Published online Jul 15, 2015. doi: 10.4251/wjgo.v7.i7.55
Peer-review started: February 1, 2015
First decision: March 6, 2015
Revised: March 28, 2015
Accepted: May 26, 2015
Article in press: May 27, 2015
Published online: July 15, 2015
Processing time: 166 Days and 16.6 Hours
Low rectal cancer is traditionally treated by abdominoperineal resection. In recent years, several new techniques for the treatment of very low rectal cancer patients aiming to preserve the gastrointestinal continuity and to improve both the oncological as well as the functional outcomes, have been emerged. Literature suggest that when the intersphincteric resection is applied in T1-3 tumors located within 30-35 mm from the anal verge, is technically feasible, safe, with equal oncological outcomes compared to conventional surgery and acceptable quality of life. The Anterior Perineal PlanE for Ultra-low Anterior Resection technique, is not disrupting the sphincters, but carries a high complication rate, while the reports on the oncological and functional outcomes are limited. Transanal Endoscopic MicroSurgery (TEM) and TransAnal Minimally Invasive Surgery (TAMIS) should represent the treatment of choice for T1 rectal tumors, with specific criteria according to the NCCN guidelines and favorable pathologic features. Alternatively to the standard conventional surgery, neoadjuvant chemo-radiotherapy followed by TEM or TAMIS seems promising for tumors of a local stage T1sm2-3 or T2. Transanal Total Mesorectal Excision should be performed only when a board approved protocol is available by colorectal surgeons with extensive experience in minimally invasive and transanal endoscopic surgery.
Core tip: The present review presents the most recent advances in the field of sphincter preserving surgery for the treatment of low rectal cancer patients, providing indications, patients’ selection, surgical techniques, multimodality approaches, postoperative course and oncological and functional outcomes. In particular, the review focuses on data deriving from prospective studies, systematic reviews and meta-analyses. The conclusion makes clear that a customized approach based on current guidelines, as well as specific pathological prognostic factors, is mandatory for obtaining the maximum favorable outcome in each patient.
- Citation: Dimitriou N, Michail O, Moris D, Griniatsos J. Low rectal cancer: Sphincter preserving techniques-selection of patients, techniques and outcomes. World J Gastrointest Oncol 2015; 7(7): 55-70
- URL: https://www.wjgnet.com/1948-5204/full/v7/i7/55.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i7.55
Low rectal cancer is defined as any tumor lying in < 5 cm from the anal verge[1]. For more than twenty years, the most fundamental advance in rectal cancer surgery was the advent of total mesorectal excision (TME), proposed by Heald et al[2] in 1982. Although TME never compared to the traditional surgical approaches in a prospective randomized fashion, TME demonstrates clear superiority in terms of local recurrence and survival as compared to historical controls[3]. Properly conducted TME reduces the recurrence rate to < 10% and increases overall 5-year survival to over 80%[4]. The Dutch TME trial[5] confirmed the above results, clearly stating an increased risk of local tumor recurrence for patients who had undergone a potentially curative procedure with incomplete mesorectal excision, as compared to patients in whom the specimen showed a completely resected mesorectum[6]. Laparoscopy offers better visualization of the pelvic cavity and therefore facilitates mobilization of the rectum[7]. Although laparoscopic TME is a standardized and reproducible procedure[8], it can be proved a technically difficult operation[9]. In the UK MRC CLASICC trial[10], a high incidence of positive circumferential radial margin (CRM) after laparoscopic anterior resection was observed. Tumor location in the mid and distal rectum may be considered per se as an important risk factor for compromised CRM[11,12].
A positive CRM (< 1 mm), places the patient at great risk for local failure and distant metastases, thus reducing the overall survival[13]. Adoption of TME resulted in decreased CRM positivity[3].
Another change in the rectal cancer surgical management was the re-evaluation of the length of distal resection margin (DRM). A 2-cm margin is quite adequate because distal intramural spread and/or retrograde lymphatic extension are rare[14]. Even more, a recent systematic review of 17 studies[15] found no negative impact of DRM < 1 cm or even < 5 mm in terms of local recurrence or overall survival in patients with good risk tumors.
Adoption of TME, tolerance of shorter DRM, and availability of circular stapling devices, have dramatically decreased the abdominoperineal resection (APR) rates.
However, pooled analysis of 14 European rectal cancer studies[16] disclosed 10% positive CRMs, 20% local recurrence rates and 59% 5-year survival for patients who had undergone APR, compared to 5% positive CRMs, 11% local recurrence rates and 70% 5-year survival for patients who had undergone LAR, concluding that the oncological outcome following APR is not superior or at least equal to the LAR, proposing that the inferior outcomes following APR could be due to deficiencies in the surgical technique and/or tumor characteristics.
In recent years, several new techniques for the treatment of very low rectal cancer patients aiming to preserve the GI continuity and to improve both the oncological as well as the functional outcomes, have been emerged. In the present article we present these new techniques providing evidence based data for the oncological and functional outcomes of each of them.
The selection of patients who may benefit from the intersphincteric resection (ISR) should be based on the results of magnetic resonance imaging (MRI), computed tomography, endoanal ultrasonography, rigid proctoscopy and digital examination[17]. Particularly, digital examination under anesthesia is important for evaluating tumor mobility, tumor relation to the anal sphincter and final decision making[18-20].
A recent systematic review[21] addressed that the method should be ideally applied in T1-3 tumors located within 30-35 mm from the anal verge, with or without internal anal sphincter (IAS) invasion[22].
Absolute contraindications for the method are T4 tumors, invasion of external anal sphincter (EAS), fixed tumors in digital examination (indication that the tumor has broken through the intersphincteric plane), poorly differentiated tumor, poor preoperative sphincter function, distant metastases and presence of mental disease[23].
ISR was firstly described by Schiessel et al[24] in 1994 and the principle of the technique is based on the dissection of the anatomical plane between the IAS, which is the prolonged muscular layer of the rectum, and the EAS. The technique is aiming to increase the preservation of sphincter and to avoid the need for a permanent stoma for low rectal cancer tumors.
The operation consists of an abdominal and a perineal phase. The abdominal phase starts with high ligation of the inferior mesenteric vein and the inferior mesenteric artery immediately after the emergence of the left colic artery[25]. In order to accomplish that, the peritoneum above the inferior mesenteric vessels is divided, and left mesocolon is mobilized via mesofascial separation[26]. After the ligation of the vessels, the parasigmoid and pararectal peritoneal folds are divided and the mesosigmoid is mobilized via mesofascial separation. Mesosigmoid is continuous with left mesocolon above and mesorectum below[27]. The dissection continues in the mesorectal plane, with the separation of the mesorectum from adjacent mesorectal fascia[2]. Although not always necessary, mobilization of the splenic flexure might be required[28]. Splenic flexure mobilization, requires freeing of both mesocolic and gastrointestinal components[29]. The dissection of left mesocolon, mesosigmoid and mesorectum via mesofascial separation, allows the removal of the specimen with intact fascial layers whilst simultaneously maximizing lymph node yield[27].
Laparoscopic, open[30] and robotic[31] approaches have been used for the abdominal phase.
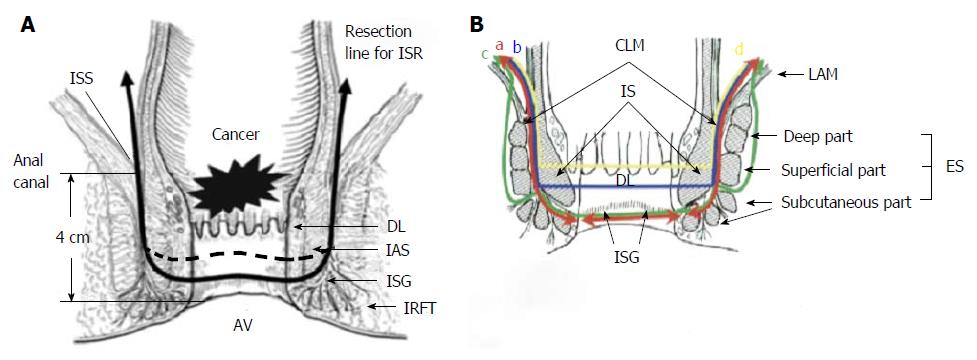
For the perineal phase, the patient is placed in the high lithotomy position, a self-retaining retractor is applied for perineal exposure[28] and 1 mg epinephrine diluted in 20 mL of saline solution is injected at several points beneath the anal mucosa, for minimization of bleeding and facilitation of intersphincteric dissection[32]. A circumferential incision in the anal mucosa, at a distance of at least 1 cm from the macroscopic distal edge of the tumor for T1 lesions and 2 cm for T2-3 lesions is made in such a way, to include in the specimen the whole rectal wall as well as a part or the whole of the IAS[32] (Figure 1). The anal orifice is then closed transanally with purse string sutures to prevent tumor cell dissemination during the perineal approach[22]. Under direct vision, the dissection is continued cephalad through the intersphincteric space to be connected with the TME plane developed transabdominally[3]. The specimen is usually delivered per anus. The continuity of GI tract is then restored by a hand-sewn coloanal anastomosis. Many types of hand sewn anastomosis have been used such as, colonic J-pouch, transverse coloplasty, or straight coloanal hand-sewn anastomosis, according to surgeons preference[22]. Finally, diverting ileostomy or colostomy is performed[21].
There are three types of ISR (partial, subtotal or total) depending on the extent of the IAS resection. Therefore, partial ISR is defined as a one-third resection of the upper part of the IAS, subtotal ISR as a two-third resection of the IAS, and total ISR as a complete resection of the IAS[21]. Combined resection of the EAS (external sphincter resection, ESR) is sometimes performed for tumors with suspected invasion into the intersphincteric space and/or external sphincter muscles[33,34].
ISR differs from conventional coloanal anastomosis performed after ultra low anterior resection, because it’s characterized by resection of internal sphincter by dissection in the intersphincteric plane[35].
Operative mortality varies between 0% and 1.7%, while postoperative morbidity rate ranges between 8% and 64%[22]. Main causes of morbidity are anastomotic leakage, anastomotic stricture, fistula formation, pelvic sepsis development, wound complications, bleeding and ileus[22]. Particularly anastomotic leakage, has been reported as related to postoperative anastomotic stricture formation, cancer recurrence, poor postoperative function and increased operative mortality[36]. In a meta-analysis by Martin et al[17], a 25.8% cumulative morbidity rate is reported, with an anastomotic leak rate of 9.1% and a pelvic sepsis rate of 2.4%. Akagi et al[37], reported Dindo Grade II complication rate of 12% and anastomotic leak rate of 5.6%, while Saito et al[38], reported a 10% leak rate.
Tilney and Tekkis[36] performed a literature search to identify studies reporting outcomes following ISR. Twenty-one studies, accumulating a total of 612 patients were identified. The pooled rate of local recurrence was 9.5%, the average 5-year survival was 81.5%, while distant metastases occurred in 9.3%.
In Martin et al[17]’s systematic review, the mean distal free resection margin was 17.1 mm (range: 12-29 mm), a CRM negative margin was achieved in 96% (range: 89%-100%) of the patients, an R0 resection was performed in 97% of the patients, the overall local recurrence rate was 6.7% (range: 0%-23%), the 5-year disease-free survival rate was 78.6% (range: 69%-87%) and the 5-year overall survival was 86.3% (range: 62%-97%), at a median follow-up of 56 mo.
A large prospective study published in 2013[37], with 124 patients with low rectal T1-3 tumors without preoperative chemo-radiotherapy (CRT), showed total (local and distant) postoperative recurrence rate of 16.1%, including 4.7% at stage I, 19.5% at stage II and 25% at stage III disease. Local recurrence (including recurrence in anastomotic site, lateral lymph nodes and pelvic floor recurrence) occurred in 4.8% of patients, including 4.7% at stage I, 4.9% at stage II and 5.0% at stage III disease. There was no anastomotic site recurrence. Lateral lymph node metastasis was observed in 2.4% of patients (2% at stage I, 2.4% at stage II and 2.5% at stage III). Pelvic floor recurrence was also found in 2.4% patients (2% at stage I, 2.4% at stage II, and 2.5% at stage III). The rate of distant metastasis was 10.5% in total. The cumulative recurrence-free 5-year survival rate at each stage was 92.2%, 81.9%, and 69.6% respectively and the cumulative cancer-specific 5-year survival rate at each stage was 90.5%, 91.0% and 83.6%, respectively. The overall 5-year survival rate at each stage was 84.2%, 85.2%, and 78.6% respectively. Moreover, the authors compared the oncologic outcomes after ISR with those after APR performed during the same period. No significant differences were noticed in the pathological stage distribution between the two operative methods. The overall recurrence-free survival and local recurrence rates after ISR were similar to those after APR.
In Saito et al[38]’s prospective study, 199 patients underwent ISR. Among them 25% had undergone neoadjuvant CRT and 20.6% underwent concomitant partial EAS resection. After a median follow-up of 6.5 years (range: 12-164 mo), pulmonary metastases were occurred in 14.1%, local recurrence with or without distant metastasis in 13.6%, liver metastasis in 7.5% and combined recurrence in 4.5% of the patients. Positive CRM was reported as high as 19.6%, but T4 tumors were also included in the study (19 T4 tumors out of 199). The estimated 7-year overall survival, disease free survival and local relapse free survival rates were 78%, 67%, and 80%, respectively.
Most of the studies comparing LAR, APR and ISR[19,37,39], concluded in non-statistically significant differences regarding their oncological outcome, although Saito et al[40] disclosed that the 5-year overall survival was worse in APR than in ISR (61.5% vs 80%).
In a recent study[41], 77 patients who underwent ISR, compared to 33 patients who underwent APR and 68 patients who underwent LAR. There were no significant differences in the disease stage status between the ISR and the LAR group of patients, although in the APR group more advance TNM stage was noticed. Overall recurrence (both local and distant), was noticed in 7.8% of the patients after ISR compared to the 11.7% in the LAR group and to the 12.1% in the APR group (P = 0.67). The local recurrence rate was 2.6% for the ISR group of patients compared to the 5.9% in the LAR group and to the 6.1% in the APR group (P = 0.57). With a median follow-up of 69 mo (range: 56-87), the 5-year local recurrence-free survival was 93.5% for the ISR group, 88.2% for the LAR group and 87.9% for the APR group; although these differences were not statistically significant. The 5-year overall survival rate after ISR was 76.4%, better than the APR (51.2%) and similar to the LAR (80.7%), probably reflecting the higher frequency of advanced cancers in APR group of patients. The 5-year overall survival rate according to the TNM stage in patients who underwent ISR was 90.0% for stage I, 79.8% for stage II, and 65.6% for stage III. In stage III patients, the 5-year overall survival rate for the ISR, LAR, and APR groups was estimated at 65.6%, 56.3%, and 33.3%, respectively (P = 0.02). These long-term results suggest that ISR is a suitable technique based on the oncologic outcome. However, T3 tumor and patients with positive microscopic resection margins were more likely to have local recurrence after ISR[42,43].
Finally, the CRM is a powerful indicator for local recurrence[44] and the CRM around the anal canal has been proposed as a risk factor for local recurrence when ISR is performed[21]. The group of patients with positive CRM displayed significantly worse overall survival, disease free survival and local relapse free survival rates than the negative CRM group of patients (P < 0.001)[38].
Other risk factors for local recurrence include, de-differentiation of the tumor, and preoperative CA19-9 levels above 37 U/mL[45], while pathological N1 and N2 tumor and poorly differentiation of the tumor, have been reported as risk factors for distant recurrence[45].
Although postoperative anal function represents a particularly important clinical outcome measure after sphincter-preserving surgery for lower rectal cancer, only few studies have reported short-term postoperative results[46-51]. After ISR, resting pressure is not greatly restored, but gradually recovers overtime[18,47]. In contrast, the maximum squeeze pressure is not affected. Anal function seems to improve over time[49,52].
Köhler et al[53] reported a 29% reduction in resting anal pressure following ISR, while the squeeze pressure recovered to preoperative levels after 12 mo.
In Martin’s et al[17] systematic review, the mean number of bowel movements per day was 2.7. Nearly half (51.2%) of the patients reported “perfect continence”, about a third (29.1%) experienced fecal soiling, 23.8% had flatus incontinence and 18.6% had urgency.
In a large study assessing functional outcomes after ISR, Denost et al[54] reported that half of the patients had a “good functional result”, 39% had minor fecal incontinence and 11% had major incontinence.
Saito et al[38] reported the long term functional results of 199 patients. In this study mean stool frequency per 24 h was 4.0 ± 3.7 and the median Wexner score was 8.5 (range: 0-20) in all patients at 5 years after stoma closure. Approximately 50% of patients had stool fragmentation and incontinence to gas, 30% still experienced soiling during the day and at the nighttime, while a quarter suffered from difficulties in evacuation. Multivariate analysis disclosed male gender and preoperative CRT as independent factors predisposing to a worse continence score, although the type of surgery (partial or total ISR) did not affect the long-term functional outcomes. Similarly, Ito et al[51] reported that preoperative CRT was the risk factor with the greatest negative impact on anal function after ISR.
In contrast, multivariate analysis in Yamada et al[32]’s study disclosed patient’s age at surgery as the only risk factor for postoperative fecal incontinence.
Bretagnol et al[48] reported that frequency, urgency, the Wexner score and the Fecal Incontinence Severity Index were significantly improved following colonic J-pouch reconstruction, compared to the straight coloanal anastomosis.
Denost et al[54] reported that the risk of fecal incontinence after ISR was directly related to the tumor level and the height of the anastomosis, stating that for a “good” continence result, distance of tumor greater than 1 cm from the anorectal ring and anastomosis higher than 2 cm from the anal verge, are required.
In a recent study[41], comparing the functional outcomes following ISR and LAR, the authors disclosed that the postoperative defecation functions in terms of the frequency of defecation, urgency, ability to distinguish gas emission and perianal skin irritation, were equal between the two techniques, the Wexner score was lower in the LAR group, but no significant difference was observed in the fecal incontinence quality of life (FIQL) score between the ISR and LAR.
Bretagnol et al[48] using both SF-36 and FIQL questionnaires to compare quality of life (QoL) between patients undergoing ISR and conventional coloanal anastomosis, found no differences in the QoL between the two groups throughout the physical and mental subscales of the SF-36.
Saito et al[38] reported that patients after ISR with or without partial EAS excision have QoL at 5 years equal to or better than those preoperatively, but patients with preoperative CRT showed significantly worse FIQL scores after long-term follow-up.
ISR was developed as an alternative to the classical surgical approaches for the treatment of low rectal cancer patients who might be benefited from a sphincter preserving technique and who otherwise, should have undergone an APR. Literature results suggest that when the method is applied in T1-3 tumors located within 30-35 mm from the anal verge with or without IAS invasion, is technically feasible, safe (in terms of early postoperative outcome), with equal oncological outcomes compared to LAP and APR and acceptable QoL. APR should be reserved for locally advanced tumors.
The Anterior Perineal PlanE for Ultra-low Anterior Resection of the Rectum (APPEAR) procedure was developed to allow sphincter-preserving rectal resection for both benign and malignant pathology, which would traditionally required APR or completion proctectomy, if treated by conventional means[55]. In recent case reports, APPEAR is indicated for patients with low rectal cancer, 2-5 cm from the anal verge[56,57].
The APPEAR technique, was firstly described by Williams et al[55] in 2008. The technique consists of abdominal and perineal approach, allowing access to low and difficult to be reached rectum between the levator ani muscle and the superior margin of EAS.
The abdominal phase of the operation is the same as the abdominal phase of ISR and is described above. The abdominal phase can be performed either laparoscopically or open.
For the perineal phase, the patient is placed in a high lithotomy position and the rectovaginal/prostatic plane is infiltrated with 1 in 300000 adrenaline saline solution. A convex crescentric skin incision is made in the perineum midway between the vagina or the base of the scrotum and the anal verge. The skin and subcutaneous tissue are dissected from the underlying external anal sphincter and transverse perinei muscles, and reflect forwards. In the female, the plane between the posterior vaginal wall and the anterior rectal wall is entered anteriorly to the perineal body. In the male, the rectourethral/prostatic plane is entered similarly, firstly isolating and then dividing bilaterally the rectourethralis muscle, close to the rectum. After dividing the rectourethralis muscle, the anterior rectal wall is mobilized from the prostate, using both blunt and sharp dissection. At the inferolateral aspect of the prostate, the dissection is performed close to the rectum, avoiding damage to the neurovascular bundles. The perineal dissection is continued cephalad until the plane created from above by the abdominal operator is reached. The rectum is then freed laterally and the specimen is delivered through the perineum. The continuity of GI tract was established with either straight coloanal anastomosis or a short colonic pouch, with protecting ileostomy[55].
Both in the initial pilot study[55] as well as the latter case report studies[56,57], no mortality was reported. The main postoperative complication was perineal wound infection with an incidence ranging from 15.4% to 60% and subsequent colonic/ileoanal pouch perineal fistulation in some of the patients[55-57]. Anastomotic stricturing occurred in 3 patients in the pilot study[55].
Oncological outcome is documented in only two studies[55,57]. In the pilot one[55], only half of the patients (7 out of 14) had rectal cancer, the median DRM was 20 mm (range: 10-37 mm) and the median circumferential resection margin was 5 mm (range: 2-21 mm). No local recurrence was noted within a median follow up of 2 years, but one patient developed distant metastases. In the recent one, no recurrence was documented, but the median follow up was only 11 mo[57].
Functional outcome were also documented in only two studies[55,57]. In the pilot one, the median Wexner score after ileostomy closure was 5 in the patients treated with coloanal anastomosis, all patients were continent to solid and liquid stool, with only one patient reporting fragmented evacuation and three patients reporting fecal urgency[55]. In the later study, the average Wexner score was 5.5 after ostomy closure[57]. Both articles showed normal resting and squeezed pressures after the APPEAR. QoL was reported in only one study[55] with the use of SF-36 questionnaire, disclosing no significant changes following the APPEAR procedure after the ileostomy closure.
Compared to ultra-low anterior resection, the APPEAR technique has the advantage of providing greater distal access to the rectum for mobilization and compared to ISR, has the advantage of not disrupting the sphincters[3]. However, the complication rate is high, mainly related to perineal wound infection, while the reports on the oncological and functional outcomes are limited. More studies are needed for evaluation of the technique.
Both methods are primarily used for local excision of lower, middle and upper rectum benign tumors via the anus[58,59].
Literature addresses that Transanal Endoscopic Microsurgery (TEM) can be applied for the resection of other benign rectal and extrarectal masses such as neuroendocrine tumors, retrorectal cysts, masses within the anovaginal septum, as well as for the repair of high rectovaginal fistulas[60], although the experience in these rare indications is limited. TEM has also been effectively used to treat anastomotic strictures, rectal prolapse, high extrasphincteric fistulas and for transrectal drainage of pelvic collections[61].
Current indications for local excision have expanded to include either the treatment of early stage rectal cancer in curative intense or as a palliation in patients with advanced rectal cancer who either refuse radical excision or they are poor surgical candidates[62,63]. Patients with incidentally found carcinoma following endoscopic polypectomy are suitable candidates for local excision, especially in the setting of a sessile polyp or when there is concern about margin positivity[64].
Applications of TransAnal Minimally Invasive Surgery (TAMIS) beyond local excision, have already been defined and they include the repair of rectouretheral fistulae[65], distal rectal mobilization[66], extraction of rectal foreign bodies[67], and most importantly, the use of TAMIS for transanal TME[68].
The right selection of the patients, who will be benefited by local excision in cases of early rectal cancer, is still an obstacle. Endorectal Ultrasound (ERUS) and/or pelvic MRI are mandatory for the preoperative staging of them. ERUS is more sensitive for the determination of the bowel wall depth invasion, while MRI is superior at evaluating mesorectal lymph nodes and the circumferential resection margin[69].
Based on the above imaging findings, NCCN guidelines[1], clearly recommend local excision as the treatment of choice for: (1) mobile/nonfixed rectal tumors, (2) less than 3 cm in size, (3) occuping less than 1/3 of the circumference of the bowel, (4) not extending beyond the submucosa (T1) which are (5) well to moderately differentiated and (6) with low-risk histopathological features. On the other hand, local excision should be avoided in cases of lymphovascular invasion, perineural invasion and mucinous components which are considered as high-risk characteristics, with high lymphnode metastatic potential.
TEM, was firstly developed in the 1980s by Buess et al[70], for the removal of endoscopically unresectable sessile rectal polyps. The author developed specific surgical rectoscope and instruments to address this problem. This facilitated a new way of operating in the rectum that was very precise and accurate due to its binocular vision and 3D visualization[71].
The equipment consists of a rigid rectoscope fixed to operating table and a unit for carbon dioxide insufflation, suction, irrigation and rectal pressure monitoring. The rectoscope is 4 cm in diameter, available in two main sizes, short (12 cm) and long (20 cm). Which one will be used, depends on the pre-operative location of the lesion in the rectum. The removable faceplate of the rectoscope has ports to facilitate the insertion of the long instruments, the suction required and to accommodate the stereoscope through which the surgeon can see the lesion magnified by six-fold. In recent times this can be connected to a laparoscopic video stack, which some surgeons prefer[72].
For anterior lesions, the patient should be placed prone and for posterior lesions in the lithotomy position[72]. The pneumorectum is maintained at a constant pressure of 10-12 mmHg which is enough for rectal wall distension and exposure of the tumor. The dissection begins by making a dotted line with the monopolar electric scalpel 10-15 mm from the macroscopic tumor margin[73].
For adenomas located within the intraperitoneal portion of the rectum, a careful mucosectomy, avoiding entry into the peritoneum, is indicated[64].
For extraperitoneally located adenomas and for all invasive carcinomas, full thickness resection should be offered as a standard treatment option[64].
Circumferential adenomas in the lower and middle rectum can be resected as complete full thickness segments, followed by an end-to-end anastomosis[64].
Invasive carcinomas in the posterior or lateral wall may be resected with some perirectal fat, often including 1 or 2 adjacent lymph nodes, which can be examined for metastatic spread[64].
With TEM, it is possible to perform local excisions with low risk of perforation at a distance up to 18-20 cm when the tumor is located in the posterior quadrant and up to 15 cm when it is located anteriorly or laterally. The limit for low located lesions is the anal verge itself[73].
The resection bed is usually closed using a running 3-0 polydioxanone (PDS) suture on a small-half needle[64]. If peritoneum is entered, the defect should be always closed, while the resection bed below the peritoneal reflection, may be left open[64]. Finally, the surgical specimen is pinned out and oriented for pathological analysis of the margins[74].
TEM has not become universally adopted by colorectal surgeons due to the considerable cost of the apparatus and the steep learning curve required for the mastering of the technique[75]. These disadvantages prompted surgeons to examine alternative methods for performing transanal surgery.
TAMIS was developed in 2009[76], and it is defined as the use of any multichannel single-port which can be placed transanally, combined with the use of ordinary laparoscopic instruments, such as a laparoscopic camera (preferably a 5-mm, 30° or 45° lens) and a standard laparoscopic carbon dioxide insufflator for performing endoluminal and more recently, extraluminal surgery[77]. A systematic review[75] of the published studies revealed that eight different types of TAMIS platforms have been used for local excision of rectal neoplasia. Regardless of which platform is used, the principles of TAMIS remain the same and the key advantages to its use are upheld.
Few deaths have been reported in the literature, mainly related to metastatic disease in the late postoperative period or due to advanced disease in patients in whom palliative TEM had been performed[59,78].
The overall complication rate for TEM has been reported between 6% and 31%, with an equal distribution between benign and malignant disease[79]. Perioperative complications include hemorrhage and intraperitoneal perforation (0%-9%), which both may require conversion to laparotomy[79]. Postoperative hemorrhage has been reported in 1% to 13% of patients. Most resolve spontaneously or conservatively with blood transfusion[64]. The conversion rate was around 5%, mainly related to technical difficulties[74].
Since TAMIS is a fairly a new technique, the appraisal of its results is mainly based on retrospective studies and case reports. Albert et al[77], reported a 6% microscopically positive margins on final pathology and a recurrence rate of 4% at 6- and 18-mo follow-up. The largest multicentre series on TAMIS for rectal lesions[80] included 75 patients (low grade rectal adenoma 33%, high grade rectal adenoma 23%, rectal adenocarcinoma 43% and carcinoid tumour 1%). Intraoperative complications occurred in 8% and postoperative morbidity rate was 19%, with only one patient requiring re-intervention.
However, the only systematic review[75] of 390 TAMIS resections published in the English literature, disclosed: a 3.0 cm average size of lesions resected, located within a 7.6 cm mean distance from the anal verge (range: 3-15 cm), an overall margin positivity rate of 4.36%, a tumor fragmentation of 4.1% and an overall complication rate of 7.4%.
The ideal goal for the treatment of T1N0M0 rectal cancers should be the maximization of the oncological outcome, with simultaneous minimization of the long-term impact of the treatment on the QoL[81].
Long-term results studies on the oncological outcome following traditional transanal local excision for T1 tumors, disclosed local recurrence rates higher than 29%[82-84].
On the other hand, the published results on the oncological outcome following TEM remain controversial, since other studies[85] reported favorable results with local recurrence rates lower than 10%, others[86] confirmed the lower local recurrence rates following TEM compared to the transanal local excision (18.5% vs 27.7%) but without statistical significance, others[87] stated alarming figures for local recurrence following TEM for T1 rectal tumors, while local recurrence rates as high as 20.5% have also been reported[88].
In an attempt to evaluate further the above findings, both Tytherleigh et al[89] in 2008, as-well-as Bach et al[90] in 2009, offered possible explanations for these unfavorable results. Both studies made clear that the depth of submucosal invasion (sm level) constituted a strong predictor for recurrence, since sm1 tumors showed low recurrence rates, but sm2-3 tumors showed recurrence rates similar to the T2 lesions[89,90]. Thus, locally excised pT1sm1 tumors without lymphovascular invasion, up to 3 cm in diameter, have a local recurrence rate of less than 5%, while locally excised pT1sm2-3 tumors have a local recurrence rate of up to 20%, similarly to T2 tumors[72].
Apart from the sm level of invasion, tumor differentiation, vascular or perineural invasion, positive resection margins, lymphocytic infiltration, lymph node spread and tumor budding (presence of neoplastic cells below the invasive front), have been proposed as additional dismal prognostic factors for local recurrence[73].
According to the NCCN guidelines[1], the standard treatment for T2N0M0 rectal adenocarcinoma is TME without adjuvant therapy per se, since such tumors have a lymph node involvement rate between 12% and 29%[58].
However, literature addresses that for T2 tumors, simple local excision (either transanal or TEM), local excision followed by postoperative CRT, as well as preoperative CRT followed by local excision, have been attempted.
TEM alone is not acceptable treatment option for fit patients with rectal cancer of local stage T2 or deeper[79].
CRT after local excision presented disappointing results, since local recurrence rate has been reported as high as 45%[91].
However, TEM after neoadjuvant CRT for downstaging of advanced tumors has been investigated demonstrating promising results[79].
Lezoche et al[92] prospectively randomized 70 patients with T2N0 rectal cancers to either TEM (n = 35) or laparoscopic radical resection (n = 35) after CRT. Patients were restaged after neoadjuvant therapy. Those in the TEM group had significantly better results in terms of hospital stay, blood loss and duration of surgery than those in the radical resection group, although there was no difference in complication rates between the two groups. Oncologic results after TEM and radical resection were comparable in terms of local (5.7% vs 2.8%), distant (2.8% vs 2.8%) and combined (9% vs 6%) recurrence rates, as well as the probability of disease-free survival (both 94%). The above oncological outcome, combined with the shorter hospital stay and the faster return to normal activities in the TEM group of patients, may suggest that TEM is a favorable and acceptable technique for selected T2 patients without nodal involvement or distant metastasis, though more evidence is required.
In their review, Borschitz et al[93] included seven studies and 237 patients who underwent local excision for T2-3 rectal tumors after neoadjuvant CRT. The authors addressed that when complete pathological response was achieved (ypT0), local recurrence was 0% and systemic recurrence was 4%, in ypT1 tumors, local recurrence was 2% and systemic recurrence was 7%, in ypT2 tumors, both local and systemic recurrence rose to 7%, while when there was no response (ypT3), the local recurrence was 21% and the systemic recurrence was 12% after local excision.
Similarly, a prospective study[94] of 27 patients with ypT0-2 tumors in the lower rectum after neoadjuvant CRT, treated by TEM, showed a local recurrence rate of 15% within a median follow-up of 15 mo. Lymphovascular invasion was disclosed as the only independent dismal prognostic factor for local failure (P = 0.04), while tumor size, ypT status, T-status downstaging, lateral/radial margins and tumor regression grade, did not reach statistical significance.
Finally, a systematic review[95] comparing the effectiveness of TEM to radical surgery for T1-2 rectal tumors, concluded that the TEM procedure was associated with a higher risk of local recurrence, but was statistically equivalent to radical surgery in terms of overall mortality, overall survival and the risk of distant metastasis. We should state however, that the main bias of this review was that low risk T1, high risk T1, as well as T2 tumors, were indiscriminately enrolled.
As a result of the dilation of the anal canal by the proctoscope and the prolonged operative time, it has been suggested that damage to the anal sphincter could cause postoperative fecal incontinence[96]. Existing data indeed suggested reduced anorectal manometric pressures (particularly the resting one) in patients who have undergone TEM, directly correlated to the length of the procedure, however this did not change continence scores or other anorectal parameters[97].
In a prospective study of 41 patients, Cataldo et al[98] found no deleterious consequences on fecal continence after TEM. They did not find any significant difference between pre- and postoperative mean Fecal Incontinence Severity Index (FISI) score (2.4 vs 2.4), mean FIQL score, number of bowel movements per day (mean 2.4 vs 1.5) and ability to defer defecation.
In a recent study of 50 patients, Doornebosch et al[99] found significantly improved FISI and FIQL scores after TEM (all P < 0.05).
Patients themselves reported improved QoL after surgery[99]. This improvement may be attributed to the fact that rectal lesions and subsequent mucous production contribute to the symptoms of fecal incontinence, which disappear once the lesion is excised. Furthermore, the presence of a large rectal mass may induce a continuous internal anal sphincter reflex, leading to a decreased anorectal function.
Allaix et al[100] studied the long-term functional outcomes and the QoL parameters after 5 years follow-up in 93 patients who underwent TEM. Similarly to previous studies, manometric values, such as anal resting pressure, rectal sensitivity threshold, maximum tolerated volume and urge to defecate threshold, declined at 3 mo but returned to preoperative level 12 mo after surgery. Compared to preoperative levels, there were no significant changes in anal squeeze pressure after surgery. Wexner incontinence scores and general QoL scores, which were increased in the early postoperative period, returned to preoperative levels at 5 years.
The functional outcome of TAMIS after rectal polyps resection are reported in only one study[101], showing excellent short-term results and comparable to functional results using the dedicated TEM equipment.
Both TEM and TAMIS are safe procedures. TEM should be used in T1 rectal cancer, with favorable pathologic features. The use of TEM after preoperative CRT is still debatable. Anal function after TEM is improving. Not enough oncological or functional outcomes are available for TAMIS.
Transanal Total Mesorectal Excision (TaTME) was developed to overcome technical difficulties associated with laparoscopic TME[68]. Most of the surgeons believe that patients with narrow pelvis, visceral obesity or large tumor diameter, are favored by this technique[102]. The procedure is feasible for mid and low rectal cancers.
In a systematic review[68] of 150 cases, rectal adenocarcinoma was the indication for TaTME, except 9 cases published by Wolthuis et al[103].
In their report, Tuech et al[102] stated as contraindications for the procedure T4 tumors invading the vagina or the prostate, tumors with no objective response to preoperative CRT as well as tumors invading EAS or levator ani. de Lacy et al[104] added as contraindications a BMI over 35, the recurrence and the intolerance of pneumoperitoneum.
Trananal TME is a new technique that allows the transanal mobilization of the rectum from distal to proximal using a variety of flexible or rigid transanal platforms[68]. The devotees of the technique support that TaTME facilitates radical dissection of the difficult distal part of the TME dissection in a narrow and/or rigid pelvis, allowing clear and safe definition of the tumor-free distal resection margin[105].
TaTME can be performed in conjunction to transabdominal assistance through multiport laparoscopy, mini-laparoscopy or a single-port access[68]. Some authors report that abdominal phase of the operation should be performed first, with the transanal phase to follow, other teams perform the two phases synchronously[102], pure TaTME has also been reported[106,107], while different type of platforms or even robotic TaTME has also been performed[108].
The standardized technique has two phases, abdominal and transanal. Most authors complete the abdominal phase with high ligation of inferior mesenteric vessels and mobilization of the left colon and the splenic flexure. The fecal stream is diverted with a loop ileostomy, unless a permanent stoma is being fashioned[12].
The transanal phase starts after the placement of a self-retaining retractor and the exploration of the rectum. For tumors located up to 3 cm from the anal verge, there is a necessity to be performed an intersphinteric dissection, after sectioning the dentate line with electrocautery. Once the full-thickness rectal wall is completely sectioned, a purse-string suture is placed through the rectum to tightly occlude it. Thereafter, it is necessary for the transanal dissection of the first 4 to 4.5 cm of the anal canal to insert a Transanal Access Platform. CO2 is insufflated to a pressure of 10 to 12 mmHg, and it is adapted during the progression of the dissection. Once introduced into the presacral plane, the mesorectum is mobilized and the posterior dissection proceeded cephalic in the avascular presacral plane in accordance to TME principles. This plane of dissection is extended medially, laterally, and interiorly to achieve circumferential rectal mobilization. The dissection is performed circumferentially and progressively to avoid retraction of the rectum that could make the division of one side difficult. Finally, the peritoneal reflection is visualized and divided to achieve sigmoid colon mobilization, with both teams collaborating to complete it. The device is removed and the specimen is carefully extracted transanally. The section of sigmoid colon is performed proximal to the vascular pedicle with scalpel. The division of the remaining mesentery and the marginal artery are completed with the specimen exteriorized. A handsewn coloanal anastomosis is then performed between the proximal sigmoid colon and the distal anorectal cuff[109].
For middle and low rectal tumors, after positioning of a self-retaining retractor, the Transanal Access Platform, is positioned in the anal canal. A purse-string suture is placed through the rectal mucosa to tightly occlude it distally to the lesion. Endoscopic transection of the full-thickness rectal wall is performed and, thereafter, another purse-string suture was placed in the distal rectal mucosa. The mesorectum mobilization is made as previously described. The specimen is exteriorized transanally, the colon is sectioned, a purse-string suture is placed and the anvil is inserted. The rectal anastomosis is performed with a EEA 33 mm circular stapler[109].
In the only systematic review enrolling 150 cases[68], no mortality was reported. The complications rate was 22.7%, mainly related to infectious complications such as pelvic abscess (n = 6) and anastomotic fistulas (n = 2). In a latter study[102], the postoperative complications rate was 26% and the anastomotic leak rate was 5.3%.
The available on the oncological outcome data of TaTME is currently derived from non-randomized retrospective comparative series, case series and case reports.
The macroscopic quality of TME was documented in all published reports, except in study of Wolthuis et al[103] and has been reported as ‘‘intact’’[110], ‘‘adequate’’[111,112], ‘‘satisfactory’’[104,113], or ‘‘complete’’[11,12,106,107,111,114].
Results from four studies[7,11,12,115], addressed positive CRM in 10 out of 136 patients (7.3%), while all studies, except two[103,113], reported a mean number of retrieved lymphnodes equal to or greater to 12.
A recent study (n = 56)[102], showed intact removal of the mesorectum in 47 cases (84%) and a nearly complete mesorectum in 9 (16%), median number of retrieved lymphnodes per patient equal to 12 (range: 7-29), median radial and distal resection margins of 8 mm (range: 0-20 mm) and 10 mm (range: 3-40 mm), respectively, CRM involvement in 5.3%, achievement of R0 resection in 53 patients (94.6%) and overall survival of 96.4% within a median follow-up period of 29 mo.
A systematic review[68], addressed that an oncological adequate TaTME operation is reproducible, with lower than APR and equal to LAR positive CRM margins and comparable extent of mesorectal excision and lymphadenectomy.
Although more studies are required to be confirmed the above findings, the comparative results published by Velthuis et al[7], indicated that TaTME may be associated to a significantly higher rate of completeness of mesorectal excision, compared to laparoscopic TME.
Functional outcomes are reported only in one study[102]. After the reversal of the ileostomy in 52 out of the 56 enrolled patients, 3 (5.7%) required a colostomy because of severe fecal incontinence, while for the remaining 49 patients without stoma (94.3%), the median Wexner score was 4 (range: 3-12) and among them, 14 (28.5%) had a score greater than 7 and 13 (28%) reported stool fragmentation and difficult evacuation.
TaTME is feasible and safe. The general consensus is that curative TaTME should be performed only when a board approved protocol is available and only by colorectal surgeons with extensive experience in minimally invasive and transanal endoscopic surgery. More studies are needed to evaluate the oncological and functional outcomes of the technique.
If ISR is applied in T1-3 rectal tumors located within 30-35 mm from the anal verge with or without IAS invasion, is technically feasible, with acceptable morbidity rates, equal oncological outcomes compared to LAP and APR and acceptable QoL, reserving APR for locally advanced tumors (Table 1).
| Patients selection(inclusion criteria) | Surgical technique | Postoperative outcome | Oncological outcome | |||
| ISR | T1-3 tumors within 30-35 mm from the anal verge | Abdominal phase: high ligation of the inferior mesenteric vessels | Morbidity: | 8%-64% | Local recurrence | 2.6%-9.5% |
| Lymphnode metastases | 2.40% | |||||
| Perineal phase: dissection on the anatomical plane between the IAS and the EAS | Mortality | 0%-1.7% | Distant metastases | 9.3%-14.1% | ||
| CRM (-) | 80.4%-96% | |||||
| 5-yr overall survival | 76.4%-86.3% | |||||
| APPEAR | Was developed to treat patients with malignant or benign disease, needing APR or completion protectomy, if treated with conventional surgery | Abdominal phase, same as TME in LAR | Morbidity: | 15.4%-60% | Distal resection margin | Median: 20 mm |
| Mostly used in rectal cancer 2-5 cm from anal verge | Perineal phase involves a convex crescentic incision in the perineum, between vagina/scrotum and anus, the dissection continues upwards to the plane made from abdominal phase. The rectum is freed laterally and posteriorly from the perineal aspect and the specimen delivered through the perineum | Mortality | 0% | CRM | Median: 5 mm | |
| Local recurrence | 0% | |||||
| TEM TAMIS | (1) Are indicated for: mobile/nonfixed tumors, less than 3 cm in size, occupying less than 1/3 of the circumference of the bowel, not extending beyond the submucosa well to moderately differ-rentiated with low-risk histopatho-logical features | Adenomas located within the intraperitoneal portion of the rectum: careful muco-sectomy, avoiding entry into the peritoneum | TEM Morbidity | 6%-31% | Without neo-adjuvant CRT | |
| 7.4%-19% | ||||||
| Extraperitoneally located adenomas: full thickness resection | TAMIS Morbidity | Occasionally | T1sm1: Local recurrence | < 5% | ||
| All invasive carcinomas: full thickness resection | TEM Mortality | Occasionally | T1sm2-3: Local recurrence | 20% | ||
| (2) As a palliation in patients with advanced rectal cancer who either refuse radical excision or they are poor surgical candidates | Circumferential adenomas in the lower and middle rectum: complete full thickness resection, followed by an end-to-end anastomosis | TAMIS Mortality | After neo-adjuvant CRT | |||
| (3) Furthermore, TAMIS is used for the repair of rectoure-theral fistulae, distal rectal mobilization, extraction of rectal foreign bodies, and for transanal TME | T2N0: | |||||
| Local recurrence | 5.7% | |||||
| Distant recurrence | 2.8% | |||||
| Combined recurrence | 9% | |||||
| After neo-adjuvant CRT | ||||||
| T2-3N0 (ypT0): | ||||||
| Local recurrence | 0% | |||||
| Systematic recurrence | 4% | |||||
| T2-3N0 (ypT1): | ||||||
| Local recurrence | 2% | |||||
| Systematic recurrence | 7% | |||||
| T2-3N0 (ypT2): | ||||||
| Local recurrence | 7% | |||||
| Systematic recurrence | 7% | |||||
| T2-3N0 (ypT3): | ||||||
| Local recurrence | 21% | |||||
| Systematic recurrence | 12% | |||||
| TaTME | Was developed to overcome technical difficulties associated with laparoscopic TME, mainly related to narrow pelvis, visceral obesity or large tumor diameter | Abdominal phase involves high ligation of inferior mesenteric vessels and mobiliazation of left colon and splenic flexure | Morbidity | 22.7%-26% | Distal resection margin | Median: 10 mm |
| Perineal phase: | Mortality | 0 | CRM | Median: 2 mm | ||
| for tumours ≤ 3 cm from anal verge, ISR and after, transanal access platform insertion, and CO2 insufflation. Dissection starts from the presacral plane, the mesorectum is mobilized and the posterior dissection proceeded cephalic in the avascular presacral plane in accordance to TME principles. The dissection continues until peritoneal reflection is visualized and divided to achieve sigmoid colon mobilization. The specimen is extracted transanaly | CRM (-) | 92.7%-96.7% | ||||
| “Intact” mesorectum | 84% | |||||
| “Nearly complete” mesorectum | 16% | |||||
| R0 resections achieved | 94.6% | |||||
| No of retrieved lymphnodes | ≥ 12 | |||||
APPEAR is a promising technique, having the advantage of not disrupting the sphincters compared to ISR. However, it carries a significant complication rate, while the long-term oncological and functional outcomes are unknown, since few studies have been published.
TEM and TAMIS should represent the treatment of choice for T1 rectal tumors, with specific criteria according to the NCCN guidelines and favorable pathologic features.
However, if the pathology report discloses depth of submucosal invasion sm2-3 level, we should advised the patient that since the locally excised pT1sm2-3 tumors have a local recurrence rate of up to 20%, they should be treated as suffering from T2 tumors.
The recommended treatment of choice for T2 rectal tumors is TME without adjuvant therapy per se.
Alternatively to that, although still debatable, preoperative (neoadjuvant) CRT followed by TEM or TAMIS seems the most promising available therapeutic option for T1sm2-3 or T2 tumors.
TaTMEs should be performed only when a board approved protocol is available and only by colorectal surgeons with extensive experience in minimally invasive and transanal endoscopic surgery.
Finally, apart from selecting the right type of operation, for every specific tumor, in every selected patient, we should also select patients that should avoid surgery. Habr-Gama et al[116] proposed the “Watch and Wait” approach for patients achieving complete clinical response (26.8%) after neoadjuvant CRT. With such an approach, the 5-year overall survival and disease free survival rates have been reported as high as 100% and 92%, respectively.
P- Reviewer: Coffey JC, Liu H, Rustagi T S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Network NCC, Rectal Cancer. Clinical Practice Guidelines in Oncology: National Comprehensive Cancer Network; version 1, 2015. Accessed 2014 Sept 17. Available from: http://www.nccn.org. |
| 2. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [PubMed] |
| 3. | Bordeianou L, Maguire LH, Alavi K, Sudan R, Wise PE, Kaiser AM. Sphincter-sparing surgery in patients with low-lying rectal cancer: techniques, oncologic outcomes, and functional results. J Gastrointest Surg. 2014;18:1358-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [PubMed] |
| 5. | Kapiteijn E, Kranenbarg EK, Steup WH, Taat CW, Rutten HJ, Wiggers T, van Krieken JH, Hermans J, Leer JW, van de Velde CJ. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg. 1999;165:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Bosch SL, Nagtegaal ID. The Importance of the Pathologist’s Role in Assessment of the Quality of the Mesorectum. Curr Colorectal Cancer Rep. 2012;8:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Velthuis S, Nieuwenhuis DH, Ruijter TE, Cuesta MA, Bonjer HJ, Sietses C. Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc. 2014;28:3494-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Lindsetmo RO, Delaney CP. A standardized technique for laparoscopic rectal resection. J Gastrointest Surg. 2009;13:2059-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Araújo SE, Seid VE, Bertoncini A, Campos FG, Sousa A, Nahas SC, Cecconello I. Laparoscopic total mesorectal excision for rectal cancer after neoadjuvant treatment: targeting sphincter-preserving surgery. Hepatogastroenterology. 2011;58:1545-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [PubMed] |
| 11. | Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, Saint-Aubert B, Colombo PE. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, Larach S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol. 2014;18:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Hall NR, Finan PJ, al-Jaberi T, Tsang CS, Brown SR, Dixon MF, Quirke P. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent. Predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41:979-983. [PubMed] |
| 14. | Ludwig KA. Sphincter-sparing resection for rectal cancer. Clin Colon Rectal Surg. 2007;20:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm Rule of Distal Bowel Resection Margin in Rectal Cancer Based on Clinical Evidence? A Systematic Review. Indian J Surg Oncol. 2012;3:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | den Dulk M, Putter H, Collette L, Marijnen CA, Folkesson J, Bosset JF, Rödel C, Bujko K, Påhlman L, van de Velde CJ. The abdominoperineal resection itself is associated with an adverse outcome: the European experience based on a pooled analysis of five European randomised clinical trials on rectal cancer. Eur J Cancer. 2009;45:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Martin ST, Heneghan HM, Winter DC. Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br J Surg. 2012;99:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 18. | Grimmeisen H. [Clinical aspects of biologically adverse weather phases]. Landarzt. 1968;44:363-366. [PubMed] |
| 19. | Portier G, Ghouti L, Kirzin S, Guimbaud R, Rives M, Lazorthes F. Oncological outcome of ultra-low coloanal anastomosis with and without intersphincteric resection for low rectal adenocarcinoma. Br J Surg. 2007;94:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Han JG, Wei GH, Gao ZG, Zheng Y, Wang ZJ. Intersphincteric resection with direct coloanal anastomosis for ultralow rectal cancer: the experience of People’s Republic of China. Dis Colon Rectum. 2009;52:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Akagi Y, Kinugasa T, Shirouzu K. Intersphincteric resection for very low rectal cancer: a systematic review. Surg Today. 2013;43:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 22. | Cipe G, Muslumanoglu M, Yardimci E, Memmi N, Aysan E. Intersphincteric resection and coloanal anastomosis in treatment of distal rectal cancer. Int J Surg Oncol. 2012;2012:581258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Spanos CP. Intersphincteric resection for low rectal cancer: an overview. Int J Surg Oncol. 2012;2012:241512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumours. Br J Surg. 1994;81:1376-1378. [PubMed] |
| 25. | Yoo JH, Hasegawa H, Ishii Y, Nishibori H, Watanabe M, Kitajima M. Long-term outcome of per anum intersphincteric rectal dissection with direct coloanal anastomosis for lower rectal cancer. Colorectal Dis. 2005;7:434-440. [PubMed] |
| 26. | Culligan K, Remzi FH, Soop M, Coffey JC. Review of nomenclature in colonic surgery--proposal of a standardised nomenclature based on mesocolic anatomy. Surgeon. 2013;11:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Sehgal R, Coffey JC. Historical development of mesenteric anatomy provides a universally applicable anatomic paradigm for complete/total mesocolic excision. Gastroenterol Rep (Oxf). 2014;2:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Kuo LJ, Hung CS, Wu CH, Wang W, Tam KW, Liang HH, Chang YJ, Wei PL. Oncological and functional outcomes of intersphincteric resection for low rectal cancer. J Surg Res. 2011;170:e93-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Coffey JC, Sehgal R, Culligan K, Dunne C, McGrath D, Lawes N, Walsh D. Terminology and nomenclature in colonic surgery: universal application of a rule-based approach derived from updates on mesenteric anatomy. Tech Coloproctol. 2014;18:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Kuo LJ, Hung CS, Wang W, Tam KW, Lee HC, Liang HH, Chang YJ, Huang MT, Wei PL. Intersphincteric resection for very low rectal cancer: clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J Surg Res. 2013;183:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Baek SJ, Al-Asari S, Jeong DH, Hur H, Min BS, Baik SH, Kim NK. Robotic versus laparoscopic coloanal anastomosis with or without intersphincteric resection for rectal cancer. Surg Endosc. 2013;27:4157-4163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Yamada K, Ogata S, Saiki Y, Fukunaga M, Tsuji Y, Takano M. Long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2009;52:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Shirouzu K, Ogata Y, Araki Y, Kishimoto Y, Sato Y. A new ultimate anus-preserving operation for extremely low rectal cancer and for anal canal cancer. Tech Coloproctol. 2003;7:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Shelygin YA, Vorobiev GI, Pikunov DY, Markova EV, Djhanaev YA, Fomenko OY. Intersphincteric resection with partial removal of external anal sphincter for low rectal cancer. Acta Chir Iugosl. 2008;55:45-53. [PubMed] |
| 35. | Nagayama S, Al-Kubati W, Sakai Y. What is the place of intersphincteric resection when operating on low rectal cancer? ISRN Surg. 2012;2012:585484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Tilney HS, Tekkis PP. Extending the horizons of restorative rectal surgery: intersphincteric resection for low rectal cancer. Colorectal Dis. 2008;10:3-15; discussion 15-16. [PubMed] |
| 37. | Akagi Y, Shirouzu K, Ogata Y, Kinugasa T. Oncologic outcomes of intersphincteric resection without preoperative chemoradiotherapy for very low rectal cancer. Surg Oncol. 2013;22:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Saito N, Ito M, Kobayashi A, Nishizawa Y, Kojima M, Nishizawa Y, Sugito M. Long-term outcomes after intersphincteric resection for low-lying rectal cancer. Ann Surg Oncol. 2014;21:3608-3615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Saito N, Sugito M, Ito M, Kobayashi A, Nishizawa Y, Yoneyama Y, Nishizawa Y, Minagawa N. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg. 2009;33:1750-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Koyama M, Murata A, Sakamoto Y, Morohashi H, Takahashi S, Yoshida E, Hakamada K. Long-term clinical and functional results of intersphincteric resection for lower rectal cancer. Ann Surg Oncol. 2014;21 Suppl 3:S422-S428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Akasu T, Takawa M, Yamamoto S, Fujita S, Moriya Y. Incidence and patterns of recurrence after intersphincteric resection for very low rectal adenocarcinoma. J Am Coll Surg. 2007;205:642-647. [PubMed] |
| 43. | Paty PB, Enker WE, Cohen AM, Lauwers GY. Treatment of rectal cancer by low anterior resection with coloanal anastomosis. Ann Surg. 1994;219:365-373. [PubMed] |
| 44. | Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Akasu T, Takawa M, Yamamoto S, Ishiguro S, Yamaguchi T, Fujita S, Moriya Y, Nakanishi Y. Intersphincteric resection for very low rectal adenocarcinoma: univariate and multivariate analyses of risk factors for recurrence. Ann Surg Oncol. 2008;15:2668-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Tiret E, Poupardin B, McNamara D, Dehni N, Parc R. Ultralow anterior resection with intersphincteric dissection--what is the limit of safe sphincter preservation? Colorectal Dis. 2003;5:454-457. [PubMed] |
| 47. | Saito N, Ono M, Sugito M, Ito M, Morihiro M, Kosugi C, Sato K, Kotaka M, Nomura S, Arai M. Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoid a permanent colostomy. Dis Colon Rectum. 2004;47:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Bretagnol F, Rullier E, Laurent C, Zerbib F, Gontier R, Saric J. Comparison of functional results and quality of life between intersphincteric resection and conventional coloanal anastomosis for low rectal cancer. Dis Colon Rectum. 2004;47:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Saito N, Moriya Y, Shirouzu K, Maeda K, Mochizuki H, Koda K, Hirai T, Sugito M, Ito M, Kobayashi A. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum. 2006;49:S13-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Yamada K, Ogata S, Saiki Y, Fukunaga M, Tsuji Y, Takano M. Functional results of intersphincteric resection for low rectal cancer. Br J Surg. 2007;94:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Ito M, Saito N, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum. 2009;52:64-70. [PubMed] [DOI] [Full Text] |
| 52. | Schiessel R, Novi G, Holzer B, Rosen HR, Renner K, Hölbling N, Feil W, Urban M. Technique and long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2005;48:1858-1565; discussion 1565-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Köhler A, Athanasiadis S, Ommer A, Psarakis E. Long-term results of low anterior resection with intersphincteric anastomosis in carcinoma of the lower one-third of the rectum: analysis of 31 patients. Dis Colon Rectum. 2000;43:843-850. [PubMed] |
| 54. | Denost Q, Laurent C, Capdepont M, Zerbib F, Rullier E. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum. 2011;54:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Williams NS, Murphy J. The APPEAR technique: a new concept in ultralow sphincter-saving resection. Dis Colon Rectum. 2008;51:369-370; author reply 371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Di Palo S, De Nardi P, Chiari D, Gazzetta P, Staudacher C. Laparoscopic TME with APPEAR (Anterior and Perineal PlanE for ultra-low Anterior Resection of the Rectum) technique for distal rectal cancer. Surg Endosc. 2013;27:3430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Qiu HZ, Xiao Y, Lin GL, Wu B, Niu BZ, Zhou JL. [Clinical application of anterior perineal plane for ultra-low anterior resection of the rectum]. Zhonghua Weichang Waike Zazhi. 2012;15:47-50. [PubMed] |
| 58. | Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064-1071; discussion 1071-1074. [PubMed] |
| 59. | Middleton PF, Sutherland LM, Maddern GJ. Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum. 2005;48:270-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Darwood RJ, Borley NR. TEMS: an alternative method for the repair of benign recto-vaginal fistulae. Colorectal Dis. 2008;10:619-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Qi Y, Stoddard D, Monson JR. Indications and techniques of transanal endoscopic microsurgery (TEMS). J Gastrointest Surg. 2011;15:1306-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Türler A, Schäfer H, Pichlmaier H. Role of transanal endoscopic microsurgery in the palliative treatment of rectal cancer. Scand J Gastroenterol. 1997;32:58-61. [PubMed] |
| 63. | Ramirez JM, Aguilella V, Gracia JA, Ortego J, Escudero P, Valencia J, Esco R, Martinez M. Local full-thickness excision as first line treatment for sessile rectal adenomas: long-term results. Ann Surg. 2009;249:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Kunitake H, Abbas MA. Transanal endoscopic microsurgery for rectal tumors: a review. Perm J. 2012;16:45-50. [PubMed] |
| 65. | Atallah S, Albert M, Debeche-Adams T, Larach S. Transanal minimally invasive surgery (TAMIS): applications beyond local excision. Tech Coloproctol. 2013;17:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Wolthuis AM, Cini C, Penninckx F, D’Hoore A. Transanal single port access to facilitate distal rectal mobilization in laparoscopic rectal sleeve resection with hand-sewn coloanal anastomosis. Tech Coloproctol. 2012;16:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Bak Y, Merriam M, Neff M, Berg DA. Novel approach to rectal foreign body extraction. JSLS. 2013;17:342-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Araujo SE, Crawshaw B, Mendes CR, Delaney CP. Transanal total mesorectal excision: a systematic review of the experimental and clinical evidence. Tech Coloproctol. 2015;19:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol. 2014;5:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Buess G, Theiss R, Hutterer F, Pichlmaier H, Pelz C, Holfeld T, Said S, Isselhard W. [Transanal endoscopic surgery of the rectum - testing a new method in animal experiments]. Leber Magen Darm. 1983;13:73-77. [PubMed] |
| 71. | Buess G, Hutterer F, Theiss J, Böbel M, Isselhard W, Pichlmaier H. [A system for a transanal endoscopic rectum operation]. Chirurg. 1984;55:677-680. [PubMed] |
| 72. | Smart CJ, Cunningham C, Bach SP. Transanal endoscopic microsurgery. Best Pract Res Clin Gastroenterol. 2014;28:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Caro-Tarrago A, Gomez-Diaz CJ, Navarro-Soto S. Transanal endoscopic surgery in rectal cancer. World J Gastroenterol. 2014;20:11538-11545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Dias AR, Nahas CS, Marques CF, Nahas SC, Cecconello I. Transanal endoscopic microsurgery: indications, results and controversies. Tech Coloproctol. 2009;13:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18:775-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 76. | Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 77. | Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 78. | Floyd ND, Saclarides TJ. Transanal endoscopic microsurgical resection of pT1 rectal tumors. Dis Colon Rectum. 2006;49:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Heidary B, Phang TP, Raval MJ, Brown CJ. Transanal endoscopic microsurgery: a review. Can J Surg. 2014;57:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Hahnloser D, Cantero R, Salgado G, Dindo D, Rega D, Delrio P. Transanal minimal invasive surgery for rectal lesions: should the defect be closed? Colorectal Dis. 2015;17:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 81. | Stitzenberg KB, Sanoff HK, Penn DC, Meyers MO, Tepper JE. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol. 2013;31:4276-4282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345-351. [PubMed] |
| 83. | Lee W, Lee D, Choi S, Chun H. Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc. 2003;17:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 84. | Madbouly KM, Remzi FH, Erkek BA, Senagore AJ, Baeslach CM, Khandwala F, Fazio VW, Lavery IC. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum. 2005;48:711-719; discussion 719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Christoforidis D, Cho HM, Dixon MR, Mellgren AF, Madoff RD, Finne CO. Transanal endoscopic microsurgery versus conventional transanal excision for patients with early rectal cancer. Ann Surg. 2009;249:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Elmessiry MM, Van Koughnett JA, Maya A, DaSilva G, Wexner SD, Bejarano P, Berho M. Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis. 2014;16:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Lezoche G, Paganini AM, Campagnacci R, Ghiselli R, Pelloni M, Rombini A, Guerrieri M. Treatment of rectal cancer by transanal endoscopic microsurgery: review of the literature. Minerva Chir. 2013;68:1-9. [PubMed] |
| 88. | Doornebosch PG, Ferenschild FT, de Wilt JH, Dawson I, Tetteroo GW, de Graaf EJ. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum. 2010;53:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 89. | Tytherleigh MG, Warren BF, Mortensen NJ. Management of early rectal cancer. Br J Surg. 2008;95:409-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, Warren B, Mortensen NJ. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 91. | Baxter NN, Garcia-Aguilar J. Organ preservation for rectal cancer. J Clin Oncol. 2007;25:1014-1020. [PubMed] |
| 92. | Lezoche G, Baldarelli M, Guerrieri M, Paganini AM, De Sanctis A, Bartolacci S, Lezoche E. A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc. 2008;22:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 93. | Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 94. | Perez RO, Habr-Gama A, Lynn PB, São Julião GP, Bianchi R, Proscurshim I, Gama-Rodrigues J. Transanal endoscopic microsurgery for residual rectal cancer (ypT0-2) following neoadjuvant chemoradiation therapy: another word of caution. Dis Colon Rectum. 2013;56:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 95. | Sajid MS, Farag S, Leung P, Sains P, Miles WF, Baig MK. Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer. Colorectal Dis. 2014;16:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Banerjee AK, Jehle EC, Kreis ME, Schott UG, Claussen CD, Becker HD, Starlinger M, Buess GF. Prospective study of the proctographic and functional consequences of transanal endoscopic microsurgery. Br J Surg. 1996;83:211-213. [PubMed] |
| 97. | Kennedy ML, Lubowski DZ, King DW. Transanal endoscopic microsurgery excision: is anorectal function compromised? Dis Colon Rectum. 2002;45:601-604. [PubMed] |
| 98. | Cataldo PA, O’Brien S, Osler T. Transanal endoscopic microsurgery: a prospective evaluation of functional results. Dis Colon Rectum. 2005;48:1366-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Doornebosch PG, Gosselink MP, Neijenhuis PA, Schouten WR, Tollenaar RA, de Graaf EJ. Impact of transanal endoscopic microsurgery on functional outcome and quality of life. Int J Colorectal Dis. 2008;23:709-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Allaix ME, Rebecchi F, Giaccone C, Mistrangelo M, Morino M. Long-term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg. 2011;98:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 101. | Schiphorst AH, Langenhoff BS, Maring J, Pronk A, Zimmerman DD. Transanal minimally invasive surgery: initial experience and short-term functional results. Dis Colon Rectum. 2014;57:927-932. [PubMed] [DOI] [Full Text] |
| 102. | Tuech JJ, Karoui M, Lelong B, De Chaisemartin C, Bridoux V, Manceau G, Delpero JR, Hanoun L, Michot F. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg. 2015;261:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 103. | Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. Dynamic article: transanal rectal excision: a pilot study. Dis Colon Rectum. 2014;57:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 104. | de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 105. | Hompes R, Arnold S, Warusavitarne J. Towards the safe introduction of transanal total mesorectal excision: the role of a clinical registry. Colorectal Dis. 2014;16:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Leroy J, Barry BD, Melani A, Mutter D, Marescaux J. No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery. JAMA Surg. 2013;148:226-230; discussion 231. [PubMed] |
| 107. | Zhang H, Zhang YS, Jin XW, Li MZ, Fan JS, Yang ZH. Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol. 2013;17:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Atallah S, Nassif G, Polavarapu H, deBeche-Adams T, Ouyang J, Albert M, Larach S. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol. 2013;17:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, DeLacy B, Balust J, Lacy AM. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 110. | Whiteford MH, Denk PM, Swanström LL. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc. 2007;21:1870-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 111. | Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 112. | Dumont F, Goéré D, Honoré C, Elias D. Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum. 2012;55:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 113. | Lacy AM, Adelsdorfer C, Delgado S, Sylla P, Rattner DW. Minilaparoscopy-assisted transrectal low anterior resection (LAR): a preliminary study. Surg Endosc. 2013;27:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 114. | Velthuis S, van den Boezem PB, van der Peet DL, Cuesta MA, Sietses C. Feasibility study of transanal total mesorectal excision. Br J Surg. 2013;100:828-831; discussion 831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 115. | Zorron R, Phillips HN, Wynn G, Neto MP, Coelho D, Vassallo RC. “Down-to-Up” transanal NOTES Total mesorectal excision for rectal cancer: Preliminary series of 9 patients. J Minim Access Surg. 2014;10:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |