5-10 |
| Adenoma with villous histology | 3 | |
| Adenoma with high grade dysplasia | 3 | |
| Adenoma ≥ 10 mm | 3 | |
| 3-10 adenomas | 3 | |
| Serrated polyps: | ||
| < 10 mm no dysplasia | 5 | |
| ≥ 10 mm | 3 | |
| Dysplasia | 3 | |
| Traditional serrated adenoma | 3 | |
| British Society of Gastroenterology[28], 2010 | 1-2 small adenomas | 5-10 |
| 3-4 small adenomas | 3 | |
| Adenoma ≥ 10 mm | 3 | |
| ≥ 5 small adenomas | 1 | |
| ≥ 3 at least one ≥ 10 mm | 1 | |
| European Society of Gastrointestinal Endoscopy[27], 2010 | High risk adenomas: | 3 |
| Adenoma ≥ 10 mm | ||
| Adenomas with high grade dysplasia | ||
| Villous component | ||
| ≥ 3 adenomas | ||
| Serrated polyp ≥ 10 mm | ||
| Serrated polyps with dysplasia | ||
| Not high risk adenomas | 10 | |
The way in which very small differences in adenoma size and number (e.g., 2 vs 3 adenomas) can affect timing of recommended surveillance (from 3 to 5 years) emphasizes the importance of the quality of the colonoscopy performed. Adenoma detection rate (ADR) is considered the most important quality metric in the performance of colonoscopy because it is a close surrogate measure for interval CRC rates and can be measured feasibly[7,29]. Other important quality metrics include cecal intubation rates and bowel preparation quality, both of which impact ADR[7]. A recent simulation study demonstrated that ADR correlates with a lower lifetime risk of CRC without an increase in cost, thus further underscoring the importance of colonoscopy quality[30]. As ADR improves overall whether from improved endoscope optics or adjunctive techniques (e.g., narrow band imaging, caps, rings)[31], the association between baseline colonic neoplasia findings and risk of future neoplasia may need to be reassessed.
Our review has certain limitations. We do not address the impact of other factors besides adenoma features on risk for CRC, which are beyond the scope of this article. However, since CRC involves the interactions of genes and the environment, other factors such as family history, age, smoking, diabetes, and obesity have the potential to impact the risk of recurrent neoplasia. Indeed, the NIH risk score looks at a variety of these factors, although its predictive ability has been modest[32]. We also did not consider the location (proximal vs distal) of adenomas in this review. Location may impart a differential neoplastic risk, with proximal lesions portending a higher risk for recurrence, and merits further clarification in terms of biological underpinnings and clinical strategies. The duration of follow-up for most of the studies ranged from 2 to 5.5 years, which does not allow for the assessment of long-term outcomes. However, this time frame is in line with current surveillance guideline recommendations and provides an adequate follow-up period for the evaluation of the risk of recurrent neoplasia. Lastly, the existing data do not explicitly compare the risk of future advanced adenomas at surveillance based on having multiple different risk factors simultaneously, likely due to limitations of sample size and loss of power with subgroup comparisons. However, if multiple independent risk factors were identified (e.g., multiplicity and size), then having those simultaneously would increase the individual’s overall risk of future advanced adenomas.
Future research should continue to evaluate the risk of CRC based on multiple factors incorporating serial colonoscopy information. A few studies have attempted to predict the risk of future neoplasia based on 2 or more examinations[11,33]. In addition, other biomarkers of the risk of CRC are needed. Since colorectal carcinogenesis involves both genetic and exogenous risk factors of which approximately half are modifiable (i.e., obesity and smoking)[34], assessment of risk at the level of the colonic mucosa where the interaction between genetics and environment plays out locally may provide a novel approach. While there are a plethora of candidate biomarkers of field carcinogenesis (e.g., molecular alterations such as methylation, gene expression, microRNA in the normal rectal epithelium), the adenoma is currently the only predictor of risk that is robust enough and practical for use in clinical practice. Future research should also explore the impact of life expectancy on surveillance colonoscopy to guide clinicians who must weigh the risks and benefits for individual patients.
In conclusion, current United States Multi-Society Task Force on CRC recommendations to perform colonoscopy surveillance at 3-5 years after baseline adenomas and serrated polyps appear appropriately tailored to the risk of future neoplasia. The data on adenoma size, adenoma multiplicity and serrated polyps in terms of risk for future adenomas and CRC are compelling, however, the absolute effect size is relatively modest. Future research should identify methods of stratifying a patient’s risk for CRC based on serial colonoscopy exams and could include composite risk scores and biomarkers.
Patients with adenomas of the colon undergo routine surveillance colonoscopy to survey for new adenomas. It is important to understand the existing evidence upon which surveillance colonoscopy recommendations are made.
This review focuses on the association between specific adenoma features and the risk of future colonic neoplasia.
This comprehensive review of the literature shows that adenoma size, adenoma multiplicity and serrated polyps increase the risk for future adenomas and colorectal cancer (CRC), however, the absolute effect size is relatively modest.
Current United States Multi-Society Task Force on CRC recommendations to perform colonoscopy surveillance at 3-5 years after baseline adenomas and serrated polyps appear appropriately tailored to the risk of future neoplasia.
“Surveillance colonoscopy” refers to colonoscopy performed at set intervals (usually 3-5 years) in patients with a history of adenomas to survey for new adenomas.
This is a well-written comprehensive review of current literature on colon adenoma features and CRC risk. To add value to the manuscript, summarising guidelines round the world to provide the readers a more comprehensive review of suggested evidence and protocols would be proposed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Baeg MK, Chew MH, Mori Y, Tsuji Y, Wang ZH S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2073] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 2. | Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46:S10-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2283] [Article Influence: 175.6] [Reference Citation Analysis (1)] |
| 5. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1443] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 6. | Schroy PC, Emmons KM, Peters E, Glick JT, Robinson PA, Lydotes MA, Mylvaganam SR, Coe AM, Chen CA, Chaisson CE. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG, Park WG, Rizk MK, Sawhney MS. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 8. | Chung SJ, Kim YS, Yang SY, Song JH, Kim D, Park MJ, Kim SG, Song IS, Kim JS. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Bonithon-Kopp C, Piard F, Fenger C, Cabeza E, O’Morain C, Kronborg O, Faivre J. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. 2004;47:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64:614-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Laiyemo AO, Murphy G, Albert PS, Sansbury LB, Wang Z, Cross AJ, Marcus PM, Caan B, Marshall JR, Lance P. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, Dekker E, Lesterhuis W, Ter Borg F, Vecht J, De Jonge V, Spoelstra P, Engels L. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Bertario L, Russo A, Sala P, Pizzetti P, Ballardini G, Andreola S, Spinelli P. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003;105:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hiraoka S, Kato J, Fujiki S, Kaji E, Morikawa T, Murakami T, Nawa T, Kuriyama M, Uraoka T, Ohara N. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139:1503-1510, 1510.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Álvarez C, Andreu M, Castells A, Quintero E, Bujanda L, Cubiella J, Salas D, Lanas Á, Carballo F, Morillas JD, Hernández C, Jover R, Sarasqueta C, Enriquéz-Navascués JM, Hernández V, Estévez P, Macenlle R, Sala T, Balaguer F, Pellisé M, Moreira L, Gil I, Peris A, González-Rubio F, Ferrández A, Poves C, Ponce M, Grau J, Serradesanferm A, Ono A, Cruzado J, Pérez-Riquelme F, Alonso-Abreu I, Carrillo-Palau M, Santander C, Díaz Tasende J, Herreros A, Cacho G, Barranco LE, Bessa X. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013;78:333-341.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Ng SC, Ching JY, Chan VC, Wong MC, Tang R, Wong S, Luk AK, Lam TY, Gao Q, Chan AW. Association between serrated polyps and the risk of synchronous advanced colorectal neoplasia in average-risk individuals. Aliment Pharmacol Ther. 2015;41:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Hazewinkel Y, de Wijkerslooth TR, Stoop EM, Bossuyt PM, Biermann K, van de Vijver MJ, Fockens P, van Leerdam ME, Kuipers EJ, Dekker E. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Holme Ø, Bretthauer M, Eide TJ, Løberg EM, Grzyb K, Løberg M, Kalager M, Adami HO, Kjellevold Ø, Hoff G. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015;64:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Moug SJ, Vernall N, Saldanha J, McGregor JR, Balsitis M, Diament RH. Endoscopists’ estimation of size should not determine surveillance of colonic polyps. Colorectal Dis. 2010;12:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Rex DK, Rabinovitz R. Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc. 2014;79:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Eichenseer PJ, Dhanekula R, Jakate S, Mobarhan S, Melson JE. Endoscopic mis-sizing of polyps changes colorectal cancer surveillance recommendations. Dis Colon Rectum. 2013;56:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 643] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 27. | Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 28. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 29. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1556] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 30. | Meester RG, Doubeni CA, Lansdorp-Vogelaar I, Jensen CD, van der Meulen MP, Levin TR, Quinn VP, Schottinger JE, Zauber AG, Corley DA. Variation in Adenoma Detection Rate and the Lifetime Benefits and Cost of Colorectal Cancer Screening: A Microsimulation Model. JAMA. 2015;313:2349-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Konda V, Chauhan SS, Abu Dayyeh BK, Hwang JH, Komanduri S, Manfredi MA, Maple JT, Murad FM, Siddiqui UD, Banerjee S. Endoscopes and devices to improve colon polyp detection. Gastrointest Endosc. 2015;81:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Park Y, Freedman AN, Gail MH, Pee D, Hollenbeck A, Schatzkin A, Pfeiffer RM. Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol. 2009;27:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Robertson DJ, Burke CA, Welch HG, Haile RW, Sandler RS, Greenberg ER, Ahnen DJ, Bresalier RS, Rothstein RI, Cole B. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med. 2009;151:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579-588. [PubMed] |