INTRODUCTION
Colorectal cancers are one of the most common cancers worldwide, and it is the second most common diagnosed cancer in women and third in men[1]. Acute malignant colorectal obstruction is a complication of colorectal cancer that can occur in 7%-29% of patients[2]. It is a life-threatening condition that needs prompt evaluation. Large bowel obstruction causes colonic dilatation, bacterial translocation, and electrolyte and fluid imbalance, and has an increased risk of colonic necrosis and perforation[3]. The main treatment of malignant colonic obstruction is resection of the tumor; however, in the past two decades, the use of self-expanding metallic stents (SEMS) has drawn interest since it was first reported in 1991 by Dohmoto[4] for palliation of malignant colonic obstruction.
The major indications of SEMS for colonic stenting are palliation of malignant obstruction and preoperative decompression[5]. Additionally, extracolonic obstructions due to other malignancies and some benign diseases have been shown to be treated by SEMS[3]. Although SEMS placement for treating malignant obstruction seems safe and effective and has some advantages over surgery, short term and long term morbidities should be kept in mind. In this review, we aimed to evaluate SEMS in the treatment of colorectal and extracolonic cancers with advantages and disadvantages of this technique over surgery.
STENT PLACEMENT TECHNIQUE
SEMS placement can be performed by using endoscopic guidance with or without the use of fluoroscopy. It can be inserted through the scope (TTS) or over the guidewire[5]. Most of the SEMS are inserted endoscopically with TTS with the use of fluoroscopy[6]. SEMS placement with endoscope has advantages over the radiologic placement when the obstruction is proximal to the rectosigmoid region or in the presence of a tortuous colon. Kim et al[7] evaluated the technical feasibility and clinical effectiveness of fluoroscopically guided placement of SEMS in 42 patients for acute malignant colorectal obstruction; clinical success was achieved in 98% of the patients. They stated that fluoroscopically guided placement was feasible without endoscopic assistance, even in lesions proximal to the splenic flexure and transverse colon. In a multicenter retrospective study, Geraghty et al[8] aimed to determine the outcomes after SEMS; TTS endoscopy technique was found to be more successful than radiological placement alone (90.3% vs 74.8%, P < 0.001) for large bowel obstruction. In another retrospective study, Kim et al[9] compared the SEMS placement technique in 111 patients; while the technical success rate was significantly higher in the endoscopic method than in the radiologic method (100% vs 92.1%, respectively, P = 0.038), the clinical success rate did not differ significantly between the two groups (91.8% vs 97.1%, respectively, P = 0.424). They concluded that endoscopic and radiologic placement technique have their own advantages and disadvantages, but when an obstructive lesion is located in the tortuous, curved angulation of the sigmoid or descending colons, it is more difficult to pass the stenotic lesion using the radiologic method alone.
Bowel preparation before stent placement is not necessary, and oral bowel cleansing is contraindicated in symptomatic bowel obstruction, but enema can be used for facilitating the stent placement by preparing the bowel distal to the stenosis[5].
Antibiotic prophylaxis before SEMS placement is not recommended routinely because of the low risk of fever and bacteremia after the insertion[5]. However, antibiotic prophylaxis should be considered especially in patients with complete obstruction who have dilated colon and a risk of microperforation during insertion[10].
Operator experience is an important matter in the placement of the stents. In a retrospective study, SEMS placement was performed in 334 patients, and technical and clinical success was higher for operators who had performed more than 10 procedures[8]. In another study, Small et al[11] reported that the complication rate was higher when stents were placed by endoscopists who were not experienced in pancreaticobiliary endoscopy.
Technical success is usually defined as stent placement appropriately across the entire length of the stenosis, and clinical success is defined as resolution of colonic obstruction within the first days after the stent placement[6]. Technical and clinical success rates vary between the studies. In a systematic review focusing on 88 studies published in 2007 by Watt et al[12] the median rate of technical success was 96.2%, ranging from 66.6% to 100%, and clinical success was achieved in 92% of the cases, ranging from 46% to 100%. It was stated that the etiology of the primary obstruction and indication for the stent placement appeared to have little effect on the rates of technical and clinical success. In a recent meta-analysis that included seven randomized clinical trials, pooled data showed a mean success rate of 76.9% (range: 46.7%-100%)[13]. In another meta-analysis, Cennamo et al[14] compared randomized trials in terms of endoscopic stenting and surgical decompression for colorectal cancer obstruction; the stents were successfully inserted in 73.5% of patients, with clinical relief of obstruction in 72% of patients.
Covered and uncovered SEMS can be used for colonic stenting. In a meta-analysis, in which Zhang et al[15] compared covered and uncovered stents, uncovered stents were found to be associated with a lower late migration rate, a higher tumor ingrowth rate, and a prolonged stent patency. No significant difference was found in technical success, clinical success, tumor overgrowth, early migration, perforation and overall complications between type of stents. In another meta-analysis including a total of 1376 patients, Yang et al[16] compared covered and uncovered SEMS in terms of technical success, clinical success and stent patency, and no significant difference was found between the two groups. Uncovered stents were found to be more prone to tumor ingrowth, but covered stents had the higher risk of stent migration over uncovered stents. Each type of stent have their own advantages and disadvantages. The main advantage of covered stents is a reduction in the risk of tissue ingrowth, whereas they are more prone to migrate.
INDICATONS OF SEMS
Palliation of malignant obstruction
Acute malignant colorectal obstruction is a complication of colorectal cancer that can occur in 7%-29% of patients[2]. Bowel obstruction can be caused by intrinsic disease or extrinsic compression. Large bowel obstruction causes colonic dilatation, bacterial translocation, electrolyte and fluid imbalance, and has an increased risk of colonic necrosis and perforation, so this gastrointestinal emergency needs urgent evaluation. The main treatment modalities for malignant colorectal obstruction are surgical resection or diverting colostomy. Resection is a suitable procedure in patients with less advanced cancer. Permanent stoma creation is a procedure for relieving symptoms of obstruction in patients with nonresectable tumors[12]. Emergent surgery should be performed for the patients with colonic perforation and ischemia/necrosis. If there are no signs of systemic toxicity, SEMS can be performed in patients with a partial obstruction or with complete obstruction. SEMS is an alternative procedure for relieving the obstruction of the colon. In the literature, several studies have been published showing the feasibility and safety of SEMS in the management of acute malignant obstruction. In 2007, Watt et al[12] compared the safety and efficacy of SEMS with surgery through a systematic review. SEMS was found to be effective and safe in overcoming left-sided malignant colorectal obstructions, with high levels of technical and clinical success, shorter hospital stay, and lower rate of serious adverse events than surgery. Zhao et al[17] compared surgery with SEMS in the relief of obstruction in a meta-analysis, and the SEMS group showed a lower clinical success rate (99.8% vs 93.1%, P = 0.0009) but shorter length of hospital stay (18.84 d vs 9.55, P < 0.00001) and time to initiation of chemotherapy (33.36 d vs 15.53 d, P < 0.00001), and lower rate of stoma formation (54.0% vs 12.7%, P < 0.00001). Hospital mortality was significantly lower in the SEMS group, and no difference was found in overall complications between the two groups. Surgery was found to be associated with short term complications and SEMS with late term complications[17]. Liang et al[18] compared SEMS with surgery in the same indication mentioned above in a meta-analysis; the success rate of SEMS was found to be 93.9%, and no significant difference was found in mortality between the groups. The hospitalization time was shorter in the SEMS group (P < 0.01); however, long term complications were higher than surgery (P = 0.03). However, it was mentioned that all of the studies reported only the complications of colostomy or Hartmann’s procedure, and none of them considered the complications of the stoma. They stated that morbidity and mortality would be much higher in the multi-stage surgery than with SEMS. In a recent study Young et al[19] compared the stent insertion and surgical decompression in patients with incurable large bowel obstruction in terms of improving quality of life. Stent related perforations or deaths were not reported. They found that surgery group had significantly reduced quality of life compared with the stent group. The patients in the stent group was found to have significantly lower permanent stoma rates, reductions in post procedure stay, earlier return of bowel function and shorter hospital stay. Thirty-day mortality for the stent group was 8% and for the surgery was 15% (P = 0.67). No significant difference was found in survival rates between treatment groups (P = 0.61)[19].
Placement of SEMS as a bridge to elective surgery
SEMS have been suggested to relieve colon obstruction and act as a “bridge to surgery” for resectable colon cancers. There are conflicting results on this subject. In the literature, systematic reviews with meta-analysis have been published in order to evaluate preoperative SEMS placement as a bridge to elective surgery with emergency resection for acute malignant left-sided colonic obstruction. In the most recent meta-analysis that included seven randomized clinical trials, Huang compared emergency surgery and SEMS group. The pooled data showed a mean success rate of colonic stent placement of 76.9% (range: 46.7%-100%). Compared with the emergency surgery group, the SEMS group achieved significantly lower rates of permanent stoma (9% vs 27.4%, P < 0.01), primary anastomosis (67.2% vs 55.1%, P < 0.01), lower overall complications (33.1% vs 53.9%, P = 0.03) and lower wound infections (6.7% vs 18.1%, P < 0.01). No significant difference was found between the two groups in anastomotic leakage, mortality, or intra-abdominal infection. In this setting, SEMS placement for relieving obstructive symptoms allows time for the optimization of the medical condition, bowel preparation, and staging the disease[13]. Although there are some advantages of SEMS placement preoperatively compared to the emergency surgery, long term oncological outcome, especially in patients with resectable colon cancer, should be kept in mind.
There has been a major concern about the oncological outcome of the patients with resectable colon cancer who received SEMS placement as a bridge to surgery. In the literature, it was shown that placement of SEMS preoperatively in patients with resectable colon cancer impairs the oncological outcome, because of the dissemination of cancer cells during the procedure[20], and because stent placement will be complicated by perforation and associated with ulceration as well as perineural and lymph node invasion of the tissues[21]. Alcántara et al[22] compared short-term results and long-term outcomes of patients who underwent stent placement preoperatively with intraoperative colonic lavage with primary anastomosis. More relapses occured in the SEMS group, but this finding was not significant, and no differences were found in survival. In another study, Sloothaak et al[23] compared 5-year overall recurrence rates in the SEMS placement as a bridge to surgery group with the emergency surgery group; the SEMS group was found to have higher recurrence rate (42% vs 25%, P = 0.027). In a larger prospective study that evaluated the long-term oncological outcome between the same groups, in patients aged ≤ 75 years, stent as a bridge to surgery was associated with a higher local recurrence rate compared with emergency surgery (32% vs 8%, P = 0.038) without a difference in the overall survival rates[24]. These findings suggest that use of SEMS in the treatment of curable patients with left-sided malignant colonic obstruction will impair the oncologic outcome. In the recent guidelines, as stent seems to impact the oncological safety with no reduction in postoperative mortality, SEMS as a bridge to elective surgery in curable patients with left-sided malignant colonic obstruction is not recommended. However, this procedure may be a good option for selected patients with a high risk of postoperative mortality, and patients over 70 years old and/or with American Society of Anesthesiologists score ≥ III[5].
Palliation of extracolonic malignant obstruction
Colonic obstructions also can occur due to tumor invasion, peritoneal seeding, or extraluminal compression resulting from advanced extracolonic malignancy[25]. Outcomes of SEMS placement in the treatment of extracolonic malignancies are unclear. There have been published studies that compared the clinical outcomes of SEMS between patients with colon cancer and with extracolonic malignancies. Kim et al[25] performed SEMS placement for colorectal cancer in 149 patients and for extracolonic malignancy in 60 patients. Advanced gastric cancer, pancreatic cancer, and ovarian cancer were the most common causes of obstruction in that study. The clinical success rates, complications, and stent patency were similar between the two groups. In another study, Kim et al[26] evaluated the clinical outcomes and complications of SEMS compared with emergency surgery for relieving obstruction; technical and clinical success rates were higher in the emergency surgery group. SEMS related complications occurred in 64.5% of the patients, including reobstruction (36.8%), stent migration (10.5%), perforation (13.2%), and bleeding (3.9%). In a retrospective study of palliative stent placement for extracolonic malignancies, clinical success was significantly higher in patients with colorectal cancer than in those with extracolonic malignancies (94.1% vs 20%, P < 0.0001). Two procedure related deaths occured in the extracolonic malignancy group. Colon stenting for this purpose was found to be less successful in comparison with patients with colorectal cancer[27].
SEMS for nonmalignant etiologies
Colonic stents have been used in avariety of nonmalignant conditions as colonic strictures, including anastomotic strictures, Crohn’s disease, radiation therapy, and diverticular disease[3]. In a retrospective study, Keränen et al[28] evaluated a total of 21 patients with 23 SEMS procedures for benign colorectal obstruction; eight of the patients had an obstruction in the surgical anastomosis, two patients had anastomotic strictures due to Crohn’s disease, 10 patients had the obstruction due to diverticular disease, and one patient had a stricture after radiation therapy. Technical success was achieved in all patients, and clinical success was achieved in 76% of the patients; complications occurred for 9 patients in 10 out of 23 procedures. They concluded that SEMS placement for benign colon strictures may be a good option for the patients who are not fit for surgery. Pommergaard et al[29] performed a retrospective study that included 45 patients with benign and malignant colonic obstruction: Technical and clinical success was 97.4% of the patients with malignant etiology, complications occurred in 21%, and mortality rate was 2.6%. For benign etiology, technical success was 85.7%, and clinical success was 71.4%, and complications occurred in 71.4% in this group with a mortality rate of 28.6%.
COMPLICATIONS OF SEMS
Stent related complications can occur in patients with malignant colon obstruction in the palliative or bridge to surgery setting. The most important complications of the stents are perforation, stent obstruction, stent migration, and bleeding. The most seen complication is the stent obstruction because of the tumor ingrowth or overgrowth[30]. The main stent related complications are discussed above.
Perforation
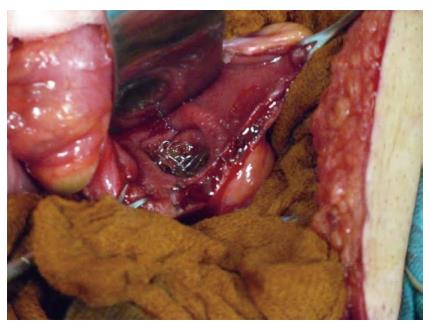
The incidence of colonic perforation after SEMS placement varies between the studies. Perforation is the most feared complication of SEMS. In a review that included a total of 2287 patients, Datye et al[31] found overall perforation rate was 4.9%. No significant difference was found in the perforation rates for palliation and bridge to surgery (4.8% vs 5.4%, P = 0.66). The mortality rate after perforation was 16.2%. Most of the perforations (over 80%) occured within 30 d of stent placement, and almost half of these were noted during or within one day of the procedure; the majority of them were related to dilation, the guidewire, or the stent. In another systematic review, Watt et al[12] reported the median rate of perforation caused by either the guidewire or stent was 4.5% (range: 0%-83%). The perforation rate was not found to be affected by the indication for stent placement. Colon perforation can be immediate or delayed, and is more likely to occur in the distal colon where sharp angulation and redundancy make stent deployment challenging[32]. Baron et al[10] identified the reasons that can cause perforation after stent placement in four different types: (1) guidewire or catheter malpositioning; (2) dilation of the stricture before or after stent placement; (3) stent-induced perforation; and (4) caused by proximal colonic distention away from the site of stent placement because of inadequate colonic decompression or excessive air insufflation. Delayed perforation can be tumor related, drug related (bevacizumab), and stent related[33]. Figure 1 shows the perforation of the rectum due to the SEMS intraoperatively.
Figure 1 Perforation of the rectum due to the self-expanding metallic stent detected intraoperatively.
In the literature, published results have shown that patients who have undergone palliative stenting can be treated with chemotherapy without antiangiogenic agents[34]. Safety of SEMS in the colon or rectum of patients who are receiving the anti-angiogenic agent bevacizumab as a component of chemotherapy has been studied in the literature. Radiotherapy and bevacizumab may increase the risk of perforation. In a retrospective study that includes 201 patients undergoing stenting for incurable malignant obstruction, bevacizumab therapy was found to increase the risk of perforation by 19.6-fold over patients who did not receive bevacizumab[35]. In another review, Small et al[11] determined long-term efficacy, incidence of complications, and risk factors of SEMS placement for colonic obstruction; the incidence of perforation was higher in patients with bevacizumab treatment compared with untreated patients (15.4% vs 6.8%). The complication rate was found to be associated with SEMS placement in men, completely obstructed bowel, with balloon-dilated strictures, and with post-stent bevacizumab treatment. Given the high risk of perforation, if a patient is treated or considered to be treated with antiangiogenic agents like bevacizumab, it is not recommended to use SEMS as a palliative treatment for obstruction[34]. However, there is no strong evidence for the newer antiangiogenic agents like aflibercept and regorafenib, and because of the similar mechanisms, perforation risk should be kept in mind[5]. Based upon these data, it is suggested that colonic stent placement be avoided if possible in patients who are or who will be receiving bevacizumab.
Stent obstruction
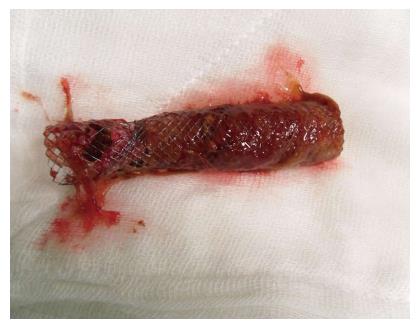
The most common complication is stent obstruction because of the tumor ingrowth or overgrowth[31]. Figure 2 shows the occluded SEMS due to the tumor ingrowth. In a meta-analysis that included 13 studies, 11 of them reported stent related complications, rate of perforation was 10.1%, stent migration was 9.2%, and stent obstruction was 18.3%[17]. It is believed that covered stents provide resistance to tumor ingrowth, thus helping to reduce reconstruction events, while uncovered stents are believed to minimize stent migration[12,36,37]. For the uncovered stents, the main disadvantage is the tumor ingrowth, but the membrane of the covered stents can provide a barrier to prevent this. In a systematic review that compared covered SEMS with uncovered SEMS, uncovered SEMS showed a higher tumour ingrowth rate (RR = 5.99; 95%CI: 2.23-16.10, P = 0.0004)[15]. The factors that are associated with the stent obstruction are: Demographic factors, underlying malignancy, length of stent, site of stricture, degree of stent expansion, and chemotherapy after stent insertion[30]. However, Im et al[38] performed a study in order to evaluate clinical outcomes, long term complications, and patency of SEMS in patients with malignant colorectal obstruction, and stent patency was not found to be associated with demographic characteristics of patients, site of obstruction, or palliative chemotherapy. In a retrospective study, Suh et al[30] analyzed the predictive factors for stent occlusion, and insufficient stent expansion (< 70%) 48 h after stent insertion was significantly associated with stent occlusion during the follow-up.
Figure 2 Occluded self-expanding metallic stent due to the tumor ingrowth.
If SEMS is inserted for the relief of obstruction in advanced and incurable colorectal cancer, the stent patency should be maintained until the death of the patients[30]. but if it is used in the preoperative setting, after 1-2 wk, the stent will be removed en bloc at the time of surgical resection[6]. In a systematic review, 14 studies reported duration of patency; the median of reported study mean durations was 106 d (range: 68-288 d) in the palliative stent population[12]. Suh et al[30] reported stent patency mean and median 184 and 141 d respectively.
There have been no accurate data in the management of occluded SEMS in malignant colorectal obstruction. In most of the patients with stent obstruction, this can be treated by stent-in-stent placement. In a retrospective case series, Yoon et al[39] determined the effectiveness of stent-in-stent SEMS insertion for the treatment of SEMS obstruction in cases with malignant colorectal obstruction. In this study the clinical success was reported in 75% of the cases; 9 of them had persistant symptoms, 8 of them underwent palliative surgery, but at the end of the follow-up, 16 of 36 patients (44.4%) remained free of obstruction symptoms until death. The success rate was found to be slightly lower than that of primary SEMS placement[40]. In another study, Yoon et al[39] compared the clinical outcomes of the patients who underwent second intervention because of the obstruction of the first successful SEMS placement for colorectal obstruction with second SEMS insertion or palliative surgery. No significant difference was found in the median overall survival (8.2 mo vs 15.5 mo) and progression-free survival (4.0 mo vs 2.7 mo) between the stent and surgery groups. However, the median lumen patency in the stent group was 3.4 mo and 7.9 mo in the surgery (P = 0.003). Male gender and having an obstruction in the right colon were identified as prognostic factors of lumen patency in second SEMS; additional chemotherapy after a second intervention was found to be a prognostic factor with a longer lumen patency in the palliative surgery group.
Stent migration
In a systematic review including 54 studies, Watt et al[12] evaluated stent for all indications reported that median rate of migration was 11%, ranging from 0% to 50%. Stent migration can occur at any time following the insertion, but is usually detected within one week of insertion. Migrations tend to occur with stents which are too narrow in diameter and/or too short in relation to the stricture they are placed in[41]. In another systematic review, Khot et al[42] reported stent migration in 54 (10%) of 551 technically successful cases; 26% of the stent migration occured within 3 d, and the remaining occured after 3 d. Factors that were associated with the migration were laser pretreatment, chemotherapy, and benign tumor. Covered and small diameter (< 24 mm) SEMS were also found to be associated with stent migration[15,35,36]. Chemotherapy with the mechanism of tumor shrinkage increases the stent migration[43-45].
Others
After the SEMS placement, abdominal pain and bleeding can occur in the follow-up. Bleeding is usually minor after the procedure, and generally no intervention will be required. Abdominal or rectal pain is common and varies between 7.4% and up to 62.5% in patients with SEMS placement within 5 cm of the anal verge[46,47]. Mild abdominal pain generally requires no specific treatment; if needed, use of analgesics will be enough for relieving the pain.