Published online Sep 15, 2014. doi: 10.4251/wjgo.v6.i9.325
Revised: September 25, 2013
Accepted: November 15, 2013
Published online: September 15, 2014
Processing time: 401 Days and 10.1 Hours
The pancreaticoduodenectomy (PD) procedure may lead to pancreatic exocrine and endocrine insufficiency. There are several types of reconstruction for this kind of operation. Pancreaticogastrostomy (PG) was introduced to reduce the rate of postoperative pancreatic fistula. Although some randomized control trials have shown no differences regarding pancreatic leakage between PG and pancreaticojejunostomy (PJ), recently some reports reveal benefits from the PG over the PJ. Some surgeons concern about the performing of the PG and inactivation of pancreatic enzymes being in contact with the gastric juice, and the detrimental results over the exocrine pancreatic function. The pancreatic exocrine function can be measured with direct and indirect tests. Direct tests have the highest sensitivity and specificity for detection of exocrine insufficiency but require tube placement. Among the tubeless indirect tests, the van de Kamer stool fat analysis remains the standard to diagnose fat malabsorption. The patient compliance and time consuming makes it not so suitable for its clinical use. Fecal immunoreactive elastase test is employed for screening of exocrine insufficiency, is not cumbersome, and has been used to study pancreatic function after resection. We analyze the FE1 levels in our patients after the PD with two types of reconstruction, PG and PJ, and we discuss some considerations about the pancreaticointestinal drainage method after pancreaticoduodenectomy.
Core tip: Many patients present pancreatic exocrine insufficiency after pancreatic resection. Exocrine insufficiency leads to steatorrhoea, flatulence, abdominal pain, weight loss and malnutrition. Extent of resection will determine the severity of insufficiency, but also changes in anatomy may be determining factors. Pancreatogastrostomy is deemed detrimental over the pancreatic function because of the hypothetical inactivation of pancreatic enzymes due to the acid juice of the stomach. In this review we discuss the physiological aspects of the changes in exocrine pancreatic function focusing on the pancreatoenterostomy after a pancreaticoduodenectomy.
- Citation: Morera-Ocon FJ, Sabater-Orti L, Muñoz-Forner E, Pérez-Griera J, Ortega-Serrano J. Considerations on pancreatic exocrine function after pancreaticoduodenectomy. World J Gastrointest Oncol 2014; 6(9): 325-329
- URL: https://www.wjgnet.com/1948-5204/full/v6/i9/325.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i9.325
After a pancreaticoduodenectomy (PD) procedure the patient has an altered upper gastrointestinal and pancreatic anatomy with potential pancreatic exocrine and endocrine insufficiency. The first known pancreaticoduodenectomy was performed by Alessandro Codivilla on february 1898 in Italy[1], followed by Kausch[2] 11 years later. There are two operation techniques performed predominantly: the classic Whipple operation, developed and modified by Whipple[3], and the pylorus-preserving procedure inaugurated by Watson[4] and popularized by Traverso et al[5]. In the classic PD, an antrectomy or distal gastrectomy is associated to the resected specimen. Gastric resection is avoided in the pylorus-preserving modification. Mortality and morbidity are not significantly different between both techniques[6], but the pylorus-preserving procedure improve postoperative weight gain[7-9].
The majority of authors consider pancreatic anastomotic leakage as the primary cause of morbidity and mortality after PD[10-13]. Pancreaticogastrostomy (PG) was introduced to reduce pancreatic leakage. Waugh and Clagett at the Mayo Clinic were the first to use this anastomosis in the clinical setting. Mackie et al[14] from the University of Pennsylvania reported their experience and observed lower operative mortality in their institution with implantation of PG. Initially infrequently used, PG is becoming more commonly performed clinically as gastrointestinal pancreatic drainage after PD. Three randomized control trials have shown PG and pancreaticojejunostomy (PJ) to be similar regarding pancreatic fistula rates[15-17] and a meta-analysis concluded that PG and PJ were not different in terms of pancreatic fistula or overall morbidity rate[18]. Nevertheless, McKay et al[19] using meta-analytical techniques found that the results suggested that PG rather than PJ for reconstruction of the pancreatic remnant after PD resulted in a significant decrease in pancreatic fistula or leakage. Recently, Shen et al[20] showed similar results in their own meta-analysis.
Many surgeons are worried about the inactivation of pancreatic enzymes and deterioration of pancreatic exocrine function due to the reflux of gastric juice into the pancreatic main duct when PG is used as the reconstruction procedure.
The aim of this article is to review the repercussion on exocrine pancreatic function according to the type of pancreatic anastomosis performed after PD. We also report our results in pancreatic exocrine function evaluated by fecal elastase test in a series of patients undergoing PD.
In a PD the possibilities regarding gastric resection are antrectomy (distal gastrectomy), or gastric-sparing techniques such as pylorus-preserving procedure, or pylorus resection with the cutting line just before the pylorus ring preserving the most part of the stomach. A greater weight loss can be expected when gastric resection is performed rather than with pylorus-preserving procedure or pylorus resection PD[2-4].
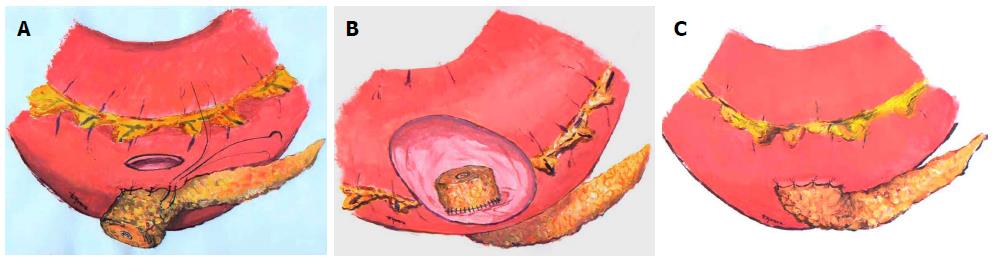
A soft, non-fibrotic gland with a small pancreatic duct increases significantly the risk of subsequent pancreatic leakage. Several techniques have been described to deal with this kind of pancreatic remnant[21]. We consider the PG anastomosis in which the pancreatic remnant is telescoped into the gastric lumen[22] as the first choice in those situations (Figures 1 and 2).
Pros and cons may be argued for each kind of anastomosis. Some groups report worse functional results with PG than PJ after PD. Their explanation for this feature is that reflux of gastric juices in the main pancreatic duct causes inactivation of the pancreatic enzymes and insufficiency of the remnant pancreas. This is a theoretical argument. In contrast, in PJ anastomosis the activation of pancreatic exocrine secretion can occur more easily in the presence of intestinal enterokinase and bile, which may irritate the remnant pancreas via the activation of trysinogen and chymotrypsinogen[9,23]. The activated enzymes may breakdown the anastomosis. This is also another theoretical argument without evidence-based clinical data.
Unless clinical consequences of the PG reconstruction result in greater deterioration than the potential benefits of this technique, PG is a safer anastomosis when dealing with a soft pancreatic parenchyma.
Classical studies[24,25] have demonstrated that the defective digestion of protein, fat, and starch is not observed until the secretion of lipase, trypsin, and amylase is less than 10% of its normal values. The most frequently described sign of pancreatic exocrine insufficiency after resectional surgery is steatorrhoea[26], i.e., stool fat content of more than 7 g/d, which may associate abdominal pain, flatus, and mostly weight loss. Fat malabsorption occurs when pancreatic lipase and trypsin decrease below 5% of normal values[27].
Halloran et al[28] considered that the high rate of impaired fat absorption in some series using PG, could be largely attributed to pancreatic enzyme degradation by gastric juice and acid. On the other hand, Johnson[29] studied gastric and pancreatic function after a Whipple operation with duct-to-mucosa PG in six out of 50 patients who agreed to undergo endoscopy and gastric intubation test. All patients had normal gastric secretion and all but one patient had demonstrable amylase and lipase activity in the gastric aspirate. The patient with no detectable enzyme activity had no clinical pancreatic insufficiency and had very high basal values of gastric secretion and a very high peak acid output (22 mmol/h). The explanation from the author is that although pancreatic enzymes are inactivated at low pH, the conditions found in the stomach immediately after a meal will be favorable for normal activity of the pancreatic enzymes. Therefore, the buffering capacity of the food may protect the pancreatic enzymes from denaturation at the time when they are required for digestion, and the PG may not be detrimental in the exocrine pancreatic function. When a PJ is performed, there is no concern about the acid pH, but the changing anatomy may provide a negative feed-back following a high-caloric jejunal load which results in reduced exocrine secretion[30].
Regardless of the type of reconstruction, PD survivors should be carefully followed up for evidence of pancreatic endocrine and exocrine insufficiency[31]. When the clinical evolution of the patient demonstrates signs of pancreatic insufficiency, pancreatic enzyme replacement therapy should be routinely considered[21].
Direct pancreatic function tests have the highest sensitivity and specificity for detection of exocrine pancreatic insufficiency and are subsequently the “gold standard” for testing pancreatic function. Nevertheless, because they are cumbersome, time-consuming, and require tube placement, tubeless indirect tests of pancreatic function have been introduced. Among them, the 72-h stool fat analysis (van de Kamer test) remains the standard test to diagnose and quantify fat malabsorption[32,33]. This test requires patient compliance that is rarely obtained, and therefore its clinical use is limited.
Fecal immunoreactive elastase test is considered one of the most satisfactory pancreatic function tests for screening of pancreatic insufficiency[34]. Fecal elastase-1 measurement has been suggested to support exocrine insufficiency after pancreatic resection[19,35]. Elastase does not interfere with pancreatic enzyme supplements, and therefore the results are not affected by pancreatic enzyme replacement therapy when patients are under oral pancreatic enzyme treatment.
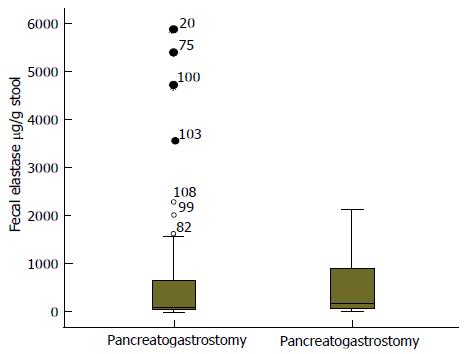
We have reviewed the data on fecal elastase from a series of PD performed at our centre by using both types of pancreatic anastomosis, PG and PJ. The pancreaticogastrostomy was performed in two layers, with intussusception of the pancreatic stump into the gastric lumen, an inner layer suturing the pancreatic stump to the gastric mucosa, and an outer layer approximating the pancreas to the gastric serosa. Elastase was measured by ELISA to estimate the postoperative pancreatic exocrine function. Stool elastase levels were available in 108 patients, 76 PG and 32 PJ. The average age was 62.7 years ± 10.9, mostly men (64.8%). Malignancy was the most predominant pathological diagnosis (pancreatic adenocarcinoma, ampuloma, or cholangiocarcinoma) and no histology of chronic pancreatitis was found in the specimens. Fecal elastase levels are considered normal when they are above 200 μg/g stool. The mean fecal elastase after PD in our series was of 57.9 μg/g ± 104.3. The mean fecal elastase in the PG group was 61.1 μg/g ± 116.4; and it was 50.20 μg/g ± 68.5 in the PY group (Figure 3). The statistical analysis did not show significant difference between elastase levels in both groups (P = 0.622). There is an evident decrease in stool elastase levels of patients after PD. This decrease is not influenced by the type of pancreatic drainage used.
Non-alcoholic fatty liver disease has been described after pancreaticoduodenectomy[36-38] as a late-phase complication. This was not assessed in our patients; nevertheless this topic may be of concern in patients with long-term survival with chronic pancreatitis and it may deserve special interest in future studies.
In summary, we consider PG as the elective pancreaticointestinal drainage method after pancreaticoduodenectomy when dealing with the soft parenchyma. Pancreatic functional concerns about this kind of reconstruction do not support its rejection. Regardless the type of pancreatic anastomosis performed in the PD, the pancreatic exocrine function after pancreatic resection should be surveyed.
P- Reviewer: Nakano H, Peng SY, Ramia JM S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Howard JM, Hess W. Tumors of the ampulla of Vater and pancreas. Mysteries of a hidden organ. New York: Kluer Academic/Plenum Publishers 2002; 421-518. [DOI] [Full Text] |
| 2. | Kausch W. Das carcinoma der papilla vateri und seine radikale entfernung. Beitr Klin Chir. 1912;78:439-486. [RCA] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 893] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 3. | Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of ampulla of Vater. Ann Surg. 1935;102:763-779. [PubMed] |
| 4. | Watson AO. Treatment of carcinoma of the ampulla of Vater: successful radical resection. Br J Surg. 1944;31:368-373. [RCA] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Traverso LW, Longmire WP. Preservation of the pylorus in pancreaticoduodenectomy a follow-up evaluation. Ann Surg. 1980;192:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Diener MK, Knaebel HP, Heukaufer C, Antes G, Büchler MW, Seiler CM. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg. 2007;245:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Seiler CA, Wagner M, Sadowski C, Kulli C, Büchler MW. Randomized prospective trial of pylorus-preserving vs. Classic duodenopancreatectomy (Whipple procedure): initial clinical results. J Gastrointest Surg. 2000;4:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Niedergethmann M, Shang E, Farag Soliman M, Saar J, Berisha S, Willeke F, Post S. Early and enduring nutritional and functional results of pylorus preservation vs classic Whipple procedure for pancreatic cancer. Langenbecks Arch Surg. 2006;391:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Fujii T, Kanda M, Kodera Y, Nagai S, Sahin TT, Hayashi M, Kanzaki A, Yamada S, Sugimoto H, Nomoto S. Preservation of the pyloric ring has little value in surgery for pancreatic head cancer: a comparative study comparing three surgical procedures. Ann Surg Oncol. 2012;19:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 268] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 11. | Machado NO. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol. 2012;2012:602478. [PubMed] |
| 12. | Oussoultzoglou E, Bachellier P, Bigourdan JM, Weber JC, Nakano H, Jaeck D. Pancreaticogastrostomy decreased relaparotomy caused by pancreatic fistula after pancreaticoduodenectomy compared with pancreaticojejunostomy. Arch Surg. 2004;139:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Aranha GV, Aaron JM, Shoup M. Critical analysis of a large series of pancreaticogastrostomy after pancreaticoduodenectomy. Arch Surg. 2006;141:574-579; discussion 579-580. [PubMed] |
| 14. | Mackie JA, Rhoads JE, Park CD. Pancreaticogastrostomy: a further evaluation. Ann Surg. 1975;181:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580-588; discussion 580-558. [PubMed] |
| 16. | Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM, Fingerhut A, Millat B, Radovanowic A, Fagniez PL. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767-771; discussion 771-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 336] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Wente MN, Shrikhande SV, Müller MW, Diener MK, Seiler CM, Friess H, Büchler MW. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, Vollmer CM, Dixon E. Meta-analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. Br J Surg. 2006;93:929-936. [PubMed] |
| 20. | Shen Y, Jin W. Reconstruction by Pancreaticogastrostomy versus Pancreaticojejunostomy following Pancreaticoduodenectomy: A Meta-Analysis of Randomized Controlled Trials. Gastroenterol Res Pract. 2012;2012:627095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Abu Hilal M, Malik HZ, Hamilton-Burke W, Verbeke C, Menon KV. Modified Cattell’s pancreaticojejunostomy, buttressing for soft pancreases and an isolated biliopancreatic loop are safety measurements that improve outcome after pancreaticoduodenectomy: a pilot study. HPB (Oxford). 2009;11:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Delcore R, Thomas JH, Pierce GE, Hermreck AS. Pancreatogastrostomy: a safe drainage procedure after pancreatoduodenectomy. Surgery. 1990;108:641-645; discussion 645-647. [PubMed] |
| 23. | Ishikawa O, Ohigashi H, Eguchi H, Yokoyama S, Yamada T, Takachi K, Miyashiro I, Murata K, Doki Y, Sasaki Y. Long-term follow-up of glucose tolerance function after pancreaticoduodenectomy: comparison between pancreaticogastrostomy and pancreaticojejunostomy. Surgery. 2004;136:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme ouputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 528] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Fogel MR, Gray GM. Starch hydrolysis in man: an intraluminal process not requiring membrane digestion. J Appl Physiol. 1973;35:263-267. [PubMed] |
| 26. | Tran TC, van Lanschot JJ, Bruno MJ, van Eijck CH. Functional changes after pancreatoduodenectomy: diagnosis and treatment. Pancreatology. 2009;9:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Keller J, Aqhdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function-clinical significance in pancreatic and non-pancreatic disorders. Best Pract Res Clin Gastroenterol. 2009;23:425-439. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 28. | Halloran CM, Cox TF, Chauhan S, Raraty MG, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: a prospective study. Pancreatology. 2011;11:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Johnson CD. Pancreaticogastrostomy after resection of the pancreatic head. Stan Pancreatic Surg. 1993;663-675. [DOI] [Full Text] |
| 30. | Sogni P, Vidon N, Chaussade S, Huchet B. Inhibitory effect of jejunal high caloric nutrient load on human biliopancreatic secretion. The role of atropine, naloxone and composition of nutrient solutions. Clin Nutr. 1993;12:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Huang JJ, Yeo CJ, Sohn TA, Lillemoe KD, Sauter PK, Coleman J, Hruban RH, Cameron JL. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231:890-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Van de kamer JH, Ten bokkel huinink H, Weyers HA. Rapid method for the determination of fat in feces. J Biol Chem. 1949;177:347-355. [PubMed] |
| 33. | Bo-Linn GW, Fordtran JS. Fecal fat concentration in patients with steatorrhea. Gastroenterology. 1984;87:319-322. [PubMed] |
| 34. | Stein J, Jung M, Sziegoleit A, Zeuzem S, Caspary WF, Lembcke B. Immunoreactive elastase I: clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem. 1996;42:222-226. [PubMed] |
| 35. | Matsumoto J, Traverso LW. Exocrine function following the whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10:1225-1229. [PubMed] |
| 36. | Nagai M, Sho M, Satoi S, Toyokawa H, Akahori T, Yanagimoto H, Yamamoto T, Hirooka S, Yamaki S, Kinoshita S. Effects of pancrelipase on nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancrea Sci. 2013;21:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Song SC, Choi SH, Choi DW, Heo JS, Kim WS, Kim MJ. Potential risk factors for nonalcoholic steatohepatitis related to pancreatic secretions following pancreaticoduodenectomy. Worl J Gastroenterol. 2011;17:3716-3723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |