Published online Mar 15, 2015. doi: 10.4251/wjgo.v7.i3.12
Peer-review started: September 29, 2014
First decision: January 8, 2015
Revised: February 25, 2015
Accepted: March 5, 2015
Article in press: March 6, 2015
Published online: March 15, 2015
Processing time: 165 Days and 19.1 Hours
Gastric metastases from lung adenocarcinoma are rare. Because gastric metastasis grossly resembles advanced gastric cancer, it is difficult to diagnose gastric metastasis especially when the histology of the primary lung cancer is adenocarcinoma. We describe a case of gastric metastasis from primary lung adenocarcinoma mimicking Borrmann type IV primary gastric cancer. A 68-year-old man with known lung adenocarcinoma with multiple bone metastases had been experiencing progressive epigastric pain and dyspepsia over one year. Esophagogastroduodenoscopy revealed linitis plastica-like lesions in the fundus of the stomach. Pathologic examination revealed a moderately differentiated adenocarcinoma with submucosal infiltration. Positive immunohistochemical staining for thyroid transcription factor-1 (TTF-1) and napsin A (Nap-A) confirmed that the metastasis was pulmonary in origin. The patient had been treated with palliative chemotherapy for the lung cancer and had lived for over fifteen months after the diagnosis of gastric metastasis. Clinicians should be aware of the possibility of gastric metastasis in patients with primary lung adenocarcinoma, and additional immunohistochemical staining for Nap-A as well as TTF-1 may help in differentiating its origin.
Core tip: This report describes the rare case of a 68-year-old patient with gastric metastasis from primary lung adenocarcinoma mimicking Borrmann type IV primary gastric cancer. When gastric carcinoma is suspected in patients with primary lung adenocarcinoma, a differential diagnosis of primary gastric cancer and gastric metastasis can be done through special immunohistochemical staining with napsin-A and thyroid transcription factor-1, especially when the biopsy results are ambiguous by histology alone.
- Citation: Kim MJ, Hong JH, Park ES, Byun JH. Gastric metastasis from primary lung adenocarcinoma mimicking primary gastric cancer. World J Gastrointest Oncol 2015; 7(3): 12-16
- URL: https://www.wjgnet.com/1948-5204/full/v7/i3/12.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i3.12
Gastric metastasis from primary lung cancer is rare. Although most gastric metastases are asymptomatic and not detected during the lifetime of the patients, when overlooked or misdiagnosed, fatal complications such as acute bleeding and perforation can occur. Here, we report a case of gastric metastasis from primary lung adenocarcinoma mimicking Borrmann type IV primary gastric cancer grossly and diagnosed through special immunohistochemical staining of tissue for thyroid transcription factor-1 (TTF-1) and napsin A (Nap-A). This report was approved by our institutional review board, and the approval number is OC14RISI0071.
A 68-year-old man with known lung cancer was referred to our hospital in March 2013 with progressive epigastric pain and dyspepsia for one year. He was a lifetime non-smoker, had been diagnosed with adenocarcinoma in situ (AIS), and had undergone left lower lobe lobectomy in 1996 at a different hospital. In June 2004, he developed a first recurrence in the lung and underwent posterior segmentectomy of the right upper lobe. In March 2007, a second recurrence was found in the left upper lobe, pleura, and diaphragm and he underwent left upper lobe wedge resection and four cycles of paclitaxel/carboplatin chemotherapy. After eighteen months, surveillance chest computed tomography (CT) showed multiple lung-to-lung metastases, and left third and fourth rib metastases. Because mutations of the epidermal growth factor receptor (EGFR) gene were not found in the tumor tissue, he received fifty-five cycles of palliative pemetrexed chemotherapy between March 2009 and February 2013, and had stable disease for approximately four years.
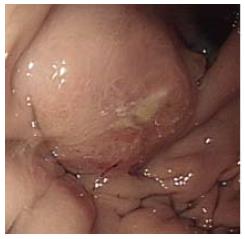
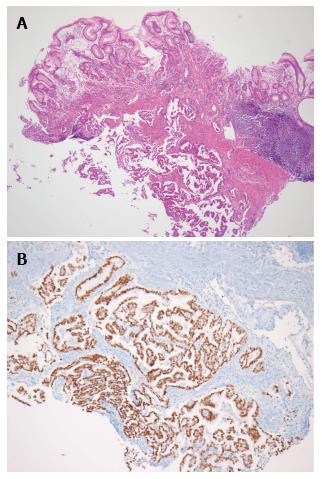
The patient had progressive epigastric pain and dyspepsia since 2012. On March 18, 2013, esophago-gastroduo-denoscopy (EGD) revealed a 4 cm × 5 cm submucosal tumor-like lesion containing central ulceration with fusion and thickened mucosal folds in the stomach fundus (Figure 1). The stomach had insufficient expansion with aeration, compatible with Borrmann type IV gastric cancer. Laboratory findings revealed mild anemia (hemoglobin level 106 g/L and hematocrit 32.8%), normal lactate dehydrogenase levels, and elevated carcinoembryonic antigen (9.67 μg/L). Abdominal CT showed irregular gastric wall thickening in the fundus associated with perigastric infiltration and diffuse nodular infiltration in the omentum and several enlarged lymph nodes in the perigastric space, suspicious of primary gastric cancer and peritoneal dissemination. Pathologic examination revealed a moderately differentiated adenocarcinoma with submucosal infiltration and presence of endolymphatic emboli (Figure 2A). Especially, the surface epithelium had no precancerous or cancerous lesions, suggesting that this lesion was metastatic or primary gastric cancer of Borrmann type IV. Positive immunohistochemical staining for TTF-1 and Nap-A confirmed that this lesion had metastasized from the lung (Figure 2B). Finally, the patient was diagnosed with known primary lung adenocarcinoma with gastric and intraperitoneal metastases.
Irinotecan/cisplatin combination regimen as second-line palliative chemotherapy for lung adenocarcinoma was initiated on March 26, 2013 and maintained for eight months. The tumor lesion remained stable for eleven cycles of chemotherapy but was discontinued on November 29, 2013 after the patient had uncontrolled diarrhea and decreasing performance status. Five months later, a surveillance chest CT showed stable lesion, but abdominal CT revealed a new hepatic nodule in segment 8. Third-line gemcitabine monotherapy was started on May 15, 2014 and to date, has been well tolerated by the patient.
Gastric metastasis from primary lung cancer is rare. A review of autopsies of 1010 cancer patients found only seventeen patients with gastric metastasis (1.7%)[1]. Breast cancer, lung cancer, esophageal cancer, and skin melanoma are the most frequent primary sites[2]. Lung cancer metastasis to the gastrointestinal (GI) tract is rare (0.5%-10%)[3], and most commonly occurs in the small bowel[4]. In one study, among eighteen patients with lung cancer and GI metastasis, nine had small bowel metastasis, four had gastric metastasis, two had colon metastasis, and one had duodenal metastasis[3]. An analysis of 473 autopsies of patients with primary lung cancer showed 3.4% with gastric metastases[5]. The prevalence of histologic types of lung cancer that metastasize to the stomach is not well known. Hasegawa et al[5] reported in 1993 that large-cell lung cancer was the most common histology, accounting for 15.6% of primary lung cancer with gastric metastases. However, recent reports have shown that pulmonary adenocarcinoma was the most frequently reported histologic type of metastasis to the gastric wall[4,6-10].
Because hematogenous metastases usually implant in the gastric submucosa[1], diagnosis can only be made after considerable growth. Therefore, most gastric metastases from primary lung cancer are asymptomatic and often discovered during autopsy[9]. Among 473 autopsies of primary lung cancer patients, only two of sixteen gastric metastasis cases had been detected clinically in living patients[5]. The most common clinical manifestations of symptomatic patients are nonspecific epigastric pain and chronic bleeding resulting in melena and anemia[1,11]. Perforation and acute bleeding have been reported in some fatal cases[4,10,12-14].
Endoscopic evaluation of 54 patients with gastric metastasis from solid malignant tumors has revealed that gastric metastases mimic submucosal tumors in 28 patients (52%) and primary gastric cancers in 21 patients (39%)[2]. When gastric metastasis mimics primary gastric cancer, it resembles advanced primary gastric cancer rather than early gastric cancer, presenting as bull’s eye signs, volcano-like ulcers, or surface umbilication[6-9,11,15]. An infiltrating “linitis plastica” pattern has been seen in only 2% of cases in lung cancer[2,8], while it has been seen in about 50% of gastric metastases from breast carcinoma[1,15].
In the present case, the patient had perigastric lymph node involvement, omental seeding, and progressive liver metastasis, in addition to Bormann type IV-like advanced gastric cancer lesion in the gastric wall. Kim et al[13] reported patients with squamous cell lung cancer with gastric wall metastasis, perigastric lymph node metastasis, and splenic invasion who received total gastrectomy and splenectomy for control of bleeding[13]. However, most gastric metastasis from primary lung cancer manifests as a solitary gastric metastasis[6-9,11]. With the exception of gastric wall metastases, these accompanying intra-abdominal metastases have only been reported in rare and unusual gastric metastasis cases.
TTF-1 regulates gene expression in the thyroid, lung, and diencephalon during embryogenesis[16]. TTF-1 has appeared to be helpful in distinguishing tissues of pulmonary origin from those of others in circumstances for which there is currently no lung-specific tumor marker[17,18]. However, there are some prior reports indicating that 13% to 45% of metastatic adenocarcinomas of pulmonary origin are TTF-1 negative, thereby limiting the sensitivity of this marker. Another marker, Nap-A, is a functional aspartic proteinase expressed in the cytoplasm of healthy lung parenchyma[16] that consists of a 38 kDa protein expressed in type II pneumocytes, alveolar macrophages, renal tubules, and exocrine glands and ducts in the pancreas. Data from tissue microarrays constructed from primary lung cancers indicate that the sensitivity of Nap-A for primary lung adenocarcinoma is similar to that of TTF-1[17]. According to recent reports, Nap-A is more sensitive than TTF-1 in distinguishing primary lung carcinoma from other adenocarcinomas[16,18-20], making it a useful additional immunohistochemical staining to TTF-1 for determining the origin of metastatic adenocarcinomas. In our present case, in addition to the EGD findings, clinical manifestation such as liver metastasis mimicked primary gastric cancer, causing us to question whether it was real gastric metastasis or not. However, immunohistochemical positivity for TTF-1 and Nap-A confirmed the diagnosis of gastric metastasis from primary lung adenocarcinoma.
Optimal management of symptomatic gastric meta-stasis from primary lung cancer remains controversial because gastrointestinal involvement is considered to represent an advanced stage. Lee et al[21] reported longer survival in patients with gastric and/or duodenal metastases that were managed by supportive treatment without surgery. However, surgery is still necessary to prevent life-threatening complication such as massive hemorrhage, obstruction and perforation thus providing effective palliation and reasonable survival in patients with only a solitary gastric metastasis[21,22]. In the present case, surgical intervention was not performed since the patient had other extrathoracic metastases outside the stomach and the symptoms were not very severe and well controlled by medical treatment.
Because gastric metastasis is a late-stage disease, in many cases, the patient’s performance status is poor owing to the high burden of the primary malignancy itself and related complications. Therefore, the prognosis of gastric metastasis from primary lung cancer is very poor. However, our patient has had stable disease with third-line chemotherapy and has lived for over five years after the initial diagnosis of lung cancer and for fifteen months after the diagnosis of gastric metastasis. A possible explanation for this extraordinarily good clinical outcome in our patient, even though his mutation status of EGFR was wild-type, could be the initial pathologic diagnosis of AIS, formerly known as bronchioloalveolar carcinoma. This is a non-mucinous or mucinous type adenocarcinoma composed of tumor cells replacing the alveolar wall without stromal invasion[23], and it is associated with better survival than other invasive adenocarcinomas[24]. Adenocarcinoma in situ and minimally invasive adenocarcinoma are known to have near 100% 5-year survival rates when completely resected[25].
In conclusion, clinicians should be aware of this rare situation of gastric metastasis from primary lung cancer. When gastric carcinoma is suspected in patients with primary lung adenocarcinoma, the differential diagnosis of primary gastric cancer from gastric metastasis should be done through special immunohistochemical staining with Nap-A and TTF-1, especially when the biopsy results are ambiguous by histology alone.
A 68-year-old man with known lung adenocarcinoma presented with epigastric pain and dyspepsia.
Malignant tumors (primary or metastatic).
Mild anemia (hemoglobin 10.6 gm/dL and hematocrit 32.8%), normal lactate dehydrogenase levels, and elevated carcinoembryonic antigen (9.67 μg/L).
Esophagogastroduodenoscopy revealed linitis plastica-like lesions in the fundus of the stomach and abdominal computed tomography scan showed diffuse nodular infiltration in the omentum, suspicious of primary gastric cancer and peritoneal dissemination.
Endoscopy and biopsy revealed an adenocarcinoma with submucosal infiltration and thyroid transcription factor-1 (TTF-1)/napsin A (Nap-A) positive confirmed that the gastric metastasis from pulmonary origin.
The patient was treated with palliative chemotherapy for the lung cancer (Irinotecan/cisplatin combination regimen).
Gastric metastases from lung adenocarcinoma are rare and difficult to diagnose especially when the histology of the primary lung cancer is adenocarcinoma because it grossly resembles advanced gastric cancer.
Nap-A is a functional aspartic proteinase expressed in the cytoplasm of healthy lung parenchyma, more sensitive than TTF-1 in distinguishing primary lung carcinoma from other adenocarcinomas.
Clinicians should be aware of the possibility of gastric metastasis in patients with primary lung adenocarcinoma, and additional immunohistochemical staining for Nap-A as well as TTF-1 may help in differentiating its origin.
This is a nicely written and interesting case-report.
P- Reviewer: Krieg A, Kim JJ, Mortensen K, Zhang SN S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975;20:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Oda H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, Yoshida S, Shimoda T. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Rossi G, Marchioni A, Romagnani E, Bertolini F, Longo L, Cavazza A, Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Garwood RA, Sawyer MD, Ledesma EJ, Foley E, Claridge JA. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg. 2005;71:110-116. [PubMed] |
| 5. | Hasegawa N, Yamasawa F, Kanazawa M, Kawashiro T, Kikuchi K, Kobayashi K, Ishihara T, Kuramochi S, Mukai M. [Gastric metastasis of primary lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:1390-1396. [PubMed] |
| 6. | Katsenos S, Archondakis S. Solitary gastric metastasis from primary lung adenocarcinoma: a rare site of extra-thoracic metastatic disease. J Gastrointest Oncol. 2013;4:E11-E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Lee MH, Kim SR, Soh JS, Chung MJ, Lee YC. A solitary gastric metastasis from pulmonary adenocarcinoma: a case report. Thorax. 2010;65:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Okazaki R, Ohtani H, Takeda K, Sumikawa T, Yamasaki A, Matsumoto S, Shimizu E. Gastric metastasis by primary lung adenocarcinoma. World J Gastrointest Oncol. 2010;2:395-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Sileri P, D’Ugo S, Del Vecchio Blanco G, Lolli E, Franceschilli L, Formica V, Anemona L, De Luca C, Gaspari AL. Solitary metachronous gastric metastasis from pulmonary adenocarcinoma: Report of a case. Int J Surg Case Rep. 2012;3:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Suzaki N, Hiraki A, Ueoka H, Aoe M, Takigawa N, Kishino T, Kiura K, Kanehiro A, Tanimoto M, Harada M. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res. 2002;22:1209-1212. [PubMed] |
| 11. | Casella G, Di Bella C, Cambareri AR, Buda CA, Corti G, Magri F, Crippa S, Baldini V. Gastric metastasis by lung small cell carcinoma. World J Gastroenterol. 2006;12:4096-4097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Fletcher MS. Gastric perforation secondary to metastatic carcinoma of the lung: a case report. Cancer. 1980;46:1879-1882. [PubMed] |
| 13. | Kim YI, Kang BC, Sung SH. Surgically resected gastric metastasis of pulmonary squamous cell carcinoma. World J Gastrointest Surg. 2013;5:278-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Schmidt G, Börsch G, von Liebe S, Böhm E. Gastric perforation secondary to metastatic bronchogenic carcinoma. Hepatogastroenterology. 1985;32:103-105. [PubMed] |
| 15. | Campoli PM, Ejima FH, Cardoso DM, Silva OQ, Santana Filho JB, Queiroz Barreto PA, Machado MM, Mota ED, Araujo Filho JA, Alencar Rde C. Metastatic cancer to the stomach. Gastric Cancer. 2006;9:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. 2012;136:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Mukhopadhyay S, Katzenstein AL. Comparison of monoclonal napsin A, polyclonal napsin A, and TTF-1 for determining lung origin in metastatic adenocarcinomas. Am J Clin Pathol. 2012;138:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Dejmek A, Naucler P, Smedjeback A, Kato H, Maeda M, Yashima K, Maeda J, Hirano T. Napsin A (TA02) is a useful alternative to thyroid transcription factor-1 (TTF-1) for the identification of pulmonary adenocarcinoma cells in pleural effusions. Diagn Cytopathol. 2007;35:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Suzuki A, Shijubo N, Yamada G, Ichimiya S, Satoh M, Abe S, Sato N. Napsin A is useful to distinguish primary lung adenocarcinoma from adenocarcinomas of other organs. Pathol Res Pract. 2005;201:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Lee PC, Lo C, Lin MT, Liang JT, Lin BR. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol. 2011;17:4314-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Goh BK, Yeo AW, Koong HN, Ooi LL, Wong WK. Laparotomy for acute complications of gastrointestinal metastases from lung cancer: is it a worthwhile or futile effort? Surg Today. 2007;37:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Maeshima A, Sakamoto M, Hirohashi S. Mixed mucinous-type and non-mucinous-type adenocarcinoma of the lung: immunohistochemical examination and K- ras gene mutation. Virchows Arch. 2002;440:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Zell JA, Ou SH, Ziogas A, Anton-Culver H. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J Clin Oncol. 2005;23:8396-8405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Van Schil PE, Sihoe AD, Travis WD. Pathologic classification of adenocarcinoma of lung. J Surg Oncol. 2013;108:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |