Published online Nov 15, 2015. doi: 10.4251/wjgo.v7.i11.361
Peer-review started: April 4, 2015
First decision: May 18, 2015
Revised: June 9, 2015
Accepted: August 25, 2015
Article in press: September 7, 2015
Published online: November 15, 2015
Processing time: 227 Days and 16.8 Hours
AIM: To evaluate neoangiogenesis in patients with colon cancer by two fluorescently labeled antibodies on fresh biopsy samples imaged with confocal laser endomicroscopy (CLE).
METHODS: CLE is an imaging technique for gastrointestinal endoscopy providing in vivo microscopy at subcellular resolution. An important question in validating tumor angiogenesis is what proportion of the tumor vascular network is represented by pre-existing parent tissue vessels and newly formed vessels. CD105 (endoglin) represents a proliferation-associated endothelial cell adhesion molecule. In contrast to pan-endothelial markers, such as CD31, CD105 is preferentially expressed in activated endothelial cells that participate in neovascularization. Thus, we evaluated CD105 and CD31 expression from samples of ten patients with primary rectal adenocarcinoma, using a dedicated endomicroscopy system. A imaging software was used to obtain the Z projection of the confocal serial images from each biopsy sample previously combined into stacks. Vascular density and vessel diameters were measured within two 50 μm x 475 μm rectangular regions of interest centered in the middle of each image in the horizontal and vertical direction. The results were averaged over all the patients and were expressed as the mean ± SE.
RESULTS: The use of an anti-CD105 antibody was found to be suitable for the detection of blood vessels in colon cancer. Whereas anti-CD31 antibodies stained blood vessels in both normal and pathologic colon equally, CD105 expression was observed primarily in malignant lesions, with little or no expression in the vessels of the normal mucosa (244.21 ± 130.7 vessels/mm3 in only four patients). The average diameter of anti-CD105 stained vessels was 10.97 ± 0.6 μm in tumor tissue, and the vessel density was 2787.40 ± 134.8 vessels/mm3. When using the anti-CD31 antibody, the average diameter of vessels in the normal colon tissue was 7.67 ± 0.5 μm and the vessel density was 3191.60 ± 387.8 vessels/mm3, while in the tumors we obtained an average diameter of 10.88 ± 0.8 μm and a vessel density of 4707.30 ± 448.85 vessels/mm3. Thus, there were more vessels stained with CD31 than CD105 (P < 0.05). The average vessel diameter was similar for both CD31 and CD105 staining. A qualitative comparison between CLE vs immunohistochemistry lead to similar results.
CONCLUSION: Specific imaging and quantification of tumor microvessels are feasible in human rectal cancer using CLE examination and CD105 immunostaining of fresh tissue samples.
Core tip: We evaluated CD105 expression from fresh tissue samples of human rectal adenocarcinoma, using confocal laser endomicroscopy (CLE). While vessels marked with fluorescent CD31 were visible in both normal and malignant tissue, CD105 was predominantly expressed in tumor lesions, having reduced affinity for normal rectal mucosa. Our data showed that CLE using CD105 antibody for tumor vascular network imaging is feasible and that CD105 represents a more specific marker for rectal cancer neoangiogenesis than panendothelial markers. To our knowledge, this is the first study to report the use of fluorescently-labeled CD105 antibody in conjunction with CLE in patients with rectal tumor.
- Citation: Ciocâlteu A, Săftoiu A, Pirici D, Georgescu CV, Cârţână T, Gheonea DI, Gruionu LG, Cristea CG, Gruionu G. Tumor neoangiogenesis detection by confocal laser endomicroscopy and anti-CD105 antibody: Pilot study. World J Gastrointest Oncol 2015; 7(11): 361-368
- URL: https://www.wjgnet.com/1948-5204/full/v7/i11/361.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i11.361
Tumor neoangiogenesis, defined as the neo-formation of blood vessels from pre-existing microvessels, represents an attractive target for both imaging and therapeutic strategies. It is thought that neovascularization is first activated by an “angiogenic switch” during premalignant phases of carcinogenesis, before tumors emerge (Folkman et al[1]; Bolontrade et al[2]; Huss et al[3]). An important question in validating tumor neoangiogenesis is what proportion of tumor vascular network is represented by pre-existing vs newly formed vessels. In this respect, new imaging and diagnostic techniques which differentiate tumors vascularization at different stages are desired[4].
Antihuman panendothelial cells antibodies are used to identify all types of blood vessels in a given tissue sample, irrespective of being mature or immature. Commonly used panendothelial markers such as CD31, CD34 or von Willebrand factor detect the parent vessels as well as the tumor vasculature, but they are not always expressed in all tumor blood vessels. Moreover, these antibodies seem to have a higher affinity for large than for microvessels[5].
Endoglin (CD105) is a co-receptor for various TGF-β family members and therefore a target for tumor vasculature[6]. The role of endoglin and the indispensable role for the TGF-β signaling pathway in developmental angiogenesis has been studied on genetically modified mice[7-9]. Unlike all other markers, endoglin mediates direct pro-angiogenic effects of TGF-β on endothelial cells and is specifically overexpressed in tumor vessels, on proliferating endothelial cells, at sites of active angiogenesis. Its expression has also been associated with metastasis and patient survival[6,10,11]. Recent reports suggest that elevated plasma levels of endoglin in patients with colorectal cancer correlate with poor prognosis (Li et al[7]; Duff et al[12]). As a result, endoglin could represent a valuable tool for the diagnosis, tumor vasculature visualization and targeted treatment of solid cancers[4].
Since endoglin is highly and specifically expressed on tumor endothelial cells, in the present study we hypothesized that it could be used as an appropriate marker to assess the vascularization of a tumor.
Confocal laser endomicroscopy (CLE) gained an important role in the study and real-time histopathological diagnosis of various gastrointestinal diseases, such as celiac disease, Barrett esophagus, microscopic colitis, inflammatory bowel disease, and recently Clostridium Difficile associated colitis[13]. Recent meta-analyses performed to determine the diagnostic accuracy of CLE in the detection of colorectal neoplasia showed high sensitivity and specificity of the method[14,15].
Recently, we have used CLE to assess tumor vasculature by fluorescence labelled antibodies targeted against endothelial markers[16,17]. In the present feasibility study, we used CLE to compare the selective expression of fluorescently labeled anti-CD105 antibodies in newly-formed vessels to fluorescently labeled anti-CD31 total vessel staining, and the gold standard of histopathology. More specifically, we aimed to answer the following questions: (1) Can the use of CLE in association with CD105 offer a more adequate quantitative and qualitative analysis of newly formed vessels than the commonly used panendothelial markers in human rectal cancer? and (2) Can this method be used in vivo for a rapid characterization of tumor microvascularization?
The current study was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1964, as revised in 2004) and approved by the local Ethics Committee. All the patients included read and accepted the written informed consent prior to study entry.
Tissue specimens from ten patients 47-80 years old (mean age of 65.2 ± 9.9 years), with histologically diagnosed rectal cancer, were collected during colonoscopy before undergoing surgical resection or neoadjuvant therapy to avoid artifacts (e.g., false positive resulted from fibrosis or inflammation increased in case of radio-chemotherapy). Fresh tissue samples from these patients were immediately processed for both CLE and immunohistochemistry assessment.
The ten patient population contained stage II-III (according to AJCC staging system) rectal adenocarcinomas without metastatic spread.
The main clinical signs the patients presented at admission in the hospital were alternating diarrhea and constipation, accelerated intestinal transit, recent constipation, unintended weight loss, rectal bleeding, abdominal pain or discomfort. Only three patients accused rectal bleeding as a single symptom, also confirmed by the physical examination (digital rectal examination). Seven patients had nonspecific findings for the laboratory tests such as moderate elevated hematological values of erythrocyte sedimentation rate (three patients), slightly elevated white blood cells count (two patients) and moderate anemia (two patients). Two patients presented slightly elevated values of both tumor markers CEA and CA19-9, while three of them had only slightly elevated CEA value. Computed tomography scan excluded the presence of metastases in all ten patients and described rectal wall thickening in four cases. Histological examination findings from endoscopic samples are summarized in Table 1.
| Patient | Gender | Age | Tumor grading | Preoperative stage | RT | CTX |
| 1 | F | 67 | G1 | T3N0M0 | No | No |
| 2 | M | 65 | G2 | T3N0M0 | Neoadj | No |
| 3 | M | 47 | G2 | T3N0M0 | Neoadj | No |
| 4 | M | 66 | G2 | T4N0M0 | Adj | Adj |
| 5 | M | 54 | G2 | T3N0M0 | No | No |
| 6 | M | 67 | G1/G2 | T3N1M0 | Neoadj | Neoadj |
| 7 | F | 80 | G1 + Mucinous areas | T3N0M0 | Neoadj | Neoadj |
| 8 | F | 78 | G2 | T3N2M0 | Neoadj | No |
| 9 | M | 59 | G1 | T3N1M0 | No | No |
| 10 | M | 69 | G1/G2 | T3N0M0 | Neoadj | No |
The biopsy samples collected with a standard colonoscope (CFQ160ZL, Olympus, Tokyo, Japan) were processed following a standardized protocol. During the endoscopic procedure, for every patient, six biopsies were taken from tumor, avoiding the ulcerated areas (paired biopsies for CLE assessment, standard immunohistochemistry and histopathological examination, respectively), as well as four biopsies from macroscopically normal surrounding tissue samples (paired biopsies for both CLE processing and standard immunohistochemistry). The biopsies were immersed immediately in 10% neutral buffered formalin for histopathological analysis, as well as in saline solution for the ex vivo immunohistochemical processing. Samples from saline solution were thoroughly washed and incubated for one hour in the dark, at 37 °C, with Alexa-Fluor 488-labeled anti-CD31 (PECAM) antibody (mouse anti-human IgG1, Exbio, Prague, Czech Republic) or respectively FITC-labeled anti-CD105/Endoglin antibody (mouse anti-human IgG2a, Exbio), diluted as 1:15 and 1:5 in saline with 1% bovine serum albumin (BSA, Sigma-Aldrich, Munich, Germany). Afterwards, the excess antibodies were washed away in saline and the samples were immediately visualized in CLE imaging to assess the microvascularization ex vivo up to a maximum depth of 250 μm. CLE images were acquired using Pentax EC-3870 CIFK, Tokyo, Japan, a dedicated endomicroscopy system with an excitation wavelength of 488 nm and with a maximum laser power output of ≤ 1 mW at the surface of the tissue[16,17].
To assess both endothelial markers more accurately, we used the color overlay function in the ImageJ image processing software (National Institutes of Health, United States). This software was used to obtain the Z projection of the confocal serial image stacks from each biopsy sample (60-250 images per biopsy sample). The vascular density and the vessel diameters were measured from the Z projections within two 50 μm × 475 μm rectangular regions of interest (ROI) centered in the middle of each image in the horizontal and vertical direction as before[17].
The results were averaged over all the patients and were expressed as the mean ± SE. We used unpaired two-tailed Student’s t-test, with the level of significance set at P≤ 0.05 to evaluate the variation of CD105 expression vs CD31 expression in microvessels from the normal mucosa tissue and from the rectal tumors.
To confirm the role of CD105 vs CD31 in tumor neoangiogenesis, adjacent samples from the same patient were processed for immunohistochemistry, for normal and tumor samples as described previously[16,17]. Briefly, after formaldehyde fixation and paraffin embedding, 4 μm tissue sections were sliced from these blocks, deparaffinized, re-hydrated and processed for antigen retrieval by microwaving for 20 min in citrate buffer pH 6. Endogenous peroxidase was next blocked utilizing 1% H2O2 for 30 min, and the false antigenic sites were further blocked by incubating the slides in 5% skimmed milk (Bio-rad, München, Germany). Paraffin-certified antibodies were next incubated alternatively on the slides overnight at 4 °C (rabbit anti-human CD105 polyclonal antibody diluted as 1:50, LabVision, Fremont, CA, United States; and mouse anti-human CD31, IgG1, clone JC70A, Dako, Glostrup, Denmark). Next day the sections were washed in saline, signal amplified with a multi-species polymeric HRP system (EnVision, Dako), and finally vessels were visualized by adding the 3-3’ diaminobenzidine substrate (DAB, Dako). Afterwards, the sections were counterstained with Hematoxylin and 3-4 hotspot high vessel density areas were captured using a Nikon Eclipse 55i microscope equipped with a 5 Megapixel CCD color camera (Nikon, Tokyo, Japan). There were selected images from the regions with the highest vascular density (“hot-spots”- according to Weidner et al[18]). Under constant illumination conditions, images were obtained using the 40 × objective, and saved as uncompressed TIF files using the Image ProPlus AMS 6 software (Media Cybernetics Inc., Bethesda, Maryland, United States). The contour for each microvessel was drawn separately with a dedicated hand tool in Adobe Photoshop software, and these ROI were filled with black RGB color and saved as layers. Images were brought back in Image ProPlus and after distance-to-pixel calibration, they were utilized for automated measurements. Total vascular area, and total vessel count were normalized to 1 mm2 and automatically measured, considering a total area of the field of 36527.48 μm2. Inflammatory plasma cells or tumor cells picking up the signal have been excluded from this interpretation by two pathologists (DP and CG).
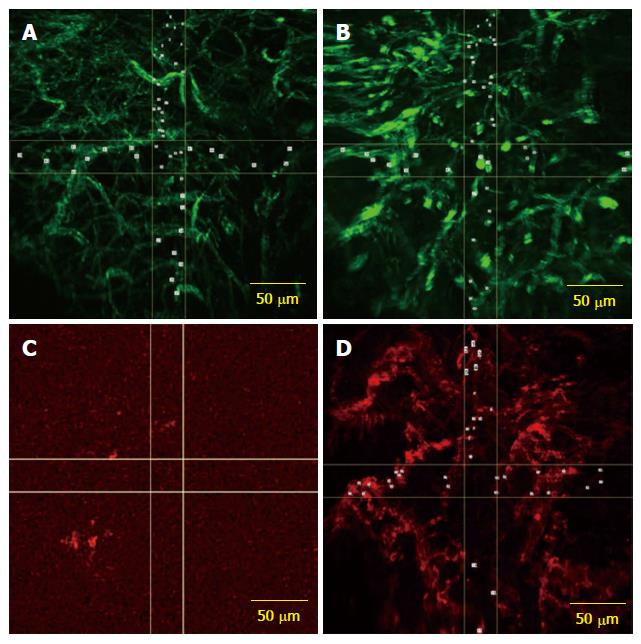
To analyze CD31 expression in rectal cancer, we evaluated tumor rectal cancer tissue and normal rectal mucosa for the vascular morphometric assessment. The CD31 antibody stained blood vessels in both normal and tumor rectal mucosa. In normal mucosa, the average diameter of vessels was of 7.67 ± 0.5 μm and the vessel density was 3191.6 ± 387.8 vessels/mm3. In the tumor sample, we obtained an average diameter of 10.88 ± 0.8 μm and a vessel density of 4707.3 ± 448.8 vessels/mm3 (Figure 1A and B).
In the CLE samples that were fluorescently labeled with both CD31 and CD105 antibodies, the typical tumor vasculature pattern was observed, with tortuous, dilated and branched vessels, but the expression of CD105 in tumor tissue was generally lower compared to CD31 vessel staining (Figure 1C and D).
Staining for CD105 was low or absent in normal mucosa (244.21 ± 130.7 vessels/mm3 in only four patients), whereas the microvascular network was visualized using CD31 as a control on samples from the same patients. The average diameter of anti-CD105 antibody stained vessels was 10.97 ± 0.6 μm in tumor tissue, and average density was 2787.4 ± 134.8 vessels/mm3.
Next we analyzed the relationship between the vascular expression with CD31 and CD105 in colorectal tumors. There were more vessels stained with CD31 than CD105 (P = 0.0006 for vascular density) in tumor. The average vessel diameter was similar for both CD31 and CD105 staining (P = 0.018 in normal samples, and P = 0.932 in malignant tissue).
The vascular density and the average diameter in tumor samples were significantly higher than the control in the 3D confocal reconstruction and in immunohistochemistry images. This fact was demonstrated by using both markers. In contrast, CD105 expression in colorectal tissues from the same patients was strongly enhanced in tumor vessels suggesting detection of the endoglin is an indication of angiogenesis particularly in malignant disease (Table 2).
| CD31 | CD105 | P-value | ||
| Vascular | Normal | 7.67 ± 0.5 | 3.46 ± 1.5 | 0.01 |
| Diameter | Tissue | |||
| (μm) | Tumor | 10.88 ± 0.8 | 10.97 ± 0.6 | 0.9 |
| Vascular | Normal | 3191.6 ± 387.8 | 244.21 ± 130.7 | < 0.001 |
| Density | Tissue | |||
| (vessels/mm3) | Tumor | 4707.3 ± 448.8 | 2787.4 ± 133.8 | 0.001 |
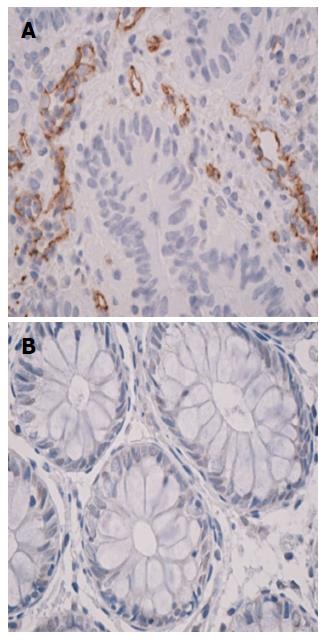
The CD105 and CD31 vascular expressions were studied in normal rectal mucosa and rectal cancer specimens. The immunohistochemical analysis revealed that the samples from normal tissue showed low detectable CD105 expression. CD105 was rarely expressed in normal mucosa, while in tumor specimens, CD105-positive vascular endothelial cells were clearly identified (Figure 2).
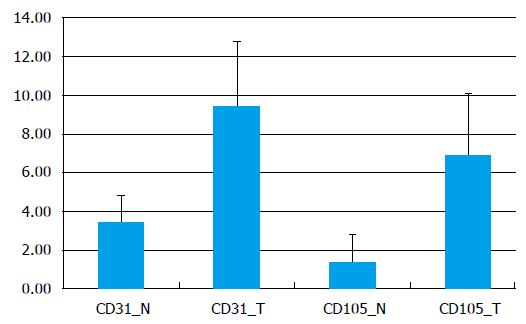
In normal tissue images CD31-stained we measured an average of 202.9 ± 91.8 vessels/mm2, with a significantly lower density of 56.5 ± 35.1 vessels/mm2 for the vascular network stained with CD105 (P = 0.00017). The intratumoral MVD average was about 298.04 ± 132.6 vessels/mm2 on CD31 stained images and on CD105 images - 205.7 ± 100.06 vessels/mm2 (P = 0.048) (Figure 3).
The values for the vascular area when using the panendothelial marker CD31 were 3.4% ± 1.3% in normal rectum and 9.4% ± 3.3% in tumors (P < 0.001). On CD105 stained sections, the total vascular area was 1.3% ± 1.4% in healthy tissue and 6.9% ± 3.1% in malignant tissue (P < 0.001).
Rectal cancer is one of the cancers which can benefit from antiangiogenic therapy with high chances of curability when the treatment is applied at an early stage. To date, no appropriate tissue biomarkers exist for staging, prediction or monitoring of the clinical response to a therapeutic intervention (e.g., antiangiogenic therapy). Beyond its already presumed roles (higher affinity for microvascularization, prognostic role), recent in vitro studies suggested that endoglin targeting could improve treatment and could reverse resistance to bevacizumab in some refractory cancer patients[19].
We hypothesized that the use of fluorescently-labeled CD105 antibodies will be suitable for identifying microvessels specific to tumor tissue. Indeed, while vessels marked with fluorescent CD31 were visible in both normal and malignant tissue, CD105 was predominantly expressed in tumor lesions, having reduced affinity for normal rectal mucosa. Thus, specific imaging and quantification of tumor microvessels were feasible using CLE examination and CD105 immunostaining of samples.
Our study proves that fluorescently labeled endoglin antibodies stained intensively intratumoral vessels, whereas vessels in non-neoplastic tissue did not or weakly expressed CD105. These results are consistent with previous observations that endoglin reacts specifically with angiogenic endothelial cells from the malignant tissues[5]. Though, the endoglin expression on macroscopically normal mucosa in four of the patients could be explained by either the existent inflammation, or the tumor spread to normal surrounding tissue.
Endoglin, as a specific marker for activated endothelium, mainly reacts with fresh or frozen tissue, while its activity in paraffin-embedded specimens is dependent on fixation[17]. In the present study, a qualitative comparison between the two methods (CLE vs IHC) lead to similar results. The major advantage of the CLE method is time efficacy and less artifacts in comparison to common IHC regarding the processing techniques[20].
Due to CD105 specific overexpression in malignant vessels, the endoglin antibodies for tumor imaging have the potential of becoming an optimal target for anticancer treatment, to improve rectal cancer diagnosis and to monitor the therapy[4]. As there are already studies regarding tumor aggressiveness and the prognostic value of vascular density on IHC when using anti-CD105 antibodies, CLE opens the possibility of applying CD105 targeted therapy, which until now was only tested in vitro and on animal models, to in vivo human subjects. Its luminal distribution on newly formed vessels makes CD105 readily accessible for the antibodies and, consequently, an interesting candidate for CLE in vivo[11].
CLE monitoring of the relationship between endothelial presence of CD105 and survival of patients would be of great interest. In our group of patients, we observed an inter-patients variation in MVD endoglin expression in tumor tissue. On one hand, this could be related to the tumor grading or staging, as an increase in MVD was demonstrated by using CD105 during progressive stages of colorectal carcinogenesis[21]. On the other hand, reduced endoglin expression could also be caused by a decreased tumor vascularization in endoglin haploinsufficiency cases[22]. There are also differences in reactivity to endothelial cells depending on tumor localization[22-24]. However, in colorectal cancer, other studies showed that, with cancer progression, endoglin signaling was lost in most of the epithelial cancer cells which became refractory to the TGF-β growth inhibiting properties[25-29]. All these factors could lead to differences in diagnostic, prognostic and therapeutic efficacy.
To our knowledge, no other studies using fluorescently-labeled CD105 with CLE imaging in patients with rectal cancer have been reported prior to this study. A larger number of patients is needed to study the correlation between MVD and tumor differentiation grade and stage, with great potential for CD105 staining combined with CLE analysis to provide a more reliable evaluation of the angiogenetic status of patients with colorectal cancer. Other studies are needed to investigate if the same CLE method could be applied to other tumor types.
In conclusion, our data showed that CLE using CD105 targeted antibodies for tumor vascular network imaging is feasible and, moreover, that this proangiogenic molecule represents a more specific marker for rectal cancer neoangiogenesis than commonly used panendothelial markers.
The main clinical signs the patients showed were alternating diarrhea and constipation, accelerated intestinal transit, recent constipation, unintended weight loss, rectal bleeding, abdominal pain or discomfort.
Only three patients accused rectal bleeding as a single symptom, also confirmed by the physical examination (digital rectal examination).
Other common digestive diseases such as hemorrhoidal disease, inflammatory bowel disease or irritable bowel syndrome were excluded.
Seven patients presented nonspecific laboratory tests findings such as moderate elevated hematological values of erythrocyte sedimentation rate (three patients), slightly elevated white blood cells count (two patients) and moderate anemia (two patients); two patients presented slightly elevated values of both tumor markers CEA and CA19-9, while three of them had only slightly elevated CEA values.
Computed tomography scan excluded the presence of metastases in all ten patients and described rectal wall thickening in four cases.
Histological examination of endoscopic samples revealed moderately differentiated adenocarcinoma (G2) in five cases, well differentiated adenocarcinoma in two cases (G1), mixed subtypes in three cases (G1/G2- two cases, G1 with mucinous areas - one case).
Tissue samples from patients with histological diagnosis of rectal cancer were collected during colonoscopy before undergoing surgical resection or neoadjuvant therapy.
Immunoendoscopy: Targeting markers of angiogenesis in association with confocal laser endomicroscopy (CLE) examination; Panendothelial markers: Present equal staining intensity in both small and large vessels and comparable reactivity in both frozen and paraffin sections, with obvious disadvantages regarding antigen specificity and sensitivity. They can identify all types of blood vessels in a given tissue sample, irrespective of being mature or immature.
Specific imaging and quantification of tumor microvessels are feasible in human rectal cancer using CLE examination and CD105 immunostaining of fresh tissue samples. A larger number of patients is needed to study the correlation between MVD and tumor differentiation grade and staging, with great potential for CD105 staining combined with CLE analysis to provide a more reliable evaluation of the angiogenetic status of patients with colorectal cancer. CLE monitoring of the relationship between endothelial presence of CD105 and survival of patients would be of great interest.
The manuscript has original results. This is an interesting study on “Tumor neoangiogenesis detection by confocal laser endomicroscopy and anti-CD105 antibody: Pilot study”. The research is limited to a small number of patients and, for this reason, this study should be considered pilot.
P- Reviewer: Baba H, Gu J, Santoro GA, Wittmann T S- Editor: Ma YJ L- Editor: A E- Editor: Jiao XK
| 1. | Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58-61. [PubMed] |
| 2. | Bolontrade MF, Stern MC, Binder RL, Zenklusen JC, Gimenez-Conti IB, Conti CJ. Angiogenesis is an early event in the development of chemically induced skin tumors. Carcinogenesis. 1998;19:2107-13. [PubMed] |
| 3. | Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res. 2001;61:2736-43. [PubMed] |
| 4. | Paauwe M, ten Dijke P, Hawinkels LJ. Endoglin for tumor imaging and targeted cancer therapy. Expert Opin Ther Targets. 2013;17:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566-1577. [PubMed] |
| 6. | Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, Letarte M, Vitetta ES, Thorpe PE. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1:1623-1634. [PubMed] |
| 7. | Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534-1537. [PubMed] |
| 8. | Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, Sedlacek HH, Müller R, Adamkiewicz J. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer. 1999;81:568-572. [PubMed] |
| 11. | Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, Natali PG, Altomonte M, Maio M. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res. 2000;6:2037-2043. [PubMed] |
| 12. | Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 371] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 13. | Neumann H, Günther C, Vieth M, Grauer M, Wittkopf N, Mudter J, Becker C, Schoerner C, Atreya R, Neurath MF. Confocal laser endomicroscopy for in vivo diagnosis of Clostridium difficile associated colitis - a pilot study. PLoS One. 2013;8:e58753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Dong YY, Li YQ, Yu YB, Liu J, Li M, Luan XR. Meta-analysis of confocal laser endomicroscopy for the detection of colorectal neoplasia. Colorectal Dis. 2013;15:e488-e495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Su P, Liu Y, Lin S, Xiao K, Chen P, An S, He J, Bai Y. Efficacy of confocal laser endomicroscopy for discriminating colorectal neoplasms from non-neoplasms: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Cârţână T, Săftoiu A, Gruionu LG, Gheonea DI, Pirici D, Georgescu CV, Ciocâlteu A, Gruionu G. Confocal laser endomicroscopy for the morphometric evaluation of microvessels in human colorectal cancer using targeted anti-CD31 antibodies. PLoS One. 2012;7:e52815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Ciocâlteu A, Săftoiu A, Cârţână T, Gruionu LG, Pirici D, Georgescu CC, Georgescu CV, Gheonea DI, Gruionu G. Evaluation of new morphometric parameters of neoangiogenesis in human colorectal cancer using confocal laser endomicroscopy (CLE) and targeted panendothelial markers. PLoS One. 2014;9:e91084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401-409. [PubMed] |
| 19. | Rosen LS, Gordon MS, Robert F, Matei DE. Endoglin for targeted cancer treatment. Curr Oncol Rep. 2014;16:365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Romani AA, Borghetti AF, Del Rio P, Sianesi M, Soliani P. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93:446-455. [PubMed] |
| 21. | Akagi K, Ikeda Y, Sumiyoshi Y, Kimura Y, Kinoshita J, Miyazaki M, Abe T. Estimation of angiogenesis with anti-CD105 immunostaining in the process of colorectal cancer development. Surgery. 2002;131:S109-S113. [PubMed] |
| 22. | Tsujie M, Tsujie T, Toi H, Uneda S, Shiozaki K, Tsai H, Seon BK. Anti-tumor activity of an anti-endoglin monoclonal antibody is enhanced in immunocompetent mice. Int J Cancer. 2008;122:2266-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Tsujie M, Uneda S, Tsai H, Seon BK. Effective anti-angiogenic therapy of established tumors in mice by naked anti-human endoglin (CD105) antibody: differences in growth rate and therapeutic response between tumors growing at different sites. Int J Oncol. 2006;29:1087-1094. [PubMed] |
| 24. | Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283-2290. [PubMed] |
| 25. | Dales JP, Garcia S, Bonnier P, Duffaud F, Andrac-Meyer L, Ramuz O, Lavaut MN, Allasia C, Charpin C. CD105 expression is a marker of high metastatic risk and poor outcome in breast carcinomas. Correlations between immunohistochemical analysis and long-term follow-up in a series of 929 patients. Am J Clin Pathol. 2003;119:374-380. [PubMed] |
| 26. | Zijlmans HJ, Fleuren GJ, Hazelbag S, Sier CF, Dreef EJ, Kenter GG, Gorter A. Expression of endoglin (CD105) in cervical cancer. Br J Cancer. 2009;100:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She X, Harada N, Uneda S, Tsujie T, Toi H. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8:135-143. [PubMed] |
| 28. | Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14:1931-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 29. | Bredow S, Lewin M, Hofmann B, Marecos E, Weissleder R. Imaging of tumour neovasculature by targeting the TGF-beta binding receptor endoglin. Eur J Cancer. 2000;36:675-681. [PubMed] |