Published online Jan 15, 2016. doi: 10.4251/wjgo.v8.i1.30
Peer-review started: June 15, 2015
First decision: August 25, 2015
Revised: October 9, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: January 15, 2016
Processing time: 215 Days and 22.7 Hours
Historically, natural products have represented a significant source of anticancer agents, with plant-derived drugs becoming increasingly explored. In particular, sanguinarine is a benzophenanthridine alkaloid obtained from the root of Sanguinaria canadensis, and from other poppy Fumaria species, with recognized anti-microbial, anti-oxidant and anti-inflammatory properties. Recently, increasing evidence that sanguinarine exibits anticancer potential through its capability of inducing apoptosis and/or antiproliferative effects on tumor cells, has been proved. Moreover, its antitumor seems to be due not only to its pro-apoptotic and inhibitory effects on tumor growth, but also to its antiangiogenic and anti-invasive properties. Although the precise mechanisms underlying the antitumor activity of this compound remain not fully understood, in this review we will focus on the most recent findings about the cellular and molecular pathways affected by sanguinarine, together with the rationale of its potential application in clinic. The complex of data currently available suggest the potential application of sanguinarine as an adjuvant in the therapy of cancer, but further pre-clinical studies are needed before such an antitumor strategy can be effectively translated in the clinical practice.
Core tip: Sanguinarine is a benzophenanthridine alkaloid isolated from the root of Sanguinaria canadensis, and other poppy Fumaria species, which exibits a clear-cut anticancer potential by inducing apoptosis and/or antiproliferative effects on tumor cells. Sanguinarine also shows antiangiogenic and anti-invasive properties, as demonstrated in vitro and in vivo. In consideration of the multiple biological effects of sanguinarine, which suggest its possible use in cancer therapy, further detailed pharmacokinetic and toxicologic studies are required to assess both the efficacy and safety of the compound before proposing a possible translation into the clinic.
- Citation: Gaziano R, Moroni G, Buè C, Miele MT, Sinibaldi-Vallebona P, Pica F. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: Evidence and perspectives. World J Gastrointest Oncol 2016; 8(1): 30-39
- URL: https://www.wjgnet.com/1948-5204/full/v8/i1/30.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i1.30
Tumor initiation is the result of multiple genetic and epigenetic events. Transformed cells are characterized by indefinite proliferation, apoptosis-resistance and the capability to metastasize and support angiogenesis[1].
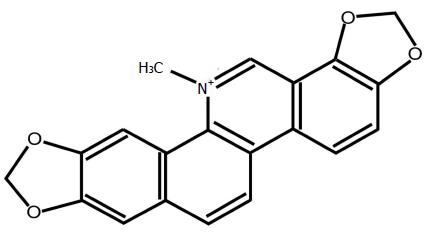
Chemotherapy, irradiation and/or immunotherapy represent the gold standard approach for the treatment of cancer worldwide. The increased frequency of tumor relapse and the toxicity of the anticancer drugs, however, often reduce the therapeutical effectiveness of several antitumor therapy protocols. Therefore, the identification of more effective therapeutic protocols is needed and, in this direction, phytochemicals may represent an attractive alternative because of their low toxicity and low cost[2]. In this scenario, sanguinarine (Figure 1) and chelerythrine are the principal members of quaternary benzo[c]phenanthridine alkaloids (QBAs)[3] obtained from Sanguinaria canadensis, Chelidonium majus, and Macleaya cordata. Alkaloids include a large group of secondary metabolites (SMs) that differ in relation to structure, function and biodistribution[4]. In the past, QBAs have attracted the attention of many pharmacologists because of their own low toxicity[5,6] and their multiple biological activities, such as the antitumor[7], antimicrobial[8,9], anti-inflammatory[10], anti-HIV[11], anti-platelet[12], anti-angiogenesis[13], and antiparasitic activities[14-16]. The influence of QBAs on the activity of various important biological enzymatic pathways has been also demonstrated[7]. For long times, sanguinarine-containing herbs were believed to possess anticancer activity but only recently evidence that sanguinarine possesses a strong anti-neoplastic activity, which is mediated mainly by the induction of tumor cell apoptosis has been proved.
This review summarize the most recent findings on the molecular mechanisms underlying the antitumor activity of sanguinarine both in vitro, in a variety of human tumor cells, and in vivo in selected experimental tumor models, together with the rationale of its potential application in clinical practice.
Physiologically, the human body controls homeostasis by eliminating damaged and aged cells by means of a genetically programmed process named apoptosis[17,18]. Tumor cells evade apoptosis and grow indefinitely. Several proteins, among which are caspases, pro-apoptotic Bax and anti-apoptotic B cell lymphoma (Bcl)-2, cytochrome c, and apoptotic protease activating factor -1, carry out the apoptotic programme either by intrinsic or extrinsic pathways. The first one is dependent on mitochondria, whereas the second one is initiated by the so-called death receptors (DRs). Selected anti-apoptotic proteins, among which Bcl-2, have been found over-expressed in different types of cancers. The down-regulation of anti-apoptotic proteins in cancer cells represents a promising therapeutic strategy of intervention in cancer therapy.
A number of plant-derived agents, have been shown to be capable of hampering disease progression by inducing cell apoptosis in multiple types of human and experimental cancers. Recently QBAs, and particularly sanguinarine, have been indicated as potential anti-cancer compounds. In detail, it has been reported that micromolar concentrations of sanguinarine are capable of inhibiting tumor cell growth, and this inhibitory effect is associated with cell cycle arrest and induction of apoptosis[19-22]. The anti-proliferative and/or pro-apoptotic activities of sanguinarine have been demonstrated in in vitro studies on several cancer cell types including epidermal[23], keratinocyte[24,25], prostate[26-28], cervical[29], breast[20,30-33], leukaemia[34,35], lymphoma[36], melanoma[37-39], colon[40,41], colorectal[21], gastric[42], pancreatic[19], lung[22], neuroendocrine[43], osteosarcoma[44], and human neuroblastoma cells[45]. By contrast, there are few studies on the in vivo effectiveness of sanguinarine administration per os[46,47] in animal tumor models[33,48].
It has been reported that sanguinarine exerts an antiproliferative activity on murine melanoma cells both in vitro and in vivo (B16 melanoma 4AS in the syngeneic host C57BL/mice), as well as in A375 human melanoma xenografts in athymic nude mice[48]. We also have conducted a study aimed at evaluating the anti-tumor effect of sanguinarine both in vitro and in vivo in a rat colorectal cancer model (DHD/K12/TRb cell line)[49]. We found that the in vitro addition of sanguinarine has a dose-dependent inhibitory effect on the proliferation of DHD/K12/TRb cells and induces tumor cell apoptosis. Sanguinarine also showed a clear-cut in vivo anti-tumor activity, leading to an inhibition of tumor growth higher than 70%[49]. The sanguinarine-induced inhibition of tumor growth was associated with its pro-apoptotic effect on tumor cells, as confirmed by the ex-vivo histopathological examinations performed on experimental tumor sections and by TUNEL assay[49].
It is known that sanguinarine-induced apoptosis occurs through multiple pathways, including the activation of nuclear factor-κB (NF-κB)[50], the mitochondrial damage resulting in activation of the caspase machinery[24] and the cell cycle arrest[27]. In detail, the sanguinarine-induced apoptosis occur either via a mithocondrial pathway dependent on caspase-9 or by the DR pathways, with the activation of caspase 8. The activation of caspase 3, which represents a key factor for apoptosis execution in both pathways, and the following cleavage of PARP together with the down-regulation of Bcl-2 and c-FLIP, may play a very important role in the apoptosis induced by sanguinarine[26,51,52]. Studies performed in human neuroblastoma cells SH-SY5Y have shown that sanguinarine reduces the expression of anti-apoptotic genes, particularly of NOL3, BCL2, and HRK genes[45]. A down-regulation of pro-caspase 3, Bcl-2, clAP2, XIAP, and c-FLIPs[20,52] has been also observed in basal cell-like MDA-MB-231 human breast carcinoma cells treated with sanguinarine. The effect of sanguinarine treatment has been evaluated also on the expression levels of Bax and Bcl-2 proteins in immortalized human keratinocytes (HaCaT)[24,25], human leukaemia JM1 and K562 cells[35] and in Hela and SiHa human cervical tumor cells[53]. These findings indicate that sanguinarine, depending on the dose employed, down-regulates the expression levels of Bcl-2 protein while increasing those of Bax protein, which is a key regulator of mitochondrial damage. Notably, Bax expression has been associated with an increased sensitivity of cancer cells to chemotherapy[54], whereas an increase of Bcl-2 has been associated with the occurrence of drug-resistance phenomena[55].
It has been proved that sanguinarine is capable of inducing DNA damage, acting as an intercalating agent[56,57], and also a very rapid cell apoptosis which does not seem to be mediated by a p53-dependent DNA damage signalling in human colon cancer[41] and in malignant melanoma cells[38].
The concentration of sanguinarine plays a key role in the induction of cell death. Consistently, both apoptotic and non-apoptotic cell death pathways have been observed in response to sanguinarine. Thus, a sanguinarine-related and bimodal cell death effect, which consists of two different types of cell death, i.e., by apoptosis (induced by low SA concentration; characterized by caspase 3 and PARP positivity) and oncosis (induced by high SA concentration; characterized by caspase 3 and PARP negativity), has been demonstrated in various cancer cells types[52].
Tumor cells are characterized by deregulated proliferation. Conversely, normal cells proliferation is the results of the action of selected growth signals [cyclins and cyclin-dependent kinases (CDKs)] and anti-growth signals (p21 and p27 proteins). Cyclins and CDKs cooperate in G1 for the initiation of the S phase and in G2 for inducing mitosis, whereas p21 and p27 selectively block the catalytic activity of CDK. Following addition of anti-mitogenic compounds or DNA injury, p21 and p27 bind to cyclin-CDK complex blocking their catalytic activity and consequently the cell cycle progression.
Actually a number of inhibitors and/or regulators of the cell cycle, among which sanguinarine, are suggested as potential antitumor agents. Sanguinarine treatment (0.2-2 mol/L for 24 h) blocks cell cycle by enhancing the expression of CDK inhibitors and by reducing not only cyclin D1, D2 and E, but also CDK2, 4 and 6 in human prostate cancer cells[27]. This alkaloid also up-regulates p27 and down-regulates cyclin D1, while inhibiting the activation of STAT3, as demonstrated in vitro in basal cell-like MDA-MB-231 human breast cancer cells and in vivo in a murine breast cancer model[33]. Holy et al[31] studied the effects of sanguinarine (5-10 μmol/L) on the cell cycle regulatory molecules, by immune-cytochemistry, that visualized the cyclin D1 and topoisomerase II in MCF-7 breast cancer cells. They reported that sanguinarine-mediated cellular events induce cell cycle arrest in G0/G1 and inhibit cell proliferation, which is associated with a striking re-localization of cyclin D1 and topoisomerase II from the nucleus to the cytoplasm.
Apoptosis induced by sanguinarine has been associated also with the production of reactive oxygen species (ROS)[20,36,52,58]. ROS are a group of highly reactive molecules, among which are superoxide anion radical, hydrogen peroxide, singlet oxygen, and hydroxyl radical. ROS are the products of the oxygen metabolism within the cell. ROS are known as key regulators of normal cell proliferation and differentiation, however, high levels of ROS have also been associated with damage of DNA and proteins and thus with the occurrence of apoptosis[59,60]. Moreover, an overdone oxidative stress has been shown capable of inducing a reduction of the normal mitochondrial membrane potential, which in turn leads to apoptosis[21,61-63]. It has been shown that ROS generation, is crucial for the apoptosis induced by sanguinarine in human breast cancer[52], SK-Mel-2 human melanoma[37], human prostate cancer[25] and in both HCT-116[21] and HT-29 human colon cancer cells[40]. Consistently, pre-treatment of tumor cells with antioxidants such as N-acetylcysteine or glutathione counteracts the apoptosis induced by sanguinarine[21,32,37,52]. Moreover, the over-expression of cyclooxygenase-2 (COX-2) also rescues prostate cancer cells from sanguinarine-induced apoptosis by inhibiting the activity of NO synthase, thus suggesting the possibility to use a combination of COX-2 inhibitors and sanguinarine in the treatment of human prostate cancer[28].
The molecular pathways associated with carcinogenesis are linked also with chronic inflammation, which emerges as an important co-factor in tumor development. The NF-κB controls the inflammatory gene expression and recently it has been suspected to be involved also in the control of tumor development[64]. Resting NF-κB localizes within the cell cytoplasm in the form of a heterodimer composed by p50, p65, and the inhibitory subunit IkBα[65]. Following activation, the IkBα protein is phosphorylated, ubiquitinated and finally degradated. Then, the p50 and p65 reach the nucleus of cell, where they interact with selected DNA sequences localized in the promoter region of various genes, leading to their transcription. Consistently, the NF-κB signalling pathway has been indicated as a key-target for the development of new chemotherapeutic approaches in cancer.
Sanguinarine has been suggested as a potential actor in the control of NF-κB-dependent pathological responses by blocking phosphorylation and degradation of IkBα. Studies by Chaturvedi et al[50] showed that in human myeloid ML-1a cells, the treatment with sanguinarine is capable of abrogating, dose- and time-dependently, the activation of NF-κB induced by tumor necrosis factor.
Many reports indicate that sanguinarine exerts antitumor activity not only by inhibiting tumor cells migration and/or invasion, but also by repressing angiogenesis[22,66]. Since solid tumors require active angiogenesis, the inhibition of endothelial cell proliferation result in the inhibition of tumor growth and progression. The best known angiogenic growth factor is represented by VEGF. Several studies have explored the relationship existing among sanguinarine, angiogenesis and metastatization. In particular, Eun and Koh[13] showed that sanguinarine inhibits the VEGF-induced endothelial cell migration, sprouting and survival in vitro, and blocks blood vessel formation in vivo in different experimental models. Furthermore, Basini et al[67] showed that sanguinarine is capable of blocking the VEGF-induced blood vessel growth. Depending on the concentration used, sanguinarine also inhibits VEGF secretion in human microvascular endothelial cells HMVEC as well as in A549 lung cancer cells[68]. This inhibitory effect has been associated with the suppression of the phosphorilation of Akt, p38 and VE-cadherin, which are well known modulators of the VEGF signal transduction pathway[67,69]. Moreover, sanguinarine enhances apoptosis in human mammary adenocarcinoma MCF-7 through the inhibition of VEGF release, induced by generation of ROS[32]. Sanguinarine also inhibits angiogenesis in preclinical experimental tumor models, such as mouse melanoma[48] and rat colorectal cancer, as we reported previously[49]. In both the experimental studies, the therapeutic efficacy of sanguinarine could not be attributed only to a direct anti-proliferative activity but also to the inhibition of tumor angiogenesis induced by this alkaloid.
The rationale of using VEGF-targeted therapies in the treatment of cancer lies in the possibility they offer to counteract the over-expression of VEGF provoked by chemotherapeutic drugs and radiation[70]. Consistently, dacarbazine, which is used in the therapy of human melanoma, induces increased VEGF-A production[71], and dacarbazine-resistant melanoma cells show an increased in vivo growth together with an increased microvessel density[72]. These studies suggest the potential application of sanguinarine, alone or in association with other VEGF inhibitors, in the control of both angiogenesis and metastatization of solid tumors.
In solid tumors, neoplastic cells can penetrate the basement membrane by proteolysis and initiate metastatization, which accounts for the majority of cancer deaths. Metastatization is the result of the cooperation between cancer cells and a sort of “inflammed” microenvironment[73]. Consistently, inflammatory cells are an important source of proteases capable of causing a degradation of extracellular matrix, which represents a crucial event in the initiation of cancer cell invasion. Matrix metalloproteinases (MMPs) are an example of agents capable to degrade the extracellular matrix[74,75] and an over-production of these enzymes has been detected in various metastatic cancers[76-78]. Indeed, there is a strong evidence that increased expression and activation of MMP-2 and MMP-9 is present in tumor tissues but not in normal tissues in patients with breast cancer[79] and that MMP-2 induces cancer cell migration by means of its interaction with collagen[80].
Recent findings show that sanguinarine inhibits the tetradecanoylphorbolmyristate acetate (TPA)-induced breast cancer cell migration and invasion while inhibiting the expression of MMP-9, NF-κB and AP-1 signaling pathways[81]. Moreover, previous studies by Sun et al[66] have showed that sanguinarine reduces prostate cancer cell growth and invasion by the inhibition of STAT3 activation. STAT3 is constitutively active in human prostate cancer metastases and has a key role in the phenomena of tumor cell migration and invasion in different types of cancer[82-84]. Since the invasivity and/or metastatic potential of a tumor parallel its maligncy, the above findings indicate that sanguinarine may play a crucial role as a therapeutic agent in anticancer therapy not only for its ability to induce apoptosis but also for its own “anti-invasive” properties.
Several plant SMs are capable of influencing effectively the multidrug resistance phenomenon in tumor cells and are able also to “chemo-sensitize” them[85-89]. Some clinical studies have explored the possible advantage of combining natural products with classical chemotherapeutic regimens[90-92]. Phytotherapy, which employs plants extracts, is still used worldwide for the treatment of various human diseases. However, evidence has been proved that combinations of individual SM in an extract may exert synergistic effects. As an example, a recent study demonstrates that the combined use of non-toxic concentrations of sanguinarine and digitonin with doxorubicin, synergistically sensitizes Caco-2 (human colorectal adenocarcinoma) and CEM/ADR5000 adryamicin-resistant leukemia cells and increases the cytotoxicity of the chemotherapeutic agent doxorubicin[93]. In this regard, it is worth mentioning that the main advantage of combination therapies is represented by the possibility of reducing the doses and thus the toxicity of chemotherapy, while retaining its own efficacy. Thus, because of its potential synergistic interaction with chemotherapeutic agents, the therapeutic use of sanguinarine as an adjuvant, in association with chemotherapy, might be considered as a theoretical option in cancer therapy.
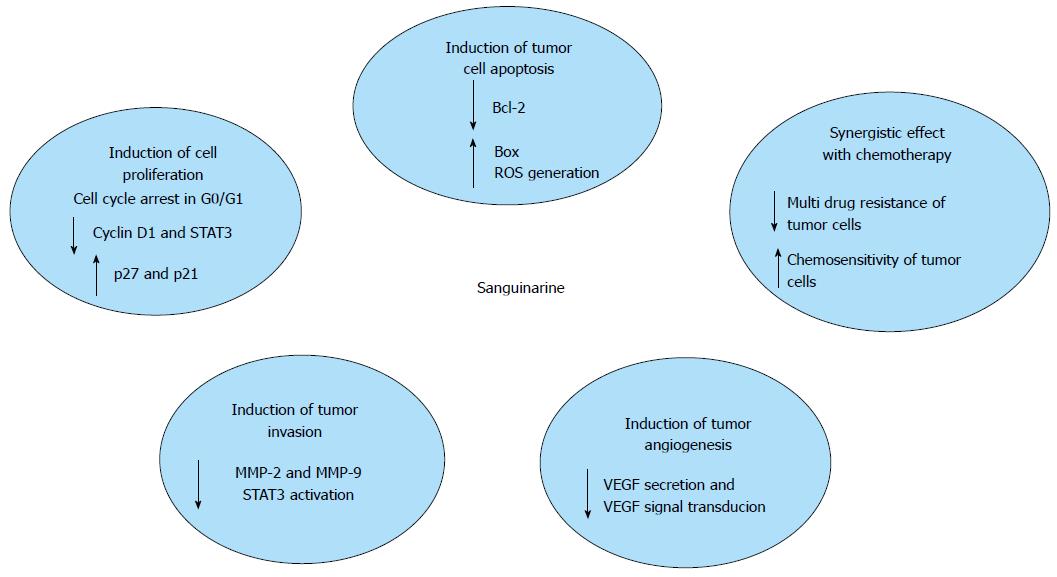
A successful resolution to the design of antitumor drugs relies, at least in part, on the possibility to overcome the intrinsic resistance to undergo apoptosis detected in many transformed cells. Findings from the studies above mentioned show that sanguinarine is capable of inhibiting tumor growth through different molecular pathways (Figure 2). A summary of the results is shown in Table 1. In conclusion, despite sanguinarine has been extensively studied, the precise mechanisms responsible for its antitumor effects still have not been completely elucidated and are strictly dependent on the cell type studied. According to the results obtained so far, it can be said that the anti-tumor action of this alkaloid is the result of a combined effect both on proliferation and invasiveness of tumor cells, that on regulation of the complex phenomena of tumor angiogenesis. In particular, owing to its pro-apoptotic potential, sanguinarine is a good candidate for the development of new anticancer therapies either when used alone or in combination with other chemotherapeutic regimens. More extensive investigation and greater caution are needed, however, to clarify the following important issues. First of all, most of the studies above mentioned have been performed in vitro using cancer cell lines, whereas there are only a few in vivo studies validating the efficacy and safety of sanguinarine administration in animal tumor models. The results of our in vivo studies confirm the effectiveness and safety of using oral sanguinarine administration to control tumor growth in rats[49]. Similar results had been previously reported in a murine melanoma model[48]. In that study, and in agreement with our findings, the anti-proliferative and anti-angiogenic effects of the oral sanguinarine administration were observed at a dosage, i.e., 5 mg/kg, devoid of apparent toxicity. On the other hand, an increase of serum levels of transaminases and LDH, hepatic vacuolization, lipid accumulation and peroxidation in the liver and a reduction of triglycerides, were observed in mice treated with high-dose sanguinarine (10 mg/kg), suggesting liver injury[94]. Previous studies showed that sanguinarine can cause physiological dysfunction in skeletal, smooth and cardiac muscles[95-97]. More recent studies clearly indicate that sanguinarine acts as a pro-apoptotic factor and alters mouse normal embryonic development at a physiological dosage, i.e., 0.5-2 μmol/L, which are obtained via dietary intake[98]. These experimental results need further confirmation in view of the possible administration of the compound in pregnancy, although at present no teratogenic effects have been reported in humans.
| Sanguinarine induces apoptosis in tumor cells through multiple pathways, including the activation of NF-κB, the mitochondrial damage and cell cycle arrest |
| Sanguinarine-induced apoptosis is associated with the decrease of Bcl-2 and the increase of Bax proteins and the generation of reactive oxygen species |
| Sanguinarine causes cell cycle arrest by increasing the expression of p27 and decreasing cyclin D1, D2 and E, and CDK2, 4 and 6 |
| Sanguinarine inhibits tumor progression associated with chronic inflammation via the inhibition of NF-κB |
| Sanguinarine inhibits tumor angiogenesis through the inhibition of VEGF secretion and VEGF signal transduction (Akt, p38 and VE-cadherin) |
| Sanguinarine has an inhibitory effect on tumor cell migration by the inhibition of MMP-9 and STAT3 activation |
| Sanguinarine exerts a synergistic effect with chemotherapeutic agents and enhances the chemosensitivity of Caco 2 and CEM/ADR5000 adryamicin-resistant leukemia cells |
Most of the studies actually known have reported that sanguinarine exerts cytotoxic activity selectively on cancer cells. Consistently, sanguinarine is a negative regulator of human epidermoid carcinoma cells (A431) but not of normal epidermal keratinocytes[23]. Evidence of this differential activity have been reported recently, showing that mouse lymphocytic leukemic cells are more sensitive to sanguinarine than normal splenocytes[99].
It is a matter of fact, however, that sanguinarine has been listed as responsible for the toxicity of Argemone mexicana seed oil[100-102]. Das et al[103] reported that topical use of argemone oil (0.15-0.3 mL) or sanguinarine (4.5-18 μmol/L) followed by application of TPA induces tumor development in a murine experimental model. Ansari et al[104] also reported that intraperitoneal administration of sanguinarine induces DNA damage in Swiss albino mice. Sanguinarine in argemone oil, is suspected to cause glaucoma[101,102]. Argemone oil increases incidence of bladder cancer in animal models[103] and of gall bladder cancer in humans[104]. Furthermore, sanguinarine extract from bloodroot (Sanguinaria canadensis), previously used in oral hygiene products, was discontinued until a link between product administration and occurrence of leukoplakia was established[105,106]. Hepatic microsomes transform sanguinarine in a mutagenic epoxide and the same sanguinarine is capable of activating polycyclic aromatic hydrocarbon signaling[107]. However, related to this topic, the results available in literature are not univocal[3]. So that is still not clear if sanguinarine may act as a carcinogenic without the cooperation of other risk factors or it is capable of acting in concert with various co-carcinogens. In light of the above facts, the possibility of obtaining beneficial effects in humans by using sanguinarine remains largely unpredictable.
Finally, since at present there is increasing interest in nanotechnology application in cancer therapy and in order to prevent the potential toxic and/or side effects induced by sanguinarine administration, in vivo studies might be performed in experimental tumor models by encapsulating the alkaloid in tumor-targeted nanoparticles[108], which accumulate preferentially in tumors recognizing single cancer cells for diagnosis and treatment. Actually, the administration of sanguinarine (10 mg/kg) per os and encapsulated by lipid nanoparticles (SG-SLNs), has been shown to induce an anti-inflammatory effect in an LPS-induced endotoxin shock murine model, and the pharmacokinetic studies have proved that the AUC0-24 and Cmax of SG-SLNs were significantly increased when compared to those of sanguinarine alone[109].
In conclusion, several studies indicate the potential application of sanguinarine as an adjuvant in the therapy of cancer, but further detailed pharmacokinetic and toxicology studies, which have to be conducted in appropriate experimental tumor models, are absolutely required to assess the efficacy and safety of this compound before such an antitumor strategy can be translated in clinical trials.
P- Reviewer: Batistoni R, Mohammad RM S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19503] [Article Influence: 780.1] [Reference Citation Analysis (0)] |
| 2. | Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 312] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Dvorak Z, Kuban V, Kledjus B, Hlavac J, Vicar J, Ulrichova J, Simanek V. Quaternary benzo[c]phenanthridines sanguinarine and chelerythrine: a review of investigations from chemical and biological studies. Heterocycles. 2006;68:2403-2422. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Dostàl J, Slavik J. Some aspects of the chemistry of quaternary benzo[c]phenanthridines alkaloids. Stud Nat Prod Chem. 2002;27:155-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Psotova J, Vecera R, Zdarilova A, Anzebacherova E, Kosina P, Svobodova A, Hrbac J, Jirovsky D, Stiborova M, Lichnovsky V. Safety assessment of sanguitrin, alkaloid fraction of Macleaya cordata, in rats. Vet Med. 2006;51:145-155. |
| 6. | Kosina P, Walterová D, Ulrichová J, Lichnovský V, Stiborová M, Rýdlová H, Vicar J, Krecman V, Brabec MJ, Simánek V. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem Toxicol. 2004;42:85-91. [PubMed] [DOI] [Full Text] |
| 7. | Slaninova I, Pencikova K, Urbanova J, Slanina J, Taborska E. Antitumor activities of sanguinarine and related alkaloids. Phytochem Rev. 2014;13:51-68. [DOI] [Full Text] |
| 8. | Miao F, Yang XJ, Zhou L, Hu HJ, Zheng F, Ding XD, Sun DM, Zhou CD, Sun W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat Prod Res. 2011;25:863-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Yang XJ, Miao F, Yao Y, Cao FJ, Yang R, Ma YN, Qin BF, Zhou L. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules. 2012;17:13026-13035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Lenfeld J, Kroutil M, Marsálek E, Slavík J, Preininger V, Simánek V. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 1981;43:161-165. [PubMed] |
| 11. | Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J Nat Prod. 1991;54:143-154. [PubMed] |
| 12. | Tsai IL, Wun MF, Teng CM, Ishikawa T, Chen IS. Anti-platelet aggregation constituents from Formosan Toddalia asiatica. Phytochemistry. 1998;48:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Eun JP, Koh GY. Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem Biophys Res Commun. 2004;317:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Miao F, Yang XJ, Ma YN, Zheng F, Song XP, Zhou L. Structural modification of sanguinarine and chelerythrine and their in vitro acaricidal activity against Psoroptes cuniculi. Chem Pharm Bull (Tokyo). 2012;60:1508-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yao JY, Li XL, Shen JY, Pan XY, Hao GJ, Xu Y, Ying WL, Ru HS, Liu XL. Isolation of bioactive components from Chelidonium majus L. with activity against Trichodina sp. Aquaculture. 2011;318:235-238. [DOI] [Full Text] |
| 16. | Wang GX, Zhou Z, Jiang DX, Han J, Wang JF, Zhao LW, Li J. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet Parasitol. 2010;171:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 1753] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 18. | Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 698] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 19. | Ahsan H, Reagan-Shaw S, Breur J, Ahmad N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007;249:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Choi WY, Kim GY, Lee WH, Choi YH. Sanguinarine, a benzophenanthridine alkaloid, induces apoptosis in MDA-MB-231 human breast carcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. Chemotherapy. 2008;54:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Han MH, Kim GY, Yoo YH, Choi YH. Sanguinarine induces apoptosis in human colorectal cancer HCT-116 cells through ROS-mediated Egr-1 activation and mitochondrial dysfunction. Toxicol Lett. 2013;220:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Jang BC, Park JG, Song DK, Baek WK, Yoo SK, Jung KH, Park GY, Lee TY, Suh SI. Sanguinarine induces apoptosis in A549 human lung cancer cells primarily via cellular glutathione depletion. Toxicol In Vitro. 2009;23:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Ahmad N, Gupta S, Husain MM, Heiskanen KM, Mukhtar H. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528. [PubMed] |
| 24. | Adhami VM, Aziz MH, Mukhtar H, Ahmad N. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182. [PubMed] |
| 25. | Reagan-Shaw S, Breur J, Ahmad N. Enhancement of UVB radiation-mediated apoptosis by sanguinarine in HaCaT human immortalized keratinocytes. Mol Cancer Ther. 2006;5:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Malíková J, Zdarilová A, Hlobilková A, Ulrichová J. The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol Toxicol. 2006;22:439-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Adhami VM, Aziz MH, Reagan-Shaw SR, Nihal M, Mukhtar H, Ahmad N. Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol Cancer Ther. 2004;3:933-940. [PubMed] |
| 28. | Huh J, Liepins A, Zielonka J, Andrekopoulos C, Kalyanaraman B, Sorokin A. Cyclooxygenase 2 rescues LNCaP prostate cancer cells from sanguinarine-induced apoptosis by a mechanism involving inhibition of nitric oxide synthase activity. Cancer Res. 2006;66:3726-3736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Ding Z, Tang SC, Weerasinghe P, Yang X, Pater A, Liepins A. The alkaloid sanguinarine is effective against multidrug resistance in human cervical cells via bimodal cell death. Biochem Pharmacol. 2002;63:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Debiton E, Madelmont JC, Legault J, Barthomeuf C. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482. [PubMed] |
| 31. | Holy J, Lamont G, Perkins E. Disruption of nucleocytoplasmic trafficking of cyclin D1 and topoisomerase II by sanguinarine. BMC Cell Biol. 2006;7:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Dong XZ, Zhang M, Wang K, Liu P, Guo DH, Zheng XL, Ge XY. Sanguinarine inhibits vascular endothelial growth factor release by generation of reactive oxygen species in MCF-7 human mammary adenocarcinoma cells. Biomed Res Int. 2013;2013:517698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kalogris C, Garulli C, Pietrella L, Gambini V, Pucciarelli S, Lucci C, Tilio M, Zabaleta ME, Bartolacci C, Andreani C. Sanguinarine suppresses basal-like breast cancer growth through dihydrofolate reductase inhibition. Biochem Pharmacol. 2014;90:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Han MH, Yoo YH, Choi YH. Sanguinarine-induced apoptosis in human leukemia U937 cells via Bcl-2 downregulation and caspase-3 activation. Chemotherapy. 2008;54:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Weerasinghe P, Hallock S, Tang SC, Liepins A. Role of Bcl-2 family proteins and caspase-3 in sanguinarine-induced bimodal cell death. Cell Biol Toxicol. 2001;17:371-381. [PubMed] |
| 36. | Hussain AR, Al-Jomah NA, Siraj AK, Manogaran P, Al-Hussein K, Abubaker J, Platanias LC, Al-Kuraya KS, Uddin S. Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer Res. 2007;67:3888-3897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Burgeiro A, Bento AC, Gajate C, Oliveira PJ, Mollinedo F. Rapid human melanoma cell death induced by sanguinarine through oxidative stress. Eur J Pharmacol. 2013;705:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Hammerová J, Uldrijan S, Táborská E, Slaninová I. Benzo[c] phenanthridine alkaloids exhibit strong anti-proliferative activity in malignant melanoma cells regardless of their p53 status. J Dermatol Sci. 2011;62:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Serafim TL, Matos JA, Sardão VA, Pereira GC, Branco AF, Pereira SL, Parke D, Perkins EL, Moreno AJ, Holy J. Sanguinarine cytotoxicity on mouse melanoma K1735-M2 cells--nuclear vs. mitochondrial effects. Biochem Pharmacol. 2008;76:1459-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Lee JS, Jung WK, Jeong MH, Yoon TR, Kim HK. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int J Toxicol. 2012;31:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Matkar SS, Wrischnik LA, Hellmann-Blumberg U. Sanguinarine causes DNA damage and p53-independent cell death in human colon cancer cell lines. Chem Biol Interact. 2008;172:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Choi WY, Jin CY, Han MH, Kim GY, Kim ND, Lee WH, Kim SK, Choi YH. Sanguinarine sensitizes human gastric adenocarcinoma AGS cells to TRAIL-mediated apoptosis via down-regulation of AKT and activation of caspase-3. Anticancer Res. 2009;29:4457-4465. [PubMed] |
| 43. | Larsson DE, Wickström M, Hassan S, Oberg K, Granberg D. The cytotoxic agents NSC-95397, brefeldin A, bortezomib and sanguinarine induce apoptosis in neuroendocrine tumors in vitro. Anticancer Res. 2010;30:149-156. [PubMed] |
| 44. | Park H, Bergeron E, Senta H, Guillemette K, Beauvais S, Blouin R, Sirois J, Faucheux N. Sanguinarine induces apoptosis of human osteosarcoma cells through the extrinsic and intrinsic pathways. Biochem Biophys Res Commun. 2010;399:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Cecen E, Altun Z, Ercetin P, Aktas S, Olgun N. Promoting effects of sanguinarine on apoptotic gene expression in human neuroblastoma cells. Asian Pac J Cancer Prev. 2014;15:9445-9451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Deroussent A, Ré M, Hoellinger H, Cresteil T. Metabolism of sanguinarine in human and in rat: characterization of oxidative metabolites produced by human CYP1A1 and CYP1A2 and rat liver microsomes using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Lopez Lozano MJ, Rios Santos V, Bullon Fernandez P. [Effectiveness of chemical products as antiplaque agents]. Rev Eur Odontoestomatol. 1991;3:115-122. [PubMed] |
| 48. | De Stefano I, Raspaglio G, Zannoni GF, Travaglia D, Prisco MG, Mosca M, Ferlini C, Scambia G, Gallo D. Antiproliferative and antiangiogenic effects of the benzophenanthridine alkaloid sanguinarine in melanoma. Biochem Pharmacol. 2009;78:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Pica F, Balestrieri E, Serafino A, Sorrentino R, Gaziano R, Moroni G, Moroni N, Palmieri G, Mattei M, Garaci E. Antitumor effects of the benzophenanthridine alkaloid sanguinarine in a rat syngeneic model of colorectal cancer. Anticancer Drugs. 2012;23:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Chaturvedi MM, Kumar A, Darnay BG, Chainy GB, Agarwal S, Aggarwal BB. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-kappaB activation, IkappaBalpha phosphorylation, and degradation. J Biol Chem. 1997;272:30129-30134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Malikova J, Zdarilova A, Hlobilkova A. Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:5-12. [PubMed] |
| 52. | Kim S, Lee TJ, Leem J, Choi KS, Park JW, Kwon TK. Sanguinarine-induced apoptosis: generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J Cell Biochem. 2008;104:895-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L, Rosen EM, Fan S. Sanguinarine inhibits growth of human cervical cancer cells through the induction of apoptosis. Oncol Rep. 2012;28:2264-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Xu ZW, Friess H, Büchler MW, Solioz M. Overexpression of Bax sensitizes human pancreatic cancer cells to apoptosis induced by chemotherapeutic agents. Cancer Chemother Pharmacol. 2002;49:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Reed JC. Bcl-2: prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995;9:451-473. [PubMed] |
| 56. | Slaninová I, Slanina J, Táborská E. Quaternary benzo[c]phenanthridine alkaloids--novel cell permeant and red fluorescing DNA probes. Cytometry A. 2007;71:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Saran A, Srivastava S, Coutinho E, Maiti M. 1H NMR investigation of the interaction of berberine and sanguinarine with DNA. Indian J Biochem Biophys. 1995;32:74-77. [PubMed] |
| 58. | Chang MC, Chan CP, Wang YJ, Lee PH, Chen LI, Tsai YL, Lin BR, Wang YL, Jeng JH. Induction of necrosis and apoptosis to KB cancer cells by sanguinarine is associated with reactive oxygen species production and mitochondrial membrane depolarization. Toxicol Appl Pharmacol. 2007;218:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Carmody RJ, Cotter TG. Signalling apoptosis: a radical approach. Redox Rep. 2001;6:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 240] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 60. | Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1409] [Cited by in RCA: 1676] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 61. | Simon L, Szilágyi G, Bori Z, Telek G, Magyar K, Nagy Z. Low dose (-)deprenyl is cytoprotective: it maintains mitochondrial membrane potential and eliminates oxygen radicals. Life Sci. 2005;78:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 63. | Kim IH, Kim SW, Kim SH, Lee SO, Lee ST, Kim DG, Lee MJ, Park WH. Parthenolide-induced apoptosis of hepatic stellate cells and anti-fibrotic effects in an in vivo rat model. Exp Mol Med. 2012;44:448-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 624] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 65. | Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 2963] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 66. | Sun M, Liu C, Nadiminty N, Lou W, Zhu Y, Yang J, Evans CP, Zhou Q, Gao AC. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate. 2012;72:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Basini G, Bussolati S, Santini SE, Grasselli F. Sanguinarine inhibits VEGF-induced angiogenesis in a fibrin gel matrix. Biofactors. 2007;29:11-18. [PubMed] |
| 68. | Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L, Rosen EM, Fan S. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Basini G, Santini SE, Bussolati S, Grasselli F. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. [PubMed] |
| 70. | Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1284] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 71. | Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003;2:753-763. [PubMed] |
| 72. | Lev DC, Onn A, Melinkova VO, Miller C, Stone V, Ruiz M, McGary EC, Ananthaswamy HN, Price JE, Bar-Eli M. Exposure of melanoma cells to dacarbazine results in enhanced tumor growth and metastasis in vivo. J Clin Oncol. 2004;22:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8174] [Article Influence: 544.9] [Reference Citation Analysis (0)] |
| 74. | Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 2887] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 75. | Jiang MC, Liao CF, Lee PH. Aspirin inhibits matrix metalloproteinase-2 activity, increases E-cadherin production, and inhibits in vitro invasion of tumor cells. Biochem Biophys Res Commun. 2001;282:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1213] [Cited by in RCA: 1145] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 77. | Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1501] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 78. | Jinga DC, Blidaru A, Condrea I, Ardeleanu C, Dragomir C, Szegli G, Stefanescu M, Matache C. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: correlations with prognostic factors. J Cell Mol Med. 2006;10:499-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 80. | Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65:130-136. [PubMed] |
| 81. | Park SY, Jin ML, Kim YH, Lee SJ, Park G. Sanguinarine inhibits invasiveness and the MMP-9 and COX-2 expression in TPA-induced breast cancer cells by inducing HO-1 expression. Oncol Rep. 2014;31:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 83. | Itoh M, Murata T, Suzuki T, Shindoh M, Nakajima K, Imai K, Yoshida K. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2006;25:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 84. | Silver DL, Naora H, Liu J, Cheng W, Montell DJ. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64:3550-3558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 85. | Möller M, Weiss J, Wink M. Reduction of cytotoxicity of the alkaloid emetine through P-glycoprotein (MDR1/ABCB1) in human Caco-2 cells and leukemia cell lines. Planta Med. 2006;72:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Ma Y, Wink M. Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine. 2008;15:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol. 2010;626:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Li S, Lei Y, Jia Y, Li N, Wink M, Ma Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine. 2011;19:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Wink M, Ashour ML, El-Readi MZ. Secondary Metabolites from Plants Inhibiting ABC Transporters and Reversing Resistance of Cancer Cells and Microbes to Cytotoxic and Antimicrobial Agents. Front Microbiol. 2012;3:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Li JH. [A study on treatment of lung cancer by combined therapy of traditional Chinese medicine and chemotherapy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16:136-138. [PubMed] |
| 91. | Cai HB, Dai FG, Min QF, Shi M, Miao JX, Luo RC. [Clinical study of the effects of radiotherapy in combination with traditional Chinese medicine on non-small cell lung cancer]. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:1112-1113. [PubMed] |
| 92. | Efferth T, Davey M, Olbrich A, Rücker G, Gebhart E, Davey R. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol Dis. 2002;28:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 93. | Eid SY, El-Readi MZ, Wink M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine. 2012;19:1288-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Choy CS, Cheah KP, Chiou HY, Li JS, Liu YH, Yong SF, Chiu WT, Liao JW, Hu CM. Induction of hepatotoxicity by sanguinarine is associated with oxidation of protein thiols and disturbance of mitochondrial respiration. J Appl Toxicol. 2008;28:945-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Hu CM, Cheng HW, Cheng YW, Kang JJ. Induction of skeletal muscle contracture and calcium release from isolated sarcoplasmic reticulum vesicles by sanguinarine. Br J Pharmacol. 2000;130:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Hu CM, Cheng HW, Cheng YW, Kan JJ. Mechanisms underlying the induction of vasorelaxation in rat thoracic aorta by sanguinarine. Jpn J Pharmacol. 2001;85:47-53. [PubMed] |
| 97. | Hu CM, Cheng YW, Liao JW, Cheng HW, Kang JJ. Induction of contracture and extracellular Ca2+ influx in cardiac muscle by sanguinarine: a study on cardiotoxicity of sanguinarine. J Biomed Sci. 2005;12:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Chan WH. Embryonic toxicity of sanguinarine through apoptotic processes in mouse blastocysts. Toxicol Lett. 2011;205:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Kaminskyy V, Lin KW, Filyak Y, Stoika R. Differential effect of sanguinarine, chelerythrine and chelidonine on DNA damage and cell viability in primary mouse spleen cells and mouse leukemic cells. Cell Biol Int. 2008;32:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Das M, Khanna SK. Clinicoepidemiological, toxicological, and safety evaluation studies on argemone oil. Crit Rev Toxicol. 1997;27:273-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Hakim SA. Argemone oil, sanguinarine, and epidemic-dropsy glaucoma. Br J Ophthalmol. 1954;38:193-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Hakim SA. Argemone oil, sanguinarine, dropsy, glaucoma and cancer. Indian Pract. 1967;20:129-141. [PubMed] |
| 103. | Das M, Ansari KM, Dhawan A, Shukla Y, Khanna SK. Correlation of DNA damage in epidemic dropsy patients to carcinogenic potential of argemone oil and isolated sanguinarine alkaloid in mice. Int J Cancer. 2005;117:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Ansari KM, Dhawan A, Khanna SK, Das M. In vivo DNA damaging potential of sanguinarine alkaloid, isolated from argemone oil, using alkaline Comet assay in mice. Food Chem Toxicol. 2005;43:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Anderson KM, Stoner GD, Fields HW, Chacon GE, Dohar AL, Gregg BR, Mallery SR. Immunohistochemical assessment of Viadent-associated leukoplakia. Oral Oncol. 2005;41:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Damm DD, Curran A, White DK, Drummond JF. Leukoplakia of the maxillary vestibule--an association with Viadent? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Karp JM, Rodrigo KA, Pei P, Pavlick MD, Andersen JD, McTigue DJ, Fields HW, Mallery SR. Sanguinarine activates polycyclic aromatic hydrocarbon associated metabolic pathways in human oral keratinocytes and tissues. Toxicol Lett. 2005;158:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 596] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 109. | Li W, Li H, Yao H, Mu Q, Zhao G, Li Y, Hu H, Niu X. Pharmacokinetic and anti-inflammatory effects of sanguinarine solid lipid nanoparticles. Inflammation. 2014;37:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |