Published online Oct 15, 2016. doi: 10.4251/wjgo.v8.i10.735
Peer-review started: April 7, 2016
First decision: June 6, 2016
Revised: June 24, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: October 15, 2016
Processing time: 176 Days and 4.8 Hours
Cancer stem cells (CSCs) constitute a small proportion of the cancer cells that have self-renewal capacity and tumor-initiating ability. They have been identified in a variety of tumors, including tumors of the digestive system. CSCs exhibit some unique characteristics, which are responsible for cancer metastasis and recurrence. Consequently, the development of effective therapeutic strategies against CSCs plays a key role in increasing the efficacy of cancer therapy. Several potential approaches to target CSCs of the digestive system have been explored, including targeting CSC surface markers and signaling pathways, inducing the differentiation of CSCs, altering the tumor microenvironment or niche, and inhibiting ATP-driven efflux transporters. However, conventional therapies may not successfully eradicate CSCs owing to various problems, including poor solubility, stability, rapid clearance, poor cellular uptake, and unacceptable cytotoxicity. Nanomedicine strategies, which include drug, gene, targeted, and combinational delivery, could solve these problems and significantly improve the therapeutic index. This review briefly summarizes the ongoing development of strategies and nanomedicine-based therapies against CSCs of the digestive system.
Core tip: There are reviews in the literature contributed to the applications of nanotechnology for the detection and treatment of gastrointestinal diseases. However this is a first review to report the current development of strategies and nanomedicine-based therapies against cancer stem cells of the digestive system.
- Citation: Xie FY, Xu WH, Yin C, Zhang GQ, Zhong YQ, Gao J. Nanomedicine strategies for sustained, controlled, and targeted treatment of cancer stem cells of the digestive system. World J Gastrointest Oncol 2016; 8(10): 735-744
- URL: https://www.wjgnet.com/1948-5204/full/v8/i10/735.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i10.735
Currently, gastrointestinal cancer is the second leading cause of cancer-related deaths worldwide. Despite some progress achieved in cancer treatment, the current therapies have limitations with respect to their ability to prevent tumor metastasis and relapse. Recent scientific studies have found that a small proportion of cancer cells (0.01%-4%) can proliferate indefinitely. These cells are similar to adult stem cells with respect to their proliferation, self-renewal, and differentiation into other cells; therefore, they were named cancer stem cells (CSCs)[1]. CSCs were first isolated from acute myeloid leukemia by Bonnet et al[2] in 1997. It was not until 2003 that CSCs in solid tumors were studied when Al-Hajj et al[3] identified CSCs with a phenotype of CD44+/CD24-/low/Lineage- in breast cancer. This provided strong evidence for the existence of CSCs in solid tumors and theoretically supported the possible identification of CSCs in other solid tumors. Subsequently, CSCs were identified in a variety of tumors, including tumors of the digestive system, such as gastric cancer[4], liver cancer[5], and colon cancer[6-9].
CSCs have many characteristics similar to those of stem cells, for example, the self-renewal and differentiation abilities, and some common signaling pathways, including the Wnt/β-catenin, Notch, and Hedgehog pathways[10-13]. However, CSCs also exhibit some unique characteristics because of abnormally regulated genetic mechanisms: (1) quiescence, conventional anticancer therapies always kill rapidly proliferating cancer cells, but have less effect on quiescent CSCs[1,14,15]; (2) high tumorigenicity, only a handful of CSCs can lead to tumor development, whereas the same number of non-CSCs are unable to form clones or tumors in vivo[16,17]; (3) resistance, CSCs highly express membrane transport proteins of the ATP binding cassette (ABC) family, which can transport and efflux a variety of materials, including metabolites, drugs, toxic substances, endogenous lipids, peptides, nucleotides, and sterols, which accounts for the drug efflux and drug resistance of CSCs[18,19]; (4) high levels of anti-apoptotic molecules[1]; and (5) enhanced DNA repair ability[20-23]. Conventional therapies including chemotherapy, radiotherapy, biotherapy, and thermal therapy mainly focus on the differentiation and proliferation of cancer cells rather than those of CSCs, resulting in an increase in the CSCs fraction, which can lead to metastasis and recurrence[24,25]. Consequently, the development of effective therapeutic strategies against CSCs plays a key role in increasing the efficacy of cancer therapy.
In recent years, the applications of nanomaterials and nanotechnology in CSC-targeted therapy have received more and more attention. As an emerging interdisciplinary field, nanotechnology can provide materials and tools with unique physical and chemical properties and biological functions for CSC-targeted therapy. In this review, we briefly discussed the properties of CSCs and the conventional strategies against CSCs of the digestive system, as well as a summary of the latest achievements in the nanomedicine approaches for CSC therapy in the digestive system.
Normal gastrointestinal tissues comprise a specific class of stem cells, named gastrointestinal stem cells, which are adult stem cells, with a capacity to self-renew and replicate. They can differentiate into any type of cells in the gastrointestinal tract and play an important role in the regeneration of gastrointestinal mucosa and maintenance of tissue homeostasis. It seems that gastrointestinal stem cells may undergo mutation and can transform into CSCs, which, in turn, participate in the initiation and progression of the gastrointestinal tumors[26]. However, Houghton found that Helicobacter pylori induced chronic inflammation in the gastric tissue of C57BL/6 mice and this inflamed tissue included bone marrow-derived cells, which could develop into intraepithelial carcinoma through dysplasia[27]. The exact origin of gastrointestinal CSCs is unknown: They may be derived directly from the mutation of normal stem cells, or it may be that mature cells acquire tumor formation potential and transform into CSCs.
The identification of CSCs plays an important role in the evaluation of the prognosis of patients and serves to guide treatment. Currently, the main method used for the isolation of CSCs is based on the surface markers (such as membrane proteins, adhesion molecules, and receptors) that distinguish CSCs from non-CSCs, which can be sorted by flow cytometry or magnetic-activated cell sorting (MACS). In recent years, great progress has been made in the study of gastrointestinal CSCs and their markers, which can theoretically support the diagnosis and treatment of gastrointestinal tumors. In addition, the identification of specific cellular markers of gastrointestinal CSCs has become a research focus. So far, some possible markers of gastrointestinal CSCs have been evaluated, such as CD24, CD133, CD44, CDl66, stage-specific embryonic antigen (SSEA), Oct-4, and Sox-2. CD133 and CD44 are the main markers of gastrointestinal CSCs. However, recent studies of CSC phenotypes have presented a new challenge[28,29]; CSCs are of different phenotypes and it is urgent and necessary to target all subsets of CSCs within the tumor to prevent a relapse. Thus, there is a need to investigate more cell surface markers in addition to CD133 and CD44, for the identification of gastrointestinal CSCs.
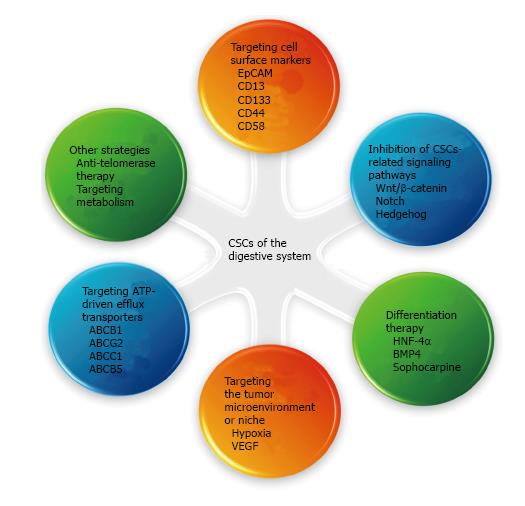
The current failure in the treatment of gastrointestinal cancer is attributable to drug resistance and recurrence after therapy in most cases, in which CSCs are thought to play a crucial role. Therefore, strategies targeting CSCs may bring new hope for the treatment of gastrointestinal cancer[19,30]. Currently, several strategies have been proposed to target CSCs of the digestive system (Figure 1). For example, specific surface markers and altered signaling pathways are attractive therapeutic targets. Induction of the differentiation of CSCs and targeting of the tumor microenvironment or the niche supporting the CSCs are also efficient strategies. Inhibition of ATP-driven efflux transporters that are overexpressed on the CSCs surface, is believed to increase the sensitivity of the tumor to chemotherapeutic drugs. Other strategies, such as anti-telomerase therapy and modulation of abnormal metabolism, are also worth evaluating.
CSCs express some unique surface markers that distinguish them from other cells; therefore, strategies targeting these specific surface markers can eradicate CSCs of the digestive system, which is an effective approach for the treatment of gastrointestinal cancer. Aptamers, which are oligonucleotide or peptide molecules that can specifically bind to a desired site and penetrate the cancer cells, have been found to target the CSCs surface markers. Shigdar et al[31] isolated the first RNA aptamer against epithelial cell adhesion molecule (EpCAM), a putative marker of gastric, colorectal, and liver CSCs. In addition, monoclonal antibodies have been developed to block CSC surface markers. Haraguchi et al[32] demonstrated that CD13 is a marker for liver CSCs and treatment with anti-CD13 antibody suppressed the self-renewal and tumor-initiating ability of dormant CSCs. Smith et al[33] reported that a murine anti-human CD133 antibody conjugated to a potent cytotoxic drug, monomethyl auristatin F, selectively targeted CD133+ cells, which is a marker for gastric and liver CSCs. Other surface markers, including CD44[34] and CD58[35], have been also utilized to specifically eradicate the CSCs of the digestive system.
The normal function of stem cells depends on the normal regulation of a variety of signaling pathways. Dysregulation of the signaling pathways results in abnormal proliferation and differentiation. Therefore, inhibition of CSCs-related signaling pathways is an effective method for cancer therapy. The common CSCs-related signaling pathways include: (1) Wnt/β-catenin pathway: Implicated in the maintenance and proliferation of CSCs[36]. Cai et al[37] suggested that the Wnt/β-catenin pathway is essential for the self-renewal of cancer stem-like cells in human gastric cancer. Another study also suggested the same role for the Wnt/β-catenin pathway in gastric CSCs and showed that salinomycin (SAL) could inhibit gastric tumor growth by suppressing Wnt/β-catenin signaling in CSCs[38]. The Wnt/β-catenin pathway plays an important role not only in gastric CSCs, but also in colon and liver CSCs[39,40]. Song et al[39] demonstrated that small molecules could target the Wnt signaling pathways in CSCs for the treatment of colorectal cancer; (2) notch pathway: Required for the maintenance of gastrointestinal stem cells[41]. Luo et al[42] suggested that the Notch pathway promotes CSCs activity in hepatocellular carcinoma (HCC). Wang et al[40] demonstrated that the Wnt/β-catenin and Notch signaling pathways play important roles in the activation of liver CSCs; and (3) hedgehog pathway: Implicated in the unchecked self-renewal and the development of metastatic tumors[43]. Song et al[44] suggested that the Sonic Hedgehog pathway is essential for the maintenance of the cancer stem-like cells in human gastric cancer. A study that investigated the molecular mechanisms of curcumin and curcumin analogs against colorectal CSCs suggested the involvement of signaling pathways, including Wnt/β-catenin, Sonic Hedgehog, Notch, and PI3K/Akt/mTOR[45].
Differentiation therapy of tumors refers to treatment of malignant tumors via induction of cell differentiation. The abnormal differentiation of CSCs is one of the important causes of cancer development, thus, inducing the differentiation of CSCs is an important method for cancer therapy[46]. Hepatocyte nuclear factor-4α (HNF-4α), a central regulator of differentiated hepatocyte phenotype, suppresses tumorigenesis and tumor development by inducing the differentiation of the hepatocarcinoma cells, especially CSCs, into mature hepatocytes[47]. Lombardo et al[48] found that bone morphogenetic protein 4 (BMP4) induces the differentiation of colorectal CSCs and increases the antitumor effects of 5-fluorouracil and oxaliplatin. Zhang et al[49] verified that sophocarpine has the ability to suppress HCC and CSCs and could act as a differentiation therapy drug.
The microenvironment is an important condition for the survival of cells, which plays an important role in the regulation of the proliferation and differentiation of cells. The stem cell microenvironment, called niche, includes the niche cells, extracellular matrix, and soluble factors derived from the niche cells. CSCs are also believed to reside in niches, which maintain the principle properties of CSCs, preserve their phenotypic plasticity, protect them from the immune system, and facilitate their metastatic potential[50]. Targeted therapy against this microenvironment is of great significance for the treatment of cancer. Vermeulen et al[51] proposed that colon cancer stemness was partly orchestrated by the microenvironment. Hypoxia, which influences the liver CSC microenvironment, has been identified as a major cause of hypervascularization in HCCs[52]. Targeting hypoxia is an effective strategy to manipulate the niche of the quiescent, drug-resistant cells. Several studies have indicated that angiogenesis can be related to CSC survival and drug resistance and shown that vascular endothelial growth factor (VEGF) is one of the most specific and critical regulators of angiogenesis, which promotes CSC activity by governing both the microvasculature formation and the intrinsic self-renewal pathways[53-55]. Targeting VEGF with inhibitors or antibodies can lead to normalization of the tumor vasculature, disruption of the CSC niche, and inhibition of tumor growth[56-58].
CSCs express high levels of ABC transporters, such as ABCB1, ABCG2, and ABCC1, which represent the three principal multidrug-resistance (MDR) genes that have been identified in tumor cells[19,59]. These transporters actively efflux the drugs outside the cells, conferring resistance to chemotherapeutic drugs[59]. Xie et al[60] suggested that the overexpression of ABCG2 is responsible for chemotherapy failure in colon cancer. Inhibition of ABCB1 (MDR1) expression, which encodes P-glycoprotein (Pgp), can increase the sensitivity of HCC cells to anticancer drugs, such as doxorubicin and daunorubicin[61]. Pgp’s cousin, ABCB5, is another ABC transporter implicated in the drug resistance of CSCs in different tumor types. For example, Cheung et al[62] found that the expression of granulin-epithelin precursor (GEP) and ABCB5 in liver CSCs was associated with chemoresistance and reduced the survival rates of patients with HCC. However, inhibition of ABC transporters is likely to have significant side effects[63], and the ability to overcome MDR clinically is rather limited[64]. Therefore, targeted and combined therapy may be required to circumvent drug resistance and nanomedicine may show tremendous potential to overcome MDR.
Anti-telomerase therapy: Telomerase activation leads to telomere maintenance, which plays an important role in the immortality of CSCs. Compared to the normal cells, the telomerase activity in CSCs is higher and the length of the telomere is shorter. Anti-telomerase therapy can specifically shorten the CSCs telomere, causing replicative senescence, apoptosis, and cell cycle arrest with little damage to the normal cells. Several anti-telomerase agents, such as the antisense oligonucleotide inhibitor GRN163L and immunotherapies that use dendritic cells (GRVAC1), hTERT peptide (GV1001), or cryptic peptides (Vx-001), are currently in clinical trials for treatment of various tumors and are speculated to efficiently target CSCs[65,66]. A recent study has implied that co-inhibition of telomerase and tankyrase 1, which elongates the telomere, may be a rational strategy for telomere-directed gastric cancer therapy[67].
Targeting the metabolism: Recently, there is growing evidence that metabolism and stemness are highly intertwined processes in tumors[68]. For example, gastrointestinal CSCs showed higher inducible nitric oxide synthase (iNOS) expression, lower reactive oxygen species (ROS) production, and a different metabolic profile with respect to non-CSCs. Aerobic glycolysis blockade, oxidative stress-based therapies, and nitric oxide synthase inhibition target the gastrointestinal CSCs and could have profound anticancer effects[69].
As discussed above, conventional therapies may not successfully eradicate CSCs owing to various problems, including solubility, stability, rapid clearance, poor cellular uptake, and unacceptable cytotoxicity. Thus, more and more attention has been drawn to the application of nanomedicine[70-72]. Nanomedicine can be defined as the application and further development of nanotechnology to solve the problems faced in medicine, i.e., to diagnose, treat, and prevent diseases at the cellular and molecular levels[73-75]. Nanomedicine is characterized by a size of less than 200 nm in general, which is smaller than the traditional medicine, thus, it has the advantages of large specific surface area, high surface reaction activity, and high adsorption capacity. Moreover, it can be optimized in the aspects of drug loading, pharmacokinetic properties, and biocompatibility by different modifications of the particle surface. In conclusion, nanomedicine has the following characteristics: Sustained, controlled, and targeted drug delivery, improved drug stability, prolonged half-life of drugs, good biocompatibility, etc. Consequently, nanomedicine strategies for sustained, controlled, and targeted treatment of CSCs of the digestive system may offer superior outcomes leading to efficient cancer therapy (Figure 1).
One important application of nanomedicine is the transport of chemotherapeutic drugs with poor solubility, stability, or severe side effects. For example, the monocarboxylic polyether antibiotic, SAL, which primarily functions as a highly selective potassium ionophore, has been shown to affect various CSCs, including liver, gastric, and colorectal CSCs[76-79]. However, it exhibits poor aqueous solubility and severe nervous and muscle toxicity, which hinder its clinical applications[80,81]. Therefore, various studies incorporate SAL into nanocarriers to address these issues. For instance, Yao et al[82] developed a gastric CSC-targeted drug delivery system (SAL-SWNT-CHI-HA complexes), which could enhance the bioavailability and cytotoxic activity of SAL. In our previous study, we developed novel iRGD (internalizing Arg-Gly-Asp peptide)-conjugated DSPE-PEG2000 nanomicelles (M-SAL-iRGD) for delivery of SAL to both liver cancer cells and CSCs. M-SAL-iRGD possessed a small size of around 10 nm and a drug encapsulation efficacy higher than 90%. It showed a superior tumor penetrating ability and therapeutic efficacy[83]. Similarly, curcumin has extraordinary anticancer properties; however, it has limited application in the treatment of cancer owing to its insolubility, instability, and poor pharmacokinetics, which greatly hamper it’s in vivo efficacy[84-86]. Wang et al[87] developed a novel nanoparticle formulation in which curcumin was encapsulated in stearic acid-g-chitosan oligosaccharide (CSO-SA) polymeric micelles to overcome these hurdles. Curcumin-loaded CSO-SA micelles could increase curcumin accumulation in cancer cells and were effective in inhibiting colorectal CSCs both in vitro and in vivo. In another study, Wang et al[88] used the CSO-SA micelles to deliver a standard chemotherapy for colorectal cancer treatment (oxaliplatin). This could also increase oxaliplatin accumulation in both colorectal cancer cells and tissues and could effectively eradicate colorectal CSCs.
Nucleic acids, especially small interfering RNAs (siRNAs) and microRNAs (miRNAs), can effectively target genes overexpressed in CSCs and involved in the maintenance of stemness and tumorigenicity. However, their characteristics, such as negative charge, high molecular weight, and low stability, limit their application. Therefore, nanomedicines have been developed to condense them for effective delivery. For instance, a novel non-cytotoxic and pH-sensitive anti-EpCAM monoclonal antibody-labeled CSCs-targeted block copolymer vesicle was synthesized as a nanocarrier for anticancer drugs and siRNA. The polymer vesicles showed good pH-regulated drug release capability and excellent stability in water, PBS, and 40% fetal bovine serum. The EpCAM-positive CSC-targeted vesicles showed a high delivery efficacy of both the anticancer drug, doxorubicin hydrochloride (DOX·HCl), and siRNA to the CSCs[89]. Similarly, Kim et al[90] developed a tumor-targeted nanodelivery platform (scL) and showed that systemic administration of scL carrying the wtp53 gene was able to induce tumor growth inhibition and promote the death of both CSCs and non-CSCs in subcutaneous colorectal cancer xenografts. Nanomedicine for siRNA delivery can also sensitize CSCs to chemotherapeutic drugs. For example, Liu et al[91] designed a novel siRNA delivery carrier system with multidrug resistance gene (MDR1)-targeted siRNA (siMDR1) and showed that it effectively reduced the expression of MDR1 in human colon CSCs, resulting in a significant increase in the chemosensitivity to paclitaxel.
Recent studies have indicated that miRNAs are important regulators of CSCs[92]. For example, miR-34, a transcriptional target of p53, inhibits the biological properties of gastric CSCs. Restoration of miR-34 expression in gastric CSCs inhibits sphere formation in vitro and tumor regeneration in vivo[93]. Liu et al[94] developed gelatinase-stimulated PEG-Pep-PCL nanoparticles to deliver miR-200c, which were reported to inhibit CSC-like properties. The miR-200c nanoparticles enhanced the radiotherapy efficacy, reduced the expression of CD44, and the percentage of CD44+ gastric cancer cells. Meanwhile, other CSCs properties, including invasiveness and resistance to apoptosis, could be suppressed by miR-200c nanoparticles.
In addition to its ability to improve drug stability and biocompatibility, nanomedicine can also be modified to direct or guide the therapeutic agents to CSCs. Since CSCs express specific cell surface biomarkers, it may be a promising strategy to use these biomarkers for targeted drug delivery. Hyaluronic acid (HA), a glycosaminoglycan widely found in the extracellular matrix, can specifically recognize its receptors, CD44, and has been identified as a potent targeting ligand to tumors possessing CD44-overexpressing cells[95]. Yao et al[82] developed SAL-loaded chitosan (CHI)-coated single-walled carbon nanotubes (SWNTs) functionalized with HA, which facilitated the uptake of SWNTs into the gastric CSCs via CD44 receptor-mediated endocytosis. In addition, anti-CD44 antibodies could also be used for CSC-targeted therapy. Wang et al[96] developed doxorubicin-loaded anti-CD44 antibody-functionalized liposomal nanoparticles, which specifically targeted CD44+ cells of HCC to mitigate the side effects of conventional chemotherapy. In a recent study, CD90+ LCSCs were isolated by magnetic-activated cell sorting from HCC cells. Therefore, Yang et al[97] prepared a CD90-targeted thermosensitive magnetoliposomes (TMs)-encapsulated 17-allylamino-17-demethoxgeldanamycin (17-AAG), which is a heat-shock protein 90 (HSP90) inhibitor, to sensitize the CD90+ LCSCs to magnetic hyperthermia and enhance its antitumor effects in vitro and in vivo.
As described above, nanomedicine-based single drug delivery systems are effective in targeting the CSCs in the digestive system. However, various CSCs-targeted drugs that are not highly cytotoxic as compared to the conventional chemotherapeutic drugs, are not very effective in reducing the bulk cancer cells, which can spontaneously and stochastically turn into CSCs again[98]. Therefore, combinational delivery of chemotherapeutics and CSC-specific agents for eliminating both the cancer cells and CSCs is a promising method to improve cancer treatment. Liu et al[99] co-loaded miR-200c and docetaxel (DOC) into an intelligent gelatinase-stimulated nanoparticle, which exhibited synergetic effects on the inhibition of both CSCs and non-CSC cancer cells. The miR-200c/DOC nanoparticles prominently suppressed the in vivo tumor growth. Shen et al[100] developed a micellar nanoparticle to deliver platinum (IV) prodrug and siNotch1 into both non-CSCs and CSCs of SMMC7721. The combined drug delivery system could remarkably augment drug delivery into tumor tissues, thus, substantially suppressing the tumor growth (Table 1).
| Tumor types | Nanomedicine | Ref. |
| Drug delivery | ||
| Gastric cancer | SAL-loaded carbon nanotubes functionalized with HA | [82] |
| Liver cancer | SAL-loaded iRGD-conjugated DSPE-PEG2000 nanomicelles | [83] |
| Colorectal cancer | Curcumin-loaded CSO-SA micelles | [87] |
| Oxaliplatin-loaded CSO-SA micelles | [88] | |
| Gene delivery | ||
| Liver cancer | anti-EpCAM-monoclonal-antibody-labeled block copolymer vesicle | [89] |
| Colorectal cancer | Wtp53 gene loaded scL nanocomplex | [90] |
| Colon cancer | MDR1 siRNA loaded lipid nanoparticles | [91] |
| Gastric cancer | miR-200c loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles | [94] |
| Targeted delivery | ||
| Gastric cancer | SAL-loaded carbon nanotubes functionalized with HA | [82] |
| Liver cancer | anti-CD44 antibody-mediated liposomal nanoparticle loaded of doxorubicin | [96] |
| CD90-targeted thermosensitive magnetoliposomes-encapsulated 17-AAG | [97] | |
| Combinational delivery | ||
| Gastric cancer | Nanoparticle co-loaded miR-200c and DOC | [99] |
| Liver cancer | micellar nanoparticle co-delivering platinum (IV) prodrug and siNotch1 | [100] |
| Colorectal cancer | Liposomes co-encapsulated irinotecan and floxuridine | [101] |
| Colon cancer | Nanoliposomes co-encapsulated vincristine and topotecan | [102] |
Additionally, nanomedicine is crucial for the delivery of dual drugs with predictable ratios at the tumor site to achieve a synergistic effect. Mayer et al[101] co-encapsulated irinotecan and floxuridine at a 1:1 molar ratio inside 100-nm-diameter liposomes composed of distearoylphosphatidylcholine/distearoylphosphatidylglycerol/cholesterol (7:2:1 molar ratio). The liposomes maintained the drug ratio in the plasma after injection, and delivered the formulated drug ratio directly to the tumor tissue of the colorectal cancer. In another study, vincristine and topotecan were successfully co-encapsulated at therapeutically relevant levels in the same nanoliposome (LipoViTo). The nanoliposomes controlled the drugs’“biofate” and maintained a fixed drug ratio in vivo, displaying an enhanced therapeutic efficacy against colon cancer[102].
The CSCs theory revealed more facts about cancer, but the CSCs in the digestive system are still not fully understood. There is a need for further investigation of the new markers, abnormal metabolism, and signal transduction pathways of CSCs, which will improve our strategies to target CSCs. In this review, we summarized the current strategies against CSCs of the digestive system. Nanomedicines have been shown to effectively deliver drugs and genes to target CSCs of the digestive system. A number of studies have shown that there is a significant increase in the therapeutic outcome with nanomedicine. However, there are still great challenges limiting the effective application of nanomedicine in clinical practice. One of the most important challenges is the biological safety issues. There is still no clear evidence that the nanomaterials can be effectively metabolized in vivo and will not accumulate to cause side effects. In addition, it is difficult to determine the safe dose of nanomedicine because of the lack of clear evaluation criteria.
Although there are still some difficulties preventing the wide application of nanomedicine in clinical practice, there is a reason to believe that, with the progress of nanotechnology and the in-depth research of CSCs, the unique advantages of nanomedicine will create good conditions for the development of personalized therapy for cancer patients and will finally be capable of conquering cancer of the digestive system.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Gharaee-Kermani M, Pixley JS S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6917] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 2. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [PubMed] |
| 3. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7730] [Article Influence: 351.4] [Reference Citation Analysis (0)] |
| 4. | Li K, Dan Z, Nie YQ. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J Gastroenterol. 2014;20:5420-5426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 927] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 6. | O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [PubMed] |
| 7. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [PubMed] |
| 8. | Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 817] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 9. | Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427-13432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 10. | Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605-R615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 553] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 11. | Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1035] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 12. | Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 845] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 13. | Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 748] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 14. | Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 363] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 15. | Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1854] [Cited by in RCA: 1942] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 16. | Pim D, Massimi P, Dilworth SM, Banks L. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene. 2005;24:7830-7838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5562] [Article Influence: 264.9] [Reference Citation Analysis (0)] |
| 18. | Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2). Int J Biochem Cell Biol. 2005;37:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2778] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 20. | Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 920] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 21. | Kim J, Wong PK. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 2009;27:1987-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4467] [Cited by in RCA: 4760] [Article Influence: 250.5] [Reference Citation Analysis (0)] |
| 23. | Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2102] [Cited by in RCA: 1935] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 24. | Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 488] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 25. | Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 605] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 26. | Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70:6-22. [PubMed] |
| 27. | Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 840] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 28. | Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 987] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 29. | Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 422] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 30. | Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 440] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 31. | Shigdar S, Lin J, Yu Y, Pastuovic M, Wei M, Duan W. RNA aptamer against a cancer stem cell marker epithelial cell adhesion molecule. Cancer Sci. 2011;102:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 33. | Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van Orden KL. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Jang BI, Li Y, Graham DY, Cen P. The Role of CD44 in the Pathogenesis, Diagnosis, and Therapy of Gastric Cancer. Gut Liver. 2011;5:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Xu S, Wen Z, Jiang Q, Zhu L, Feng S, Zhao Y, Wu J, Dong Q, Mao J, Zhu Y. CD58, a novel surface marker, promotes self-renewal of tumor-initiating cells in colorectal cancer. Oncogene. 2015;34:1520-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 37. | Cai C, Zhu X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol Med Rep. 2012;5:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 39. | Song L, Li Y, He B, Gong Y. Development of Small Molecules Targeting the Wnt Signaling Pathway in Cancer Stem Cells for the Treatment of Colorectal Cancer. Clin Colorectal Cancer. 2015;14:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo J, Huang H, Du Q, Geller DA, Cheng B. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7:5754-5768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Luo J, Wang P, Wang R, Wang J, Liu M, Xiong S, Li Y, Cheng B. The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma. Oncotarget. 2016;7:9525-9537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 44. | Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:e17687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Ramasamy TS, Ayob AZ, Myint HH, Thiagarajah S, Amini F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006;17:1620-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Yin C, Lin Y, Zhang X, Chen YX, Zeng X, Yue HY, Hou JL, Deng X, Zhang JP, Han ZG. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48:1528-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Lombardo Y, Scopelliti A, Cammareri P, Todaro M, Iovino F, Ricci-Vitiani L, Gulotta G, Dieli F, de Maria R, Stassi G. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology. 2011;140:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Zhang PP, Wang PQ, Qiao CP, Zhang Q, Zhang JP, Chen F, Zhang X, Xie WF, Yuan ZL, Li ZS. Differentiation therapy of hepatocellular carcinoma by inhibiting the activity of AKT/GSK-3β/β-catenin axis and TGF-β induced EMT with sophocarpine. Cancer Lett. 2016;376:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1184] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 51. | Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1446] [Article Influence: 96.4] [Reference Citation Analysis (1)] |
| 52. | Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GC, Poon RT, Fan ST. An Akt/hypoxia-inducible factor-1alpha/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res. 2009;15:3462-3471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Dimova I, Popivanov G, Djonov V. Angiogenesis in cancer - general pathways and their therapeutic implications. J BUON. 2014;19:15-21. [PubMed] |
| 54. | Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 55. | Pandya NM, Dhalla NS, Santani DD. Angiogenesis--a new target for future therapy. Vascul Pharmacol. 2006;44:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 56. | Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 963] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 57. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 58. | Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 574] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 59. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 4152] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 60. | Xie ZY, Lv K, Xiong Y, Guo WH. ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from colon cancer. Oncol Res Treat. 2014;37:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Li B, Ye T, Zhao L, Li DH, Gou XH, Zhao LY, Han L, Chen L, Yan LN, Gong JP. Effects of multidrug resistance, antisense RNA on the chemosensitivity of hepatocellular carcinoma cells. Hepatobiliary Pancreat Dis Int. 2006;5:552-559. [PubMed] |
| 62. | Cheung ST, Cheung PF, Cheng CK, Wong NC, Fan ST. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159-165. [PubMed] |
| 65. | Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 537] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 66. | Ruden M, Puri N. Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev. 2013;39:444-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 67. | Zhang H, Yang MH, Zhao JJ, Chen L, Yu ST, Tang XD, Fang DC, Yang SM. Inhibition of tankyrase 1 in human gastric cancer cells enhances telomere shortening by telomerase inhibitors. Oncol Rep. 2010;24:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Menendez JA, Alarcón T. Metabostemness: a new cancer hallmark. Front Oncol. 2014;4:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Di Francesco AM, Toesca A, Cenciarelli C, Giordano A, Gasbarrini A, Puglisi MA. Metabolic Modification in Gastrointestinal Cancer Stem Cells: Characteristics and Therapeutic Approaches. J Cell Physiol. 2016;231:2081-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Zhao Y, Alakhova DY, Kabanov AV. Can nanomedicines kill cancer stem cells? Adv Drug Deliv Rev. 2013;65:1763-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | Malhi S, Gu X. Nanocarrier-mediated drugs targeting cancer stem cells: an emerging delivery approach. Expert Opin Drug Deliv. 2015;12:1177-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Shen S, Xia JX, Wang J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials. 2016;74:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Feng SS. New-concept chemotherapy by nanoparticles of biodegradable polymers: where are we now? Nanomedicine (Lond). 2006;1:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 801] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 74. | Pan J, Liu Y, Feng SS. Multifunctional nanoparticles of biodegradable copolymer blend for cancer diagnosis and treatment. Nanomedicine (Lond). 2010;5:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1439] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 76. | Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012;2012:950658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 77. | Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL, Wu SM, Cheng P, Zhang Y, Shen M. Salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. PLoS One. 2012;7:e50638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 78. | Zhi QM, Chen XH, Ji J, Zhang JN, Li JF, Cai Q, Liu BY, Gu QL, Zhu ZG, Yu YY. Salinomycin can effectively kill ALDH(high) stem-like cells on gastric cancer. Biomed Pharmacother. 2011;65:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Zhang C, Tian Y, Song F, Fu C, Han B, Wang Y. Salinomycin inhibits the growth of colorectal carcinoma by targeting tumor stem cells. Oncol Rep. 2015;34:2469-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Boehmerle W, Endres M. Salinomycin induces calpain and cytochrome c-mediated neuronal cell death. Cell Death Dis. 2011;2:e168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Boehmerle W, Muenzfeld H, Springer A, Huehnchen P, Endres M. Specific targeting of neurotoxic side effects and pharmacological profile of the novel cancer stem cell drug salinomycin in mice. J Mol Med (Berl). 2014;92:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Yao HJ, Zhang YG, Sun L, Liu Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials. 2014;35:9208-9223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 83. | Mao X, Liu J, Gong Z, Zhang H, Lu Y, Zou H, Yu Y, Chen Y, Sun Z, Li W. iRGD-conjugated DSPE-PEG2000 nanomicelles for targeted delivery of salinomycin for treatment of both liver cancer cells and cancer stem cells. Nanomedicine (Lond). 2015;10:2677-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 84. | Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1167] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 85. | Tønnesen HH, Másson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 544] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 86. | Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1194] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 87. | Wang K, Zhang T, Liu L, Wang X, Wu P, Chen Z, Ni C, Zhang J, Hu F, Huang J. Novel micelle formulation of curcumin for enhancing antitumor activity and inhibiting colorectal cancer stem cells. Int J Nanomedicine. 2012;7:4487-4497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Wang K, Liu L, Zhang T, Zhu YL, Qiu F, Wu XG, Wang XL, Hu FQ, Huang J. Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. Int J Nanomedicine. 2011;6:3207-3218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Chen J, Liu Q, Xiao J, Du J. EpCAM-Antibody-Labeled Noncytotoxic Polymer Vesicles for Cancer Stem Cells-Targeted Delivery of Anticancer Drug and siRNA. Biomacromolecules. 2015;16:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Kim SS, Rait A, Rubab F, Rao AK, Kiritsy MC, Pirollo KF, Wang S, Weiner LM, Chang EH. The clinical potential of targeted nanomedicine: delivering to cancer stem-like cells. Mol Ther. 2014;22:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Liu C, Zhao G, Liu J, Ma N, Chivukula P, Perelman L, Okada K, Chen Z, Gough D, Yu L. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release. 2009;140:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Res. 2011;71:5950-5954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 93. | Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 94. | Cui FB, Liu Q, Li RT, Shen J, Wu PY, Yu LX, Hu WJ, Wu FL, Jiang CP, Yue GF. Enhancement of radiotherapy efficacy by miR-200c-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer cells. Int J Nanomedicine. 2014;9:2345-2358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Platt VM, Szoka FC. Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5:474-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 96. | Wang L, Su W, Liu Z, Zhou M, Chen S, Chen Y, Lu D, Liu Y, Fan Y, Zheng Y. CD44 antibody-targeted liposomal nanoparticles for molecular imaging and therapy of hepatocellular carcinoma. Biomaterials. 2012;33:5107-5114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 97. | Yang R, Tang Q, Miao F, An Y, Li M, Han Y, Wang X, Wang J, Liu P, Chen R. Inhibition of heat-shock protein 90 sensitizes liver cancer stem-like cells to magnetic hyperthermia and enhances anti-tumor effect on hepatocellular carcinoma-burdened nude mice. Int J Nanomedicine. 2015;10:7345-7358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108:7950-7955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 885] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 99. | Liu Q, Li RT, Qian HQ, Wei J, Xie L, Shen J, Yang M, Qian XP, Yu LX, Jiang XQ. Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials. 2013;34:7191-7203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 100. | Shen S, Sun CY, Du XJ, Li HJ, Liu Y, Xia JX, Zhu YH, Wang J. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials. 2015;70:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 102. | Zucker D, Barenholz Y. Optimization of vincristine-topotecan combination--paving the way for improved chemotherapy regimens by nanoliposomes. J Control Release. 2010;146:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |