Published online Jan 15, 2016. doi: 10.4251/wjgo.v8.i1.55
Peer-review started: May 30, 2015
First decision: July 18, 2015
Revised: August 18, 2015
Accepted: November 17, 2015
Article in press: November 25, 2015
Published online: January 15, 2016
Processing time: 230 Days and 21 Hours
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies with a five-year survival rate of approximately 5%. Several target agents have been tested in PDAC, but almost all have failed to demonstrate efficacy in late phase clinical trials, despite the better understanding of PDAC molecular biology generated by large cancer sequencing initiatives in the past decade. Eroltinib (a small-molecule tyrosine-kinase inhibitor of epidermal growth factor receptor) plus gemcitabine is the only schedule with a biological agent approved for advanced pancreatic cancer, but it has resulted in a very modest survival benefit in unselected patients. In our work, we report a summary of the main clinical trials (closed and ongoing) that refer to biological therapy evaluation in pancreatic cancer treatment.
Core tip: Our study aims to give an overview of the progress made in molecular targeted therapy for pancreatic cancer in recent years and the current status of clinical trials in the field. Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies with a five-year survival rate of approximately 5%. Several target agents have been tested in PDAC but almost all have failed to demonstrate efficacy in late phase clinical trials, even with a better understanding of PDAC molecular biology generated by large cancer sequencing initiatives in the past decade. Eroltinib (small-molecule tyrosine-kinase inhibitor of epidermal growth factor receptor) plus gemcitabine is actually the only schedule with a biological agent approved for advanced pancreatic cancer, but it resulted in a very modest survival benefit in unselected patients. In our work, we reported a summary of the main clinical trials (close and ongoing) that refer to biological therapy evaluation in pancreatic cancer treatment.
- Citation: Di Marco M, Grassi E, Durante S, Vecchiarelli S, Palloni A, Macchini M, Casadei R, Ricci C, Panzacchi R, Santini D, Biasco G. State of the art biological therapies in pancreatic cancer. World J Gastrointest Oncol 2016; 8(1): 55-66
- URL: https://www.wjgnet.com/1948-5204/full/v8/i1/55.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i1.55
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies, representing the fourth leading cause of cancer death. The five-year survival rate is approximately 5%, and surgery remains the most effective treatment[1].
Unfortunately, only 20% of patients are suitable for radical resection, and recurrence of disease occurs in 80% of patients who undergo resection[2].
The most important improvement concerns the conventional chemotherapy, represented by FOLFIRINOX and gemcitabine plus nab-paclitaxel regimens, but it results in a modest outcome advantage[3,4].
No significant progress has been made in the field of targeted therapy. Eroltinib [a small-molecule tyrosine-kinase inhibitor of epidermal growth factor receptor (EGFR)] plus gemcitabine is actually the only schedule with a biological agent approved for pancreatic cancer, but it results in a very modest survival benefit in unselected patients[5].
In recent decades, several combinations of classic chemotherapy and novel biological agents have been studied, but they have not improved overall survival, and furthermore, those trials did not use biomarkers to select responder patients[6].
Our study aims to give an overview of the progress made in molecularly targeted therapy for pancreatic cancer in recent years and the current status of clinical trials in the field, as summarized in Table 1, Table 2, and Table 3.
| Agent | Target pathway | Treatment | Setting | n | mOS (mo) | PFS (mo) | FDA approval | Ref. |
| Erlotinib | EGFR signaling | GEM plus erlotinib | M/LA | 569 | 6.24 vs 5.91 | 3.75 vs 3.55 | Yes | [5] |
| vs GEM plus P | (P = 0.038) | (P = 0.004) | ||||||
| Cetuximab | EGFR signaling | GEM plus cetuximab | M/LA | 766 | 6.5 vs 6 | 3.5 vs 3 | No | [17] |
| vs GEM | (P = 0.14) | (P = 0.058) | ||||||
| Tipifarnib | KRAS pathway | GEM plus tipifarnib | M/LA | 688 | 6.3 vs 6 | 3.7 vs 3.6 | No | [30] |
| vs GEM | (P = 0.75) | (P = 0.72) | ||||||
| Ganitumab | IGFR pathway | GEM plus ganitumab (12 mg/kg or 20 mg/kg) | M | 800 | 12 mg/kg arm | 12 mg/kg arm | No | [35] |
| vs GEM plus P | 7.0 vs 7.2 | 3.7 vs 3.6 | ||||||
| (P = 0.494) | (P = 0.520) | |||||||
| 60 mg/kg arm | 60 mg/kg arm | |||||||
| 7.1 vs 7.2 | 3.7 vs 3.7 | |||||||
| (P = 0.397) | (P = 0.403) | |||||||
| Bevacizumab | Angiogenesis | GEM plus bevacizumab | M/LA | 602 | 5.7 vs 6.0 | 4.8 vs 4.3 | No | [36] |
| vs GEM plus P | (P = 0.40) | (P = 0.99) | ||||||
| Aflibercept | Angiogenesis | GEM plus aflibercept | M/LA | 546 | 6.5 vs 7.8 | 3.7 vs 3.7 | No | [38] |
| vs GEM plus P | (P = 0.203) | (P = 0.864) | ||||||
| Axitinib | Angiogenesis | GEM plus axitinib | M/LA | 632 | 8.5 vs 8.2 | 4.4 vs 4.4 | No | [41] |
| vs GEM plus P | (P = 0.543) | (P = 0.520) | ||||||
| Marimastat | Tumor stroma | GEM plus marimastat | M/LA | 239 | 5.4 vs 5.4 | 3 vs 3.1 | No | [75] |
| vs GEM | (NA) | (NA) |
| Agent | Target pathway | Treatment | Setting | n | Ref. |
| Cetuximab | EGFR signaling | GEM plus cisplatin plus cetuximab | M/LA | [16] | |
| vs | 84 | ||||
| GEM plus cisplatin | |||||
| Gefitinib | EGFR signaling | GEM plus gefitinib | M/LA | 57 | [18] |
| (single arm) | |||||
| Trastuzumab | EGFR signaling | GEM plus trastuzumab | M/LA | 34 | [20] |
| (single arm) | 2+/3+ HER-2 expression | ||||
| Trastuzumab | EGFR signaling | Capecitabine plus trastuzumab | M/LA | 17 | [21] |
| (single arm) | 3+ HER-2 expression or gene amplification | (212 screened) | |||
| Nimotuzumab | EGFR signaling | GEM plus nimotuzumab | M/LA | 18 | [23] |
| (single arm) | |||||
| Nimotuzumab | EGFR signaling | Nimotuzumab monotherapy | Refractory to first line standard chemotherapy M/LA | 56 | [24] |
| (single arm) | |||||
| Selumetinib | KRAS/MEK pathway | Capecitabine plus selumetinib | Refractory to first line standard chemotherapy M/LA | 70 | [31] |
| vs Capecitabine | |||||
| Trametinib | KRAS/MEK pathway | GEM plus trametinib | M/LA | 160 | [32] |
| vs GEM plus P | |||||
| Sorafenib | Angiogenesis | GEM plus sorafenib | M/LA | 70 | [40] |
| (single arm) | |||||
| RO4929097 | Hedgehog signaling | RO4929097 monotherapy | Refractory to first line standard chemotherapy M | 18 | [57] |
| (single arm) | |||||
| Everolimus | mTOR pathway | Everolimus plus capecitabine | M/LA | 31 | [67] |
| (single arm) |
| ClinicalTrials.gov identifier | Agent | Target | Status |
| NCT01728818 | Afatinib | EGFR signaling | Recruiting |
| NCT01659502 | TL-118 | Angiogenesis | Not yet recruiting |
| NCT01621243 | Necuparanib | Angiogenesis | Recruiting |
| NCT01088815 | Vismodegib | Hedgehog signaling | Recruiting |
| NCT01096732 | Vismodegib | Hedgehog signaling | Terminated |
| NCT01431794 | LDE-225 | Hedgehog signaling | Recruiting |
| NCT00515866 | KU-0059436 | PARP inhibitor | Completed |
| NCT01585805 | Veliparib | PARP inhibitor | Recruiting |
| NCT01571024 | BKM120 | mTOR and PI3K/Akt pathway | Recruiting |
| NCT01028495 | RX-0201 | mTOR and PI3K/Akt pathway | Completed |
| NCT01337765 | BEZ235 + MEK162 | mTOR and PI3K/Akt pathway | Completed |
| NCT00560963 | Everolimus | mTOR pathway | Completed |
| NCT00075647 | Temsirolimus | mTOR pathway | Completed |
| NCT01839487 | PEGPH20 | Tumor stroma | Recruiting |
Large cancer sequencing initiatives generated a large quantity of data in the past decades. Those findings showed a complex genomic landscape characterized particularly by inter-tumoural and intra-tumoural heterogeneity involving genomic aberration[7].
With the exception of the well-known KRAS, TP53, CDKN2A and SMAD4 alterations occurring at respective frequencies of 71%, 49%, 22% and 20%, a large number of genomic rearrangements with mutational frequencies less than 2% were found[8,9].
The majority of single gene mutations in pancreatic cancer can be grouped into common cellular pathways. Jones et al[10] identified 69 mutated gene sets in most of the 24 samples analysed in their pioneering sequencing study, of which 31 could be grouped into 12 core signalling pathways. These pathways included KRAS signalling, the transforming growth factor β (TGF-β) pathway, DNA damage control, apoptosis, regulation of G1/S cell cycle transition, Hedgehog signalling, the homophilic cell adhesion pathway, integrin signalling, TGF-β signalling, Wnt/Notch signalling, and the invasion pathway[10].
Genomic heterogeneity, a characteristic of PDAC, implies genomic instability, which is due to the acquisition of telomere dysfunction and abnormal cell-cycle control occurring predominantly in early cancer stages, but it persists after cancer dissemination, resulting in parallel evolution among different metastases. Cell clones arranging metastasis may require other driver mutations compared with primary tumour cells implementing genetic variation in pancreatic cancer[11,12].
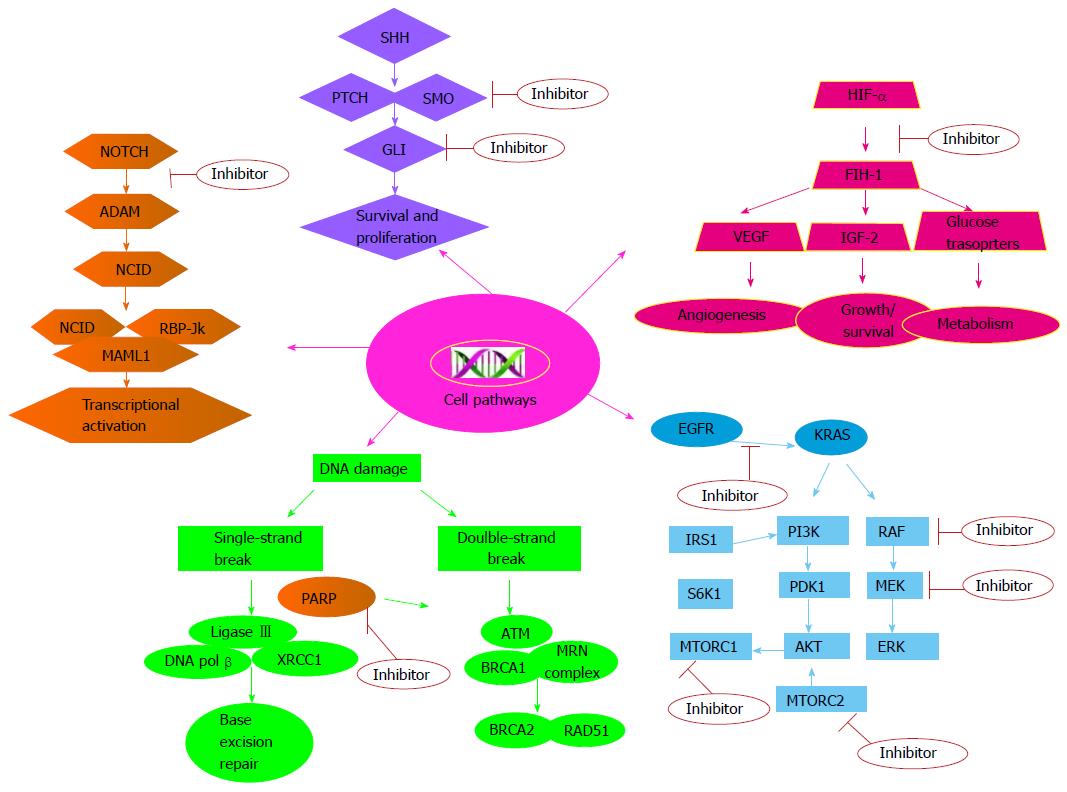
Given this molecular complexity, it is very difficult to separate passenger from driver mutations, to identify molecular mutations with a crucial role in pancreatic carcinogenesis that can be developed into actionable molecular targets of novel biological agents or to identify patients potentially responsive to existing agents already approved for human use in other cancers (Figure 1), and currently no predictive or prognostic biological factors are employed in clinical practice.
EGFR is a transmembrane receptor member of the ErbB family with a tyrosine kinase domain that is activated by many ligands including epidermal growth factor (EGF), TGF-α, heparin-binding EGF, amphiregulin, epiregulin, betacellulin and neuregulin (an epidermal growth factor). EGFR is involved in cell cycle regulation, cell survival, adhesion and differentiation through activation of the Ras/MAP kinase, phosphatidylinositol 3’-kinase (PI3K)/Akt, Janus kinase/Stat and phospholipase C/protein kinase C pathways. Several trials showed that EGFR is overexpressed in up to 90% of pancreatic cancer samples. Therefore, inhibitors targeting EGFR have been considered a promising therapeutic agent[13].
Eroltinib is a tyrosine kinase inhibitor (TKI) molecule that competes with ATP for binding to the kinase domain, thereby blocking downstream signal transduction. A possible therapeutic role was evaluated in a large phase III trial, enrolling 569 chemotherapy naïve patients with locally advanced or metastatic pancreatic adenocarcinoma randomized to receive gemcitabine plus placebo or gemcitabine plus erlotinib 100-150 mg daily. The median overall survival (mOS) and progression free survival (PFS) were modestly, but statistically significantly, improved in the combination arm, 6.24 mo vs 5.91 mo (P = 0.038) and 3.75 mo vs 3.55 mo (P = 0.004), respectively[5].
Neither EGFR status nor KRAS status analysed in the subgroup of patients treated with erlotinib was shown to be predictive of a survival benefit in patients receiving the combination schedule[14].
Erlotinib has been approved by the FDA in combination with gemcitabine as a first-line treatment for advanced pancreatic adenocarcinoma.
Cetuximab is a monoclonal antibody binding the extracellular domain of EGFR. After encouraging results in a phase I trial, subsequent studies in association with gemcitabine-based chemotherapy have failed to demonstrate any survival benefit[15,16].
A phase II study has evaluated the possible therapeutic role of gefitinib, a competitive inhibitor of ATP binding to the intracellular kinase domain of EGFR, in combination with gemcitabine in inoperable or metastatic pancreatic cancer patients. The combination demonstrated promising activity with a mOS and PFS in the combination arm of 7.3 and 4.1 mo, respectively, but other evidence supporting a role of gefitinib in PDAC treatment is lacking[17].
Another ErbB family of transmembrane tyrosine kinase receptors is HER-2, which is overexpressed in 11% of pancreatic adenocarcinoma cases. HER2-positive status has also been correlated with shorter survival[18].
Trastuzumab plus gemcitabine was tested in 34 metastatic pancreatic cancer patients with HER-2 overexpression as determined by immunohistochemistry, and partial responses were observed in 6% of cases[19]. Harder et al[20] in a multicentre phase II study, investigated the efficacy and toxicity of the HER2 antibody, trastuzumab, plus capecitabine in patients with pancreatic cancer and HER2 overexpression, but this treatment did not perform favourably with respect to either PFS or OS compared with standard chemotherapy.
After FDA approval of lapatinib, clinical trials have been initiated to test the effect of this HER-2 inhibitor combined with chemotherapy in pancreatic carcinoma. In particular, lapatinib was tested in combination with capecitabine as a second-line treatment in advanced pancreatic cancer with promising preliminary results. Further studies are needed to evaluate the real effectiveness and role of this molecule in the treatment of PADC[21].
Nimotuzumab, another anti-EGFR monoclonal antibody, showed promising results[22]. In a phase II trial where advanced pancreatic cancer patients were randomized to receive second-line monotherapy with nimotuzumab, Strumberg et al[23] showed PFS after 1 year of 10.3% and median overall survival of 18.1 wk with a tolerable toxicity profile.
Based on preclinical evidence, afatinib, an inhibitor of EGFR, HER2 and HER4, is under evaluation in an ongoing phase II trial[24,25].
KRAS activating mutations are present in 70% to 90% of cases of pancreatic cancer. K-Ras is a GTPase protein belonging to the Ras protein family, which has oncogenic activity, and gain-of-function mutations resulting in constitutive activation promote proliferation and inhibit apoptosis through the RAF/MEK/ERK and PIK3/AKT pathways. K-Ras is very difficult to target, and no inhibitors are actually available to use in clinical practice[26].
Preclinical study has shown that farnesylation is an important post-translational modification required for Ras activation, allowing the protein to be attached to the plasma membrane for signal transduction[27].
After promising results in terms of anti-proliferative activity in pancreatic tumour cell lines, farnesyl-transferase inhibitors, particularly tipifarnib, failed to improve overall survival either as a single agent or in combination with gemcitabine in a phase III trial[28,29].
Due to the difficulty of targeting Ras directly, a possible solution could be to block targets downstream of KRAS, such as the protein kinase MEK. Selumetinib is an oral small molecule that inhibits MEK1/2. In a phase II trial, patients were randomized to receive single-agent capecitabine or selumetinib as a second-line treatment for advanced pancreatic cancer. The selumetinib arm showed a median overall survival of 5.4 mo vs 5.0 mo in the capecitabine arm, but this result was not statistically significant[30].
Another MEK1/2 inhibitor, trametinib, was tested in pancreatic cancer in combination with gemcitabine against a regimen of gemcitabine plus placebo in a phase II randomized multicentre study. Nevertheless, no significant advantages were demonstrated in terms of overall survival or PFS[31].
Rigosertib, a first-in-class Ras mimetic and small molecule inhibitor of multiple signalling pathways, including polo-like kinase 1 and phosphoinositide 3-kinase (PI3K), was assessed in combination with gemcitabine in patients with treatment-naïve metastatic pancreatic adenocarcinoma in a phase II/III randomized study, but the combination regimen did not improve survival or response, as recently presented at the 2015 ASCO Annual Meeting[32].
Research in this field is in development, but the available trials have failed to show any survival benefit.
Another possible target in ductal pancreatic cancer is represented by insulin like growth factor 1 receptor, which is highly expressed in pancreatic cells, and upon ligand binding activates several pathways involved in cell proliferation and cell survival such as the PIK3/AKT pathway[33].
Monoclonal antibodies against IGFR (cixutumumab, ganitumab) were evaluated in PDAC treatment, but unfortunately, they failed to show a statically significant survival benefit[34].
In particular, the phase III trial assessing ganitumab in combination with gemcitabine was closed early based on a pre-planned futility analysis: The median overall survival was 7.1 mo in the maximum dose ganitumab arm vs 7.2 mo in the placebo arm (HR, 0.97, P = 0.397)[35].
Neoangiogenesis is essential for tumour progression and metastatization mechanisms. Vascular endothelial growth factor (VEGF) stimulates the proliferation of endothelial cells and is overexpressed in human pancreatic cancer. Nevertheless, neoangiogenesis inhibitors, particularly VEGF inhibitors, failed to improve overall survival in combination with gemcitabine in advanced pancreatic cancer. After encouraging results, phase III trials that tested the efficacy of bevacizumab in association with gemcitabine alone, or gemcitabine plus erlotinib, did not confirm previous findings[36,37].
Aflibercept, a new recombinant fusion protein with extracellular portions of VEGFR-1 and VEGFR-2, which binds VEGF-A, VEGF-B and placental growth factors 1 and 2 thereby inhibiting VEGF-ligand-dependent signalling processes, suppresses tumour growth in pancreatic cell lines and xenografts. Nevertheless, a phase III study aiming to investigate OS in metastatic pancreatic cancer patients receiving standard gemcitabine and either aflibercept or placebo demonstrated that adding aflibercept to gemcitabine did not improve OS in metastatic pancreatic cancer patients[38].
Similarly sorafenib, an oral multikinase inhibitor of Raf-kinase, VEGF-R2/-R3 and PDGFR-β, tested alone or in combination with gemcitabine in small phase I and II trials, and axitinib, an anti-angiogenesis agent assessed in combination with gemcitabine, showed no statistically significant efficacy in a phase III trial in advanced PDAC[39-41].
Phase II studies combining chemotherapy with promising new anti-angiogenic molecular agents, such as TL-118, a nonsteroidal anti-inflammatory oral medication, or necuparanib, which is re-engineered from heparin with possible anti-tumour activity, are underway[42,43].
Hedgehog signalling has a critical role in cell proliferation and survival during embryonic development. Normal pancreatic cells silence this pathway, but pathological activation is observed in many solid tumours, particularly in PADC. Hedgehog binds to the extracellular receptor Patched, which, in the absence of Hedgehog, suppresses activation of the G-protein-coupled receptor Smoothened and upregulates glioma associated oncogene homolog1 transcriptional activity. Cancer cell lines show both Hedgehog ligand-dependent and -independent mechanisms of aberrant signalling[44].
Bailey et al[45] showed how Sonic hedgehog (SHH) and other proteins downstream of the Hedgehog pathway, detected in precursor lesions and in PDAC primary tumour samples, contribute to the formation of the desmoplastic reaction, an important characteristic of pancreatic cancer that limits the effective delivery of anticancer agents to pancreatic cancer cells. Genetically engineered mouse models demonstrated a depletion of tumour matrix from SHH pathway inhibition, which could be a promising strategy in pancreatic cancer therapy[46].
Vismodegib (GDC-0449), an oral small-molecule inhibitor targeting Smoothened[47], is under assessment in open phase II trials in combination with gemcitabine in advanced cancer, in combination with gemcitabine and nab-paclitaxel in metastatic settings with promising preliminary data[48], and as a single agent in neoadjuvant settings followed by surgery[49-51].
The Smoothened inhibitor saridegib (IPI-926) was tested in association with gemcitabine against gemcitabine plus placebo in a randomized, double-blind, placebo-controlled phase II trial enrolling patients with metastatic disease. Unfortunately, this study was closed ahead of time due to evidence of decreased patient survival in the saridegib arm[52].
Hedgehog inhibitors are an active research field, and several clinical trials are ongoing[53]. Notch signalling is another embryonic pathway crucial for pancreatic organogenesis, but after pancreas development, it is active only in a stem cell subgroup. This pathway is upregulated in PDAC and promotes tumourigenesis. Binding of Notch ligand to its receptor promotes a cascade of proteolytic cleavages, mediated by γ-secretase (presenilin). The activated form ICN (intra cellular notch) forms part of a transcription complex that, after translocating to the nucleus, regulates transcription of several genes involved in proliferation and differentiation of cells, interacting with other pathways such as Hedgehog, KRAS and NF-κB signalling[54,55].
RO4929097 is a selective inhibitor of the γ-secretase enzyme with anti-tumour activity in preclinical studies[56].
A recent phase II single-arm trial assessed the possible role of RO4929097, enrolling 18 previously treated advanced PDAC patients. The treatment was well tolerated; the median survival was 4.1 mo, and the median progression-free survival was 1.5 mo[57].
Encouraging clinical results were observed testing demcizumab, an anti- Delta-like ligand 4 antibody, plus gemcitabine and nab-paclitaxel in advanced PDAC in a phase Ib trial. Further evidence is needed to confirm these preliminary data[58].
Mutations affecting BRCA pathway components, especially the tumour suppressor gene BRCA2, which is associated with hereditary predisposition to breast, ovarian and pancreatic cancer, promote deficiency in DNA damage repair mechanisms and genomic instability[11].
Poly ADP-ribose polymerase (PARP) is a nuclear enzyme recruited to repair cell DNA damage, and as recent evidence showed, patients with defects in the homologous DNA recombination pathway may benefit from the use of PARP inhibitors as monotherapy or in combination with radiation or other chemotherapeutic agents. Clinical trials testing those new agents in selected patients are currently in the development phase[59-61].
After activation, Ras can phosphorylate PI3K, which activates Akt, a serine/threonine kinase. Signal transduction by activated PI3K/Akt plays a role in tumour cell proliferation, survival and metabolism, usually through several downstream targets, including the mammalian target of rapamycin (mTOR)[62].
Several trials testing PI3K/AKT axis inhibitors are currently ongoing in advanced pancreatic cancer patients after encouraging preclinical model results[63]. These trials included the following PI3K/AKT axis inhibitors: BKM120, a PI3K inhibitor tested in combination with the mFOLFOX-6 schedule; RX-0201, an Akt antisense oligonucleotide tested in a phase II study plus gemcitabine; and BEZ235, a combined inhibitor of PI3K and mTOR assessed in a phase study in combination with the MEK inhibitor MEK162[64-66].
Wolpin et al[67] evaluated a possible role of everolimus, an oral mTOR inhibitor, as monotherapy in 33 gemcitabine-refractory pancreatic cancer patients. The PFS and OS were 1.8 and 4.5 mo, respectively.
Recently, the results of a single arm phase II study where everolimus was tested in combination with capecitabine were published. The median OS was 8.9 mo and PFS was 3.6 mo[68].
The results of a phase I/II study testing everolimus in combination with gemcitabine in advanced settings and the results of a phase II trial testing temsirolimus, another mTOR inhibitor, in locally advanced or metastatic settings are anticipated[69,70].
The stroma is a dynamic compartment of pancreatic tumours that is critically involved in tumour formation, progression and the metastasis process. Therefore, targeting stromal microenvironment elements could be an efficient therapeutic strategy in addition to previously described trials evaluating Hedgehog signalling inhibitors[71].
After promising data derived from a preliminary clinical study on the possible role of PEGPH20, a pegylated formulation of recombinant hyaluronidase, a phase II trial is currently in the recruitment phase. The purpose of that study is to enrol untreated patients with metastatic disease to receive a combination of PEGPH20, nab-naclitaxel and gemcitabine or a combination of nab-paclitaxel and gemcitabine[72,73].
Additionally, inhibition of PDGFR, a receptor expressed in stromal cells with a critical role in recruiting pericytes and in interstitial fluid pressure in the tumour stroma, could be an interesting molecular target, as suggested by preclinical studies using an orthotopic pancreatic tumour mouse model[74].
TKI258, a PDGFR inhibitor, is under evaluation in a phase I dose assessment for advanced pancreatic cancer patients[75].
In the past, matrix metalloproteinase inhibitors such as marimastat were tested. Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes responsible for the degradation of connective tissue proteins, and aberrant MMP expression is observed in PDAC. Nevertheless, the results of a phase III trial provided no evidence to support a combination of marimastat with gemcitabine in patients with advanced pancreatic cancer[76].
Knowledge of the molecular biology of PDAC has important potential clinical relevance, but current efforts to improve understanding of the mutational profile of this tumour have not provided any significant advantage in the use of targeted therapy. Several agents have been tested in PDAC, but almost all have failed to demonstrate efficacy in late phase clinical trials. Only erlotinib has been approved by the FDA for advanced pancreatic cancer treatment, but the improvement of overall survival is barely 2 wk compared with gemcitabine alone[5].
There could be many reasons for those unsatisfying results. First of all, the extreme genomic heterogeneity of PDAC is an important block to identifying new candidate actionable molecular targets or to testing existing biological therapies already approved for human use for other cancers. In addition, no significant results have been observed by matching targeted agents with patients harbouring the cognate molecular abnormality, such as, for example, the use of trastuzumab in HER2 overexpression cases. Due to poor results derived from targeting a single molecule, new strategies using multitargeted agents or molecular agent combinations are in the development phase in order to block more than one driving genomic aberration and to prevent or evade resistance.
Additionally, the type of chemotherapy used in combination could be a failure factor. Indeed, the majority of trials have combined target agents with gemcitabine, but actually, the first-line schedules are represented by FOLFIRINOX or gemcitabine plus Nab-paclitaxel. Therefore, greater efficacy may be obtained from the combination of target agents with those chemotherapeutic drugs.
Furthermore, most studies in which molecular or chemotherapeutic agents in pancreatic cancer were tested enrolled an unselected population of patients to treat. In the last 3 years, approximately 116 trials specific for PDAC systemic therapy were registered of which only about 8% applied criteria to select a patient subset based upon molecular biomarkers[77].
Most studies in which molecular or chemotherapeutic agents in pancreatic cancer were tested enrolled an unselected population of patients to treat. In the last 3 years, approximately 116 trials specific for PDAC systemic therapy were registered of which only about the 8% applied criteria to select a patient subset based upon molecular biomarkers[77].
To stratify patients, the Australian Pancreatic Cancer Genome Initiative has started a pilot study to evaluate the feasibility of assessing a more stratified approach in the management of pancreatic cancer through predefined actionable molecular phenotypes. Patients are enrolled in this trial, called IMPaCT (Individualised Molecular Pancreatic Cancer Therapy), after a preliminary phenotype screening in order to compare the use of gemcitabine in an unselected population to a stratified approach. The aim of the study is to create a tailored approach to pancreatic cancer treatment, which seems to be one of the major challenges for the future[78].
Finally, thanks to biotechnology advancement, biological agents can find application in cancer treatment by tumour-targeted delivery of cytotoxic drugs. Particularly, Ahn et al[79] developed antibody fragment-installed polymeric micelles via maleimide-thiol conjugation for selective delivery of platinum drugs to pancreatic tumours. This antibody-drug conjugate significantly suppressed the growth of pancreatic tumour xenografts. This technology, with potential activity in vitro and in a mouse model, could be a promising future strategy in pancreatic cancer therapy[79].
In conclusion, the lack of efficacy of targeted therapy in PDAC represents a challenge for the future, and more efforts are needed in order to make pancreatic cancer a curable disease.
We would like to thank the Interdepartmental Center of Cancer Research “Giorgio Prodi” for technical support.
P- Reviewer: Aoyagi K, Nigam P, Park JY, Sperti C S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 2. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. [PubMed] |
| 3. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5642] [Article Influence: 403.0] [Reference Citation Analysis (1)] |
| 4. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4890] [Article Influence: 407.5] [Reference Citation Analysis (0)] |
| 5. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [PubMed] |
| 6. | Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, Biasco G. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review). Oncol Rep. 2010;23:1183-1192. [PubMed] |
| 7. | Chang DK, Grimmond SM, Biankin AV. Pancreatic cancer genomics. Curr Opin Genet Dev. 2014;24:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | 8 Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabé RR, Bhan MK, Calvo F, Eerola I, Gerhard DS. International network of cancer genome projects. Nature. 2010;464:993-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1982] [Cited by in RCA: 1705] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 9. | Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1647] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 10. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3027] [Article Influence: 178.1] [Reference Citation Analysis (0)] |
| 11. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1995] [Article Influence: 199.5] [Reference Citation Analysis (1)] |
| 12. | Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1119] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 13. | Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11:305-309. [PubMed] |
| 14. | da Cunha Santos G, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S, Squire J, Parulekar W, Moore MJ, Tsao MS. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010;116:5599-5607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Cascinu S, Berardi R, Labianca R, Siena S, Falcone A, Aitini E, Barni S, Di Costanzo F, Dapretto E, Tonini G. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol. 2008;9:39-44. [PubMed] |
| 16. | Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 17. | Fountzilas G, Bobos M, Kalogera-Fountzila A, Xiros N, Murray S, Linardou H, Karayannopoulou G, Koutras AK, Bafaloukos D, Samantas E. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer Invest. 2008;26:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Kimura K, Sawada T, Komatsu M, Inoue M, Muguruma K, Nishihara T, Yamashita Y, Yamada N, Ohira M, Hirakawa K. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin Cancer Res. 2006;12:4925-4932. [PubMed] |
| 19. | Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, Hesketh P, Rathore R, Wolff R, Tantravahi U. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706-712. [PubMed] |
| 20. | Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Safran H, Miner T, Resnick M, Dipetrillo T, McNulty B, Evans D, Joseph P, Plette A, Millis R, Sears D. Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: a phase I study for advanced pancreaticobiliary cancer. Am J Clin Oncol. 2008;31:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Su D, Jiao SC, Wang LJ, Shi WW, Long YY, Li J, Bai L. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol. 2014;35:2313-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Strumberg D, Schultheis B, Scheulen ME, Hilger RA, Krauss J, Marschner N, Lordick F, Bach F, Reuter D, Edler L. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs. 2012;30:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Ioannou N, Dalgleish AG, Seddon AM, Mackintosh D, Guertler U, Solca F, Modjtahedi H. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br J Cancer. 2011;105:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | PD Dr. med. Volker Heinemann. Afatinib as Cancer Therapy for Exocrine Pancreatic Tumours (ACCEPT). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01728818 NLM Identifier: NCT01728818. |
| 26. | di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 316] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 27. | Takashima A, Faller DV. Targeting the RAS oncogene. Expert Opin Ther Targets. 2013;17:507-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1364] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 29. | Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430-1438. [PubMed] |
| 30. | Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, Tebbutt NC. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 32. | Scott AJ, O’Neil BH, Ma WW, Cohen SJ, Aisner D, Menter AR, Abdulaziz Tejani M. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. ASCO Annual Meeting. J Clin Oncol 33. 2015;abstr 4117. |
| 33. | Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1-12. [PubMed] |
| 34. | Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer. 2014;120:2980-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, Moore MJ, Peeters M, Bodoky G. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 36. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 675] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 37. | Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 502] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 38. | Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49:2633-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Chiorean EG, Schneider BP, Akisik FM, Perkins SM, Anderson S, Johnson CS, DeWitt J, Helft P, Clark R, Johnston EL. Phase 1 pharmacogenetic and pharmacodynamic study of sorafenib with concurrent radiation therapy and gemcitabine in locally advanced unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2014;89:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, Nattam S, Agamah E, Stadler WM, Vokes EE. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2012;30:382-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 42. | Tiltan Pharma Ltd. Investigator’s Initiated Phase II Study for Pancreatic Cancer Patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01659502 NLM Identifier: NCT01659502. |
| 43. | Momenta Pharmaceuticals, Inc . M402 in Combination With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01621243 NLM Identifier: NCT01621243. |
| 44. | Di Marco M, Macchini M, Vecchiarelli S, Sina S, Biasco G. Hedgehog signaling: from the cuirass to the heart of pancreatic cancer. Pancreatology. 2012;12:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995-6004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 46. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2543] [Article Influence: 158.9] [Reference Citation Analysis (0)] |
| 47. | Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One. 2011;6:e27306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | De Jesus-Acosta A, O’Dwyer P, Ramanathan R, Von Hoff D, Maitra A, Rasheed Z. A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (Pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). ASCO Gastrointestinal Cancer Symposium. 2014;Abstract No. 257. |
| 49. | National Cancer Institute (NCI). Gemcitabine Hydrochloride With or Without Vismodegib in Treating Patients With Recurrent or Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01064622 NLM Identifier: NCT01064622. |
| 50. | Sidney Kimmel Comprehensive Cancer Center. Pancreas, Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01088815 NLM Identifier: NCT01088815. |
| 51. | Lisa Bax. Hedgehog Inhibition for Pancreatic Ductal Adenocarcinoma (PDAC) in the Preoperative Setting (HIPPoS). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01096732 NLM Identifier: NCT01096732. |
| 52. | Available from: http://www.businesswire.com. |
| 53. | Sidney Kimmel Comprehensive Cancer Center. Gemcitabine + Nab-paclitaxel With LDE-225 (Hedgehog Inhibitor) as Neoadjuvant Therapy for Pancreatic Adenocarcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01431794 NLM Identifier: NCT01431794. |
| 54. | Sjölund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer. 2005;41:2620-2629. [PubMed] |
| 55. | Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert Opin Ther Targets. 2010;14:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Plentz R, Park JS, Rhim AD, Abravanel D, Hezel AF, Sharma SV, Gurumurthy S, Deshpande V, Kenific C, Settleman J. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2009;136:1741-9.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek MA, Dasari A, Blatchford PJ, Quackenbush K, Messersmith W. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs. 2014;32:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Hidalgo M, Cooray P, Jameson MB, Carrato A, Parnis F, Jeffery M, Grimison PS, Stagg RJ, Kapoun AM, Dupont J. A phase Ib study of the anti-cancer stem cell agent demcizumab (DEM) & gemcitabine (GEM) /- paclitaxel protein bound particles (nab-paclitaxel) in pts with pancreatic cancer. 2015 ASCO Annual Meeting. J Clin Oncol. 2015;abstr 4118. |
| 59. | Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas. 2015;81:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 60. | Tangutoori S; AstraZeneca. Study to Assess the Safety & Tolerability of a PARP Inhibitor in Combination With Gemcitabine in Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT00515866 NLM Identifier: NCT00515866. |
| 61. | National Cancer Institute (NCI). Gemcitabine Hydrochloride and Cisplatin With or Without Veliparib or Veliparib Alone in Patients With Locally Advanced or Metastatic Pancreatic Cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01585805 NLM Identifier: NCT01585805. |
| 62. | Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218-1249. [PubMed] |
| 63. | Ito D, Fujimoto K, Mori T, Kami K, Koizumi M, Toyoda E, Kawaguchi Y, Doi R. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer. 2006;118:2337-2343. [PubMed] |
| 64. | UNC Lineberger Comprehensive Cancer Center. BKM120 + mFOLFOX6 in Advanced Solid Tumors With Expansion Cohort Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01571024 NLM Identifier: NCT01571024. |
| 65. | Rexahn Pharmaceuticals, Inc . A Safety and Efficacy Study of RX-0201 Plus Gemcitabine in Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01028495 NLM Identifier: NCT01028495. |
| 66. | Novartis Pharmaceuticals. Safety, Pharmacokinetics and Pharmacodynamics of BEZ235 Plus MEK162 in Selected Advanced Solid Tumor Patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01337765 NLM Identifier: NCT01337765. |
| 67. | Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 68. | Kordes S, Klümpen HJ, Weterman MJ, Schellens JH, Richel DJ, Wilmink JW. Phase II study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2015;75:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Novartis Pharmaceuticals. Treatment of Patients Suffering From a Progressive Pancreas Carcinoma With Everolimus (RAD001) and Gemcitabine. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT00560963 NLM Identifier: NCT00560963. |
| 70. | National Cancer Institute (NCI). CCI-779 in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT00075647 NLM Identifier: NCT00075647. |
| 71. | Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 72. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1640] [Article Influence: 126.2] [Reference Citation Analysis (0)] |
| 73. | Halozyme Therapeutics. PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Subjects With Stage IV Untreated Pancreatic Cancer (HALO-109-202). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01839487 NLM Identifier: NCT01839487. |
| 74. | Hwang RF, Yokoi K, Bucana CD, Tsan R, Killion JJ, Evans DB, Fidler IJ. Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res. 2003;9:6534-6544. [PubMed] |
| 75. | Roswell Park Cancer Institute. Dovitinib Lactate, Gemcitabine Hydrochloride, and Capecitabine in Treating Patients With Advanced or Metastatic Solid Tumors, Pancreatic Cancer and Biliary Cancers. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01497392 NLM Identifier: NCT01497392. |
| 76. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 397] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 77. | Bramhall SR; ClinicalTrials. gov. gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2015; May] Available from: http: //www.clinicaltrials.gov. |
| 78. | Chantrill LA, Nagrial AM, Watson C, Johns AL, Martyn-Smith M, Simpson S, Mead S, Jones MD, Samra JS, Gill AJ. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res. 2015;21:2029-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 79. | Ahn J, Miura Y, Yamada N, Chida T, Liu X, Kim A, Sato R, Tsumura R, Koga Y, Yasunaga M. Antibody fragment-conjugated polymeric micelles incorporating platinum drugs for targeted therapy of pancreatic cancer. Biomaterials. 2015;39:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |