Published online Jul 15, 2014. doi: 10.4251/wjgo.v6.i7.194
Revised: March 19, 2014
Accepted: May 8, 2014
Published online: July 15, 2014
Processing time: 210 Days and 7.5 Hours
Due to a wide range of clinical response in patients undergoing neo-adjuvant chemoradiation for rectal cancer it is essential to understand molecular factors that lead to the broad response observed in patients receiving the same form of treatment. Despite extensive research in this field, the exact mechanisms still remain elusive. Data raging from DNA-repair to specific molecules leading to cell survival as well as resistance to apoptosis have been investigated. Individually, or in combination, there is no single pathway that has become clinically applicable to date. In the following review, we describe the current status of various pathways that might lead to resistance to the therapeutic applications of ionizing radiation in rectal cancer.
Core tip: Treatment of locally advanced rectal cancer stage II and III includes neoadjuvant chemo-radiation followed by surgery if clinically feasible. A strategy of observing patients without an operation has been proposed by some surgeons, but this is still the center of much debate. Moreover, the therapeutic effect of ionizing radiation in treatment of rectal cancer varies significantly from one person to another. This has led investigators to identify the molecular targets and pathways in rectal tumors resistant to ionizing radiation in a bid to improve the therapeutic effect of radiation by advanced biomedical and genetic engineering.
- Citation: Ramzan Z, Nassri AB, Huerta S. Genotypic characteristics of resistant tumors to pre-operative ionizing radiation in rectal cancer. World J Gastrointest Oncol 2014; 6(7): 194-210
- URL: https://www.wjgnet.com/1948-5204/full/v6/i7/194.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i7.194
There are approximately 40340 patients diagnosed with rectal cancer annually in United States[1]. Cancer of the colon and rectum combined claimed 51690 deaths in 2012[1]. Rectal cancer, though staged similarly to colon cancer, is managed differently due to the pelvic location of the rectum. The rectum is in close proximity to the urogenital organs and anal sphincters. Hence, surgery for rectal cancer is associated with complications ranging from 15% to 70%[2]. Moreover, many patients will have local as well as distant metastasis during post-op surveillance[3,4]. Hence, careful and methodical planning is required to avoid unnecessary surgery with potential short and long term complications. Recent studies have underscored the importance of ionizing radiation (as neoadjuvant therapy) in patients with stage II and III rectal cancer. There are many benefits to the use of IR in the neoadjuvant compared to the adjuvant setting[5]. Additionally, in some cases, this approach allows the tumors to be down-staged resulting in complete pathological response (pCR, i.e., complete obliteration of the tumor following preoperative chemoradiation at laparotomy) or complete clinical response (cCR, i.e., complete obliteration of the tumor following preoperative chemoradiation during repeat colonoscopy or other diagnostic modalities such as MRI).
However, the benefit from preoperative radiation varies significantly in trials with a substantially wide pCR (9%-37%)[6-10]. Patients who achieve a pCR have better outcomes compared to patients who do not[11]. Some surgeons have elected a watchful waiting approach for patients who achieve cCR[12-17].
The logical clinical and pre-clinical question is to devise methods by which we can personalize treatment for rectal cancer, such that the most effective therapy with the least side effect profile can be offered consistently to patients affected by rectal cancer. In order to achieve this objective, extensive research has been performed over the last few decades to identify biological markers and genetic phenotypes that can predict successful response to radiation and translate into improved survival. We present a review of the current status of these markers.
The therapeutic effect of IR is largely the result of double stranded DNA breaks that result from IR-induced DNA damage. DNA breaks are difficult to repair and typically result in apoptosis. DNA double-strand break (DSB) can be repaired by one of the following three pathways: homologous recombination[18], non-homologous end-joining (NHEJ) pathway, or an alternate NHEJ pathway (characterized by larger deletions and translocations)[19]. The details behind the selection and execution of these pathways are not entirely clear, but it seems that NHEJ is the major pathway as it is the only one that occurs in all stages of cell cycle.
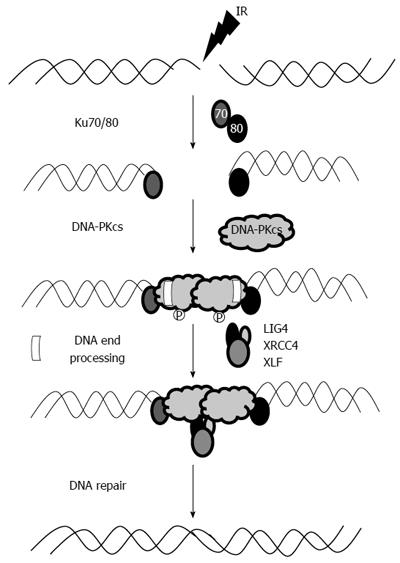
The NHEJ pathway is essential for DSB repair and is also important for V (D) J recombination during T and B cell lymphocyte development. The catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) is an integral part of the NHEJ pathway. The actual mechanism of this pathway is rather complex (Figure 1), but can be broadly classified into three steps. In the first phase, Ku 70/80 heterodimer identifies DSB, facilitates the activation and recruitment of DNA-PKcs, and then ties the DNA ends in a synaptic complex[20]. The next step involves enzymatic processing of the DNA ends followed by ligation (by DNA ligase IV) in the last phase. The order and timing of this sequence of events is not well defined; however, it is widely regarded that Ku 70/80 protein is the most important and integral part of this sequence as it recruits DNA-PKcs as well as interacts with a host of other important proteins. Moreover, Ku has lyase activity allowing it to process DNA ends during NHEJ[21].
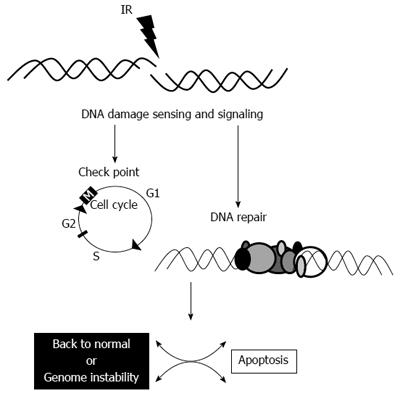
Following successful DNA repair, the cell might undergo back to the normal cell cycle. If some error occurs during the repair, the cell might undergo genomic instability and if the cell is unable to repair the radiation-induced damage, it undergoes apoptosis (Figure 2)[22]. Thus, a logical place to begin investigating marker of radioresistance is by interrogating the NHEJ pathway of DNA repair in cancer cells.
DNA-PKcs has multiple roles in DNA repair and carcinogenesis. DNA-PKcs facilitates DSB repair, thus ensuring stability and integrity of genetic chromosomes. Hence, low levels of DNA-PKcs might result in mutations promulgating the cascade of carcinogenesis. A cell with low levels of DNA-PKcs might be unable to repair the DNA damage incurred by IR and destine the cell for apoptosis. In this scenario, low levels of DNA-PKcs should be a surrogate for radiosensitivity.
On the other hand, cancer cells might contain higher DNA-PKcs levels induced by the rapid cell turnover. In this scenario, increases in DNA-PKcs activity will enhance cancer cell resistance and decrease susceptibility to chemotherapy and ionizing radiation[23-26].
Pre-clinical studies have demonstrated that DNA-PKcs deficient Chinese hamster ovary cells showed profound cell death following treatment with IR compared to the DNA-PKcs complimented V3-YAC cells[27]. Colon cancer HCT-116 DNA-PKcs-/- cells and xenografts were exquisitely sensitive to IR[28,29]. Unfortunately, the role of DNA-PKcs activity in development of various cancers has been investigated in multiple studies and has shown conflicting results in carcinogenesis as well as being a poor predictor of a response to IR, but more data is needed in this area (Table 1).
| Tumor type | Assay | Specimen | Sample size | DNA-PKcs activity | Interpretation |
| Nasopharyngeal cancer | IHC | Tumor | 66 | ↑ in 70% of tumor tissue | No association with locoregional control and survival |
| Nasopharyngeal cancer | IHC | Tumor | 223 | ↑ in 37% of tumor tissue | Overexpression associated with advanced stage and poor survival |
| Esophageal cancer | IHC, IB, Kinase activity | Tumor, normal | 13 paired | ↑ in tumor tissue | NA |
| Gastric cancer | IHC | Tumor | 279 | ↑ in 73% of tumor tissue | Loss of expression associated with lymphatic invasion, lymph node metastasis, advanced pathological stage, and poor survival |
| Gastric cancer | IHC | Tumor, normal | 791 | ↑ in 80% of tumor tissue | Loss of expression associated with intratumoral neutrophils, microsatellite instability, mutations in DNA-PKcs and poor survival |
| Colorectal cancer | RT-PCR, IB, kinase activity | Tumor, normal | 12 paired | ↑ in tumor tissue | NA |
| Colorectal cancer | IHC, IB | Tumor, normal | 359 (35 paired) | ↑ in 64% of tumor tissue | Overexpression associated with clinical stage, lymphatic invasion, distant metastasis and poor survival |
| Non-small cell lung cancer | IHC | Tumor | 113 | ↑ in 89% of tumor tissue | Overexpression associated with tumor grade |
| Non-small cell lung cancer | IHC | Tumor | 86 | ↑ in 87% of tumor tissue | No association with clinical characteristics or outcome |
| Non-small cell lung cancer | RT-PCR | Tumor, normal | 140 paired | ↑ in tumor tissue | Overexpression associated with poor survival |
| Non-small cell lung cancer | IHC | Tumor, normal | 116 (12 paired) | ↑ in 75% of tumor tissue | No association with clinical characteristics or outcome |
| Glioma | Kinase activity | Tumor | 36 | ↑ in tumor tissue | Hyperactivity correlates with rumor grading |
| Ovarian cancer | IHC | Tumor, normal | 100 | ↓ in 40% of tumor tissue | loss of expression associated with tumor progression, advanced clincal stage, and lymph node metastasis |
| ALL, CLL, lymphoma, multiple myeloma | IHC, IB | Lymphoid tissue | 86 | ↑ During lymphoid development and in lymphoid malignancies | Overexpression associated with higher lymphoma grading and degree of maturation in lymphoid malignancies other than multiple myeloma |
| B-cell CLL | IB, kinase activity | Lukemia cells | 54 | ↑ in del(17p) and del(11q) | Overexpression associated with shorter treatment free interval |
| B-cell CLL | RT-PCR | Lukemia cells | 50 | ↑ in del(17p) | Overexpression associated with poor survival |
| Cancer of breast, cervix, head and neck esophageal and lymphoma | Kinase activity | PBLs | 167 | ↓ in advanced stage | Hypoactivity associated with advanced stage and distant metastasis |
| Radiation response | |||||
| Esophageal cancer | IHC | Tumor | 67 | ↑ in 54% of tumor tissue | Overexpression predicts better response to chemoradiation |
| Oral squamous cell carcinoma | IHC | Tumor | 42 | ↑ in residual tumor after RT | Not predictive of radiation response |
| Cervical cancer | IHC | Tumor | 22 | ↑ in residual tumor after RT | No association with clinical characteristics |
| Breast cancer | IHC | Tumor | 224 | ↑ in 43% of tumor tissue | Overexpression predicts better locoregional control of radiation alone versus chemotherapy alone in early stage |
| Cancer risk | |||||
| Lung cancer | Kinase activity | PBLs | Cancer 41/healthy 41 | ↓ in cancer patients | Hypoactivity associated with cancer of the lung |
| Breast, cervix, head and neck, esophagus and lymphoma | Kinase activity | PBLs | Cancer 93/healthy 41 | ↓ in cancer patients | Hypoactivity associated with chromosomal instability and cancer of breast and cervix |
Significant increases of DNA-PKcs activity have been observed in certain gastrointestinal cancers such as colorectal cancer[30,31], esophageal cancer[32], nasopharyngeal cancer, and non-small cell lung cancer[33]. Conversely, loss of DNA-PKcs expression has been linked to gastric tumors correlating with signs of invasion and poor survival[34,35].
Levels of DNA-PKcs in cancer cells before treatment (radiation or chemotherapy) has been compared to levels after treatment, and have shown mixed results. The expression of DNA-PKcs was noted to be directly proportional to a favorable response with radiation in esophageal and early breast cancer but not in nasopharyngeal cancer[36-38]. On the other hand, studies have revealed increased levels of DNA-PKcs and Ku proteins in residual tumors after radiation treatment, suggesting a means of survival and a marker of radioresistance in recurrent tumors[39].
While the cellular status of the DNA-PKcs as a predictor of IR remains to be investigated, DNA-PKcs inhibition might have a therapeutic role in rectal cancer. Pre-clinical studies showed that pharmacological inhibition of DNA-PKcs led to substantial chemo- and radio-sensitization[27,40-42]. The effect of DNA- PKcs inhibitors has been examined in mouse xenograft tumor models with favorable results. There has been significant tumor growth delay and improved survival in mice treated with combined DNA-PKcs inhibition and ionizing radiation. The combination treatment reduces levels of cell proliferation marker Ki67 and increases activity of certain proteins known for its anti-tumor properties[43,44].
Inhibitors of DNA-PKcs have been shown to have a synergistic effect along with cisplatinum/platinum based drugs in treatment of ovarian, colon, and breast cancer[45-47]. Multiple DNA-PKcs kinase activity inhibitors are not only in various stages of development but a few are being tested in clinical trials (Table 2). Similarly, new in vivo substrates of DNA-dependent protein kinase (Akt1/PKBa, Hsp90a, NR4A[48-52]), which can be induced by ionizing radiation have been identified.
| Inhibitor | Mechanism/comments |
| A12B4C3 | PNKP inhibitor, sensitizes cells to camptothecin |
| BTW3 | A small peptide DNA-PK inhibitor, proposed to compete for DNA-PKcs autophosphorylation |
| KU0060648 | DNA-PK and P13K inhibitor |
| NU7441/KU57788 | DNA-PK inhibitor, competitive with ATP |
| ScFv 18-2 | An antibody-derived DNA-PK inhibitor that can bind to an epitope unique to DNA-PKcs |
| ZSTK474 | DNA-PK and P13K inhibitor, competitive with ATP; in phase 1 clinical trials (NCT01280487 and NCT01682473) |
| CC-115 | Dual inhibitor of DNA-PKcs and mTOR, in phase 1 clinical trials |
| CC-122 | DNA-PK inhibitor, in phase 1 clinical trials |
Furthermore, additional DNA-PKcs inhibitors have been developed such as anti-DNA-PKcs ScFv 18-2 (derived from an existing anti-DNA PKcs monoclonal antibody)[53], and anti-DPK3-scFv (selected from a humanized semi-synthetic scFV library)[44]. These anti-DNA PKcs sensitize cells to radiation induced injury[44,54,55] in a similar fashion to RNA inhibition of DNA-PKcs transcripts[56-58].
The interaction between epidermal growth factor receptor (EGFR) and the DNA-PKcs has also been explored. This interaction is required for radiation induced nuclear AKT phosphorylation and cell survival[52,59,60]. Similarly, blockage of EGFR signaling pathway with a monoclonal antibody can inhibit DNA-PKcs activation and thereby decrease DNA repair capacity. This could enhance sensitization and susceptibility of cells to ionizing radiation[61,62].
Clinically, deficiency in DNA-PK activity led to sensitivity to nitrogen mustards in patients with chronic lymphocytic leukemia[25]. The drug 2-N-morpholino-8-dibenzothiophenyl-chromen-4-one (NU7441) is a potent and specific DNA-PK inhibitor[63]. Treatment with NU7441 and topoisomerase inhibitors combined with IR caused potent chemo-radio sensitization in SW620 colorectal cancer cells as well as xenografts[27]. The various mechanisms by which DNA-PKcs inhibitors facilitate radiation induced death include apoptosis[64,65], acceleration of senescence, induction of mitotic catastrophe, and autophagy[43,66,67].
Studies evaluating expression of DNA-PKcs in peripheral blood lymphocytes (PBLs) as a marker of host immunity and cancer development have shown an additional role in cancer development as it relates to host immunity. Data from multiple studies demonstrated that cancer patients have a lower level of DNA-PKcs activity in PBLs[23,68,69], suggesting impaired ability to recognize cancer cells leading to a poor prognosis. Whether this is mediated by activation of natural killer (NK) cells or release of pro-inflammatory cytokines is not clearly understood[70]. Destruction of NK cells leading to increases in spontaneous tumor development in mouse models[71] leans in favor to the former hypothesis. Moreover, an inverse association between DNA-PKcs activity in PBLs and stage of cancer was also observed in patients who were treated with radiotherapy for advanced cancer, displaying poorer prognosis and higher frequency of distant metastasis[68].
In addition to its role in NHEJ pathway, DNA-PKcs regulates the DNA damage repair mechanisms by a variety of mechanisms. These include DNA interstrand crosslink (ICL) repair[72,73], AKT activation, EGFR nuclear translocation, or activation/mobilization of chromatin remodeling factor structure-specific recognition protein 1 (SSRP1) from nucleolus[60,74,75]. Biomedical engineering aiming to mimic some of the activities of the DNA-PKcs has been instrumental in developing novel agents that might be useful for cancer therapeutics.
It is clear that the status of the DNA-PKcs plays a fundamental role in ionizing radiation-induced cell death. Many aspects of its role in cancer therapeutics are currently under investigation. In rectal cancer, the role of DNA-PKcs is still in its infancy. As markers of a response to ionizing radiation, the role of the DNA-PKcs is complicated by the fact that there is paucity of high quality data. In rectal cancer, our group demonstrated counter-intuitive results with regards to the role of DNA-PKcs in the response to IR (discussed below). In prostate cancer, nuclear positivity for DNA-PKcs was associated with chemical recurrence[76]. Further studies are required to shed more light into these issues.
Ku70 and Ku80 proteins are essential components of the NHEJ pathway. These proteins serve as a medium by which multiple other DNA-repair proteins can be attached to the pathway cascade[77]. Importantly, the Ku proteins have a high affinity for broken DNA strands and rapidly bind to them. This initial process also recruits DNA-PKcs for DNA repair, though the exact mechanism is still unknown[78,79]. Additionally, Ku proteins play a major role in recruitment of XRCC4[80,81], XLF[82], APLF (APTX and PNK-like factor)[83] to DSBs helping with the repair process and promoting NHEJ. Moreover, Ku has the ability to enzymatically process DNA ends during NHEJ using the 5’-deoxyribose-5-phosphate (5’-dRP)/AP lyase activity[21]. Ku also excises abasic sites near DSBs suggesting a potential role in repairing damage by IR[21].
Intuitively, tumors that express high levels of Ku proteins should be able to repair the damage induced by IR more efficiently and thus become more resistant to therapy. In vitro studies have failed to show an association between the Ku proteins and radiosensitivity[84]. Ex vivo studies have also interrogated the role of the Ku proteins as surrogates of a response to IR.
Lack of Ku70 immunoreactivity correlated with radiosensitivity in patients with carcinoma of the cervix. In these patients, survival was better in tumors that had lower nuclear expression of Ku70[84]. In squamous cell carcinoma of the head and neck, Ku80 over expression was an independent predictor of regional recurrence and mortality in patient treated with IR[85]. Similarly, in rectal cancer low levels of Ku70 and Ku80 were associated with pCR. Ku70 was associated with down-staging. Disease free survival was 42% in patients with high Ku70 expression compared to 78% in patients with low expression of the same protein. Similar results were observed for Ku80[86]. Elevated levels of Ku proteins occur in high grade lymphoid malignancies[87]. The Ku70/Ku80 heterodimer DNA end-binding activity was 2- to 3-fold higher in the resistant B-CLL cell subset compared with the sensitive B-CLL cell subset[88], highlighting a possible mechanism behind increased DNA-PKcs activity in resistant CLL cells. The authors showed that novel DNA-dependent protein kinase (DNA-PK) inhibitor, NU7026 (2-(morpholin-4-yl)-benzo[h]chomen-4-one), and the phosphatidylinositol 3 (PI-3) kinase inhibitor, wortmannin, restored sensitivity to DNA damage-induced apoptosis of otherwise resistant cells.
Ku proteins can be upregulated after radiation treatment[39,89]. In one such study, expression of DNA-PK complex proteins (including Ku 70 proteins) increased after radiation treatment in residual tumors, and the increased values correlated with the tumor radiation resistance[89]. Various mechanisms have been postulated behind the role of Ku proteins in radioresistance. A distinct cell-interdependent signal is conveyed through gap junctions during chemotherapy with cisplatin, mediated by the kinase function of Ku70, Ku80 and DNA-dependent protein kinase complex. This communication may explain the resistance to cisplatin-induced death of cancer cells[90]. It is also possible that the role of Ku proteins and DNA-PKcs in DNA damage repair depends upon the extent and complexity of damage by IR. Studies have revealed that simple DSBs induced by laser irradiation are repaired rapidly involving Ku70/80 and XRCC4/Ligase IV/XLF. In contrast, DSBs with greater chemical complexity are repaired slowly and requires additional use of DNA-PKcs[91].
While these data seem compelling, more research is required prior to establishing the role of the Ku proteins in a response to radiation in rectal cancer. Current data on this subject, while promising, is currently limited and not clinically available. In rectal cancer, our group demonstrated counter-intuitive results with regards to the role of DNA-PKcs in the response to IR (discussed below).
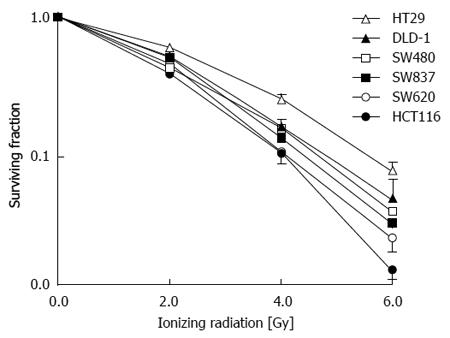
Examination of factors leading to radioresistance can practically be approached in vitro. Analysis of five colon cancer cell lines (HT29, DLD-1, SW480, SW620, and HCT116) as well as one rectal cancer cell line (SW837) have demonstrated a similar pattern of response to a group of patients treated for rectal cancer with pre-operative IR (Figure 3)[92]. The cell lines that have been treated with IR and examined originate from patients with different characteristics.
SW480 cells were derived from a primary Duke’s stage B colon adenocarcinoma from a 50-year-old Caucasian male, while the SW620 cell line was cultured from a lymph node metastasis from the same patient at a later time. The DLD-1 cell line was established from an adult male with adenocarcinoma of the colon. The SW837 cell line was derived from a 53-year-old Caucasian male with rectal cancer. HCT-116 cells were cultured from an adult male with colon cancer. HT-29 cells were derived from a 44-year-old Caucasian woman with colorectal adenocarcinoma. All of these cells have mutations of the p53 gene, except for HCT-116 cells (p53-Wt). HT-29 cell have mutations of both alleles of the p53 gene (p53-null)[92]. HCT-116 cells display microsatellite instability.
These cells have been extensively studied and a number of properties are known. Analysis of these factors and a response to IR has not yielded any uniform patter of predictability that could be surrogate markers in ex vivo studies. For instance, the inhibitor of apoptosis, survivin, has been shown to play a significant role in resistance to IR (discussed below)[93]. Analysis of this model of rectal cancer in vitro (Figure 3) has not consistently corroborated this finding. For instance, survivin was expressed in higher levels in the radiosensitive SW620 compared to the relative more radioresistant SW480 cell line. Interestingly, these two cells originated from the same patient one at the time of stage II colon cancer (SW480) and the second one from a lymph node metastasis (SW620) such that these two cell lines contain similar genetic background.
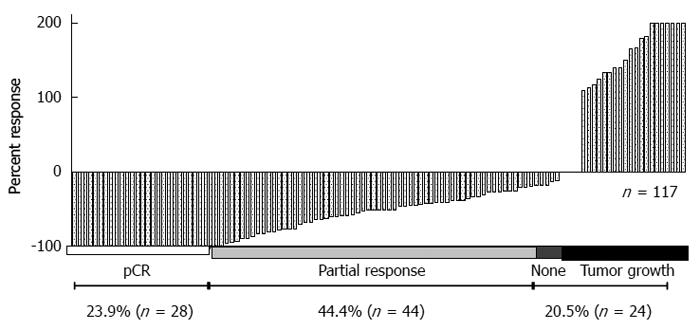
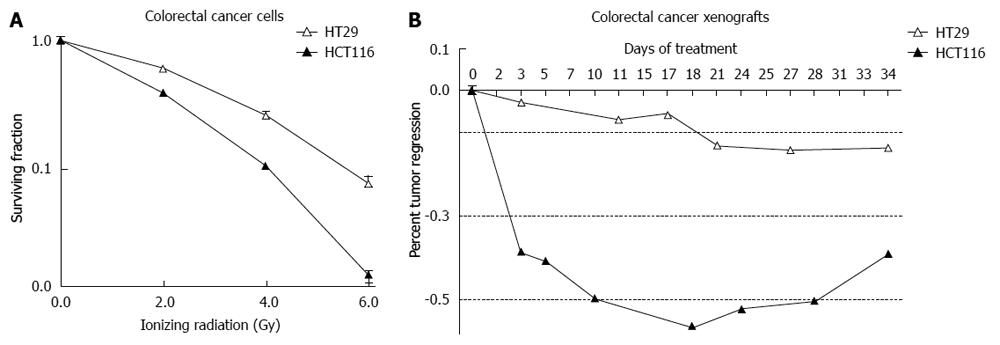
Analysis of these cell lines is representative of the response that was observed in 117 patients who were treated with preoperative ionizing radiation and underwent surgical resection (Figure 4). A pivotal question is to determine what causes these differences in patients and cell lines receiving the same treatment. A simple approach in the laboratory is to take the more radioresistant and the more radiosensitive cells and analyze specific differences. This approach has been undertaken in vitro and in vivo. HCT-116 cell and xenografts are substantially more sensitive to IR compared to HT-29 cells and xenografts (Figure 5).
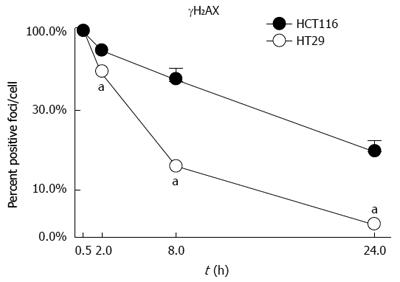
Analysis of DNA induced damage (by γH2AX) indicated that the radioresistant HCT-116 cells suffer more DNA damage when exposed to IR and that this damage persists over time indicating a poor ability of the cells to repair the DNA affected by IR (Figure 6)[94]. Predictably, HT-29 cells should be able to repair DNA more effectively and should have increased levels of DNA-PKcs and Ku proteins. In fact, the opposite results have been observed in our studies. Our results showed that compared to HCT-116 cells, HT-29 cells expressed lower levels of DNA-PKcs and Ku proteins[95].
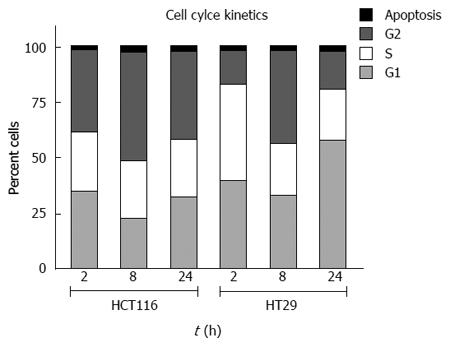
Examination of cell cycle kinetics demonstrates that the radiosensitive HCT-116 cells substantially accumulate in the G-2 phase of the cell cycle. HT-29 cells proceed through the cell cycle in spite of receiving the same dose of IR (Figure 7)[22,28,92,94,96,97]. According to these observations, there should be differences in cell cycle regulators and apoptotic factors that could be used to predict a response to IR.
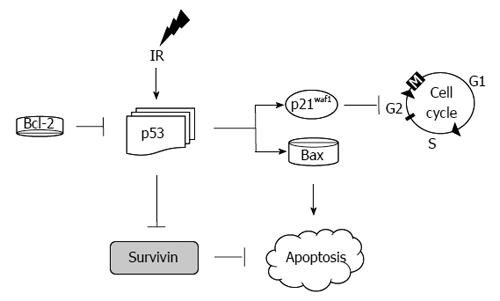
Analysis of this model with regards to the central mediators of apoptosis (as depicted in Figure 8) has demonstrated the following in HCT116 (vs HT29 cells): marked over expression of p21, decreased expression of p53, Bax, Bcl-2 and survivin[92]. Examination of these findings is intuitive in some areas while counterintuitive in others. For instance, p21 elevation in response to IR is an expected response of these radiosensitive cells. This was associated with an appropriate response of p53 leading to activation of p21 culminating in apoptosis as demonstrated by an elevation of the cleaved PARP-1. In HT29 cells, on the other hand, p53 was markedly elevated. This is the result of the mutated status of p53 in HT29 cells. However, the results with regards to Bax and survivin are not clear in these experiments as a decrease in survivin and Bax was expected in these radioresistant cells.
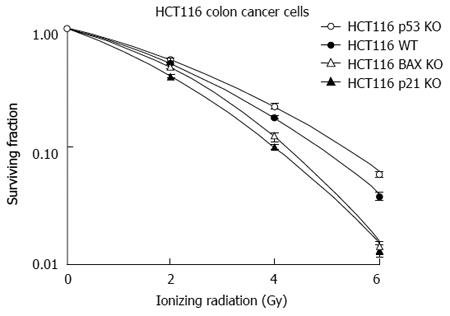
In separate in vitro studies, analysis with colorectal cancer cells with stable knock out (KO) of genes responsible for apoptosis from IR-induced injury was undertaken. This demonstrated that the p21 and the Bax KO genotypes were associated with radiosensitivity rather than radioresistance (Figure 6)[28]. The results with regards to p21 have been previously reported and indicate that it is mitotic catastrophe that leads these cells to undergo cellular death rather than becoming more radioresistant. The Bax KO genotype leading to a more radiosensitive phenotype as opposed to radioresistance was partly mediated by apoptosis inducing factor (AIF) and not to caspase mediated apoptosis[28]. AIF is an important mediator of cellular death that requires further studies as a predictor of a response to IR in rectal cancer[98].
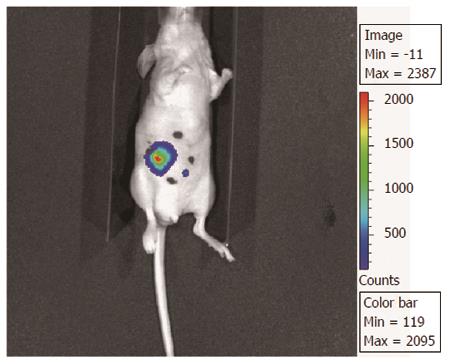
These observations in vitro have been noted in vivo models of rectal cancer as well. However, one of the limitations of the studies in vivo is that these studies have relied on xenograft models of rectal cancer. We have previously described an orthotropic model in which cells have been implanted in the cecum and then the cecum was secured to the abdominal wall for targeted IR. Because these cells can be labeled with luciferase, the response to IR can be followed over time by biluminenscence imaging (Figure 9). However, this model requires further validation[97].
In summary, observations from these studies demonstrate that there are good models for the study of rectal cancer in response to IR in vitro and in vivo. We have identified some molecules that can be used to predict a response to IR in HT-29 and HCT-116 cells. Application of these factors to the rest of the cells as depicted in Figure 3 has yielded mixed results. There is no unifying pathway that has been identified to date. Moreover, identification of predictors for a response to IR remain at large. For instance, many inhibitors of apoptosis examined (IAPs; survivin, XIAP, cIAP 1/2) were all increased in the more radiosensitive SW620 cells compared to the SW480 cells. Survivin, in response IR in colorectal cancer cells (0, 2, 4, and 6 Gy) was expressed in the following order in several cells: SW620 > HT-29 > HCT-116. Apoptosis was interrogated by PARP-1 cleavage and demonstrated that apoptosis in response to IR occurred in the following pattern: DLD-1 > HCT-116 > SW480 > HT-29 > SW480. p27 demonstrated the following pattern: HT-29 > HCT-116 > SW480. There was no particular pattern of expression of these factors nor was there a correlation to a response to IR noted. Thus, there is further need for identification of a unifying pathway that could be used to determine a response to IR.
The additional advantage of the current in vitro and in vivo models is that they can be utilized for the study of radiosensitizing agents and some of these have demonstrated promising results[92,94]. The effects of the radiosensitizing agents on specific pathways can also be explored in this fashion.
We then proceeded with a review of literature to determine how these observations compared to other studies. The result of this review have been previously documented to some extent and are presented and updated in the following discussion[22,99].
If cells are unable to repair the damage induced by IR, the cell is destined to undergo programmed cell death. In the classical pathway, the stressed cell leads to an up-regulation of p53, which then stops the cell cycle via induction of the cyclin depended kinase inhibitor p21. Failure to repair the damage causes BAX to induce apoptosis[22,100] (Figure 8).
It is conceivable that defects in any of these molecules (apoptotic or cell cycle proteins) alone or in combination could serve as a surrogate to predict a response to IR in rectal cancer. In vitro studies with colon cancer cells exposed to radiation have been in agreement with the classical response to apoptosis with p53, but not uniformly with p21 and BAX (as discussed in the previous section)[28] (Figure 8).
p53:In vitro, HCT-116 cells deficient of p53 are more radioresistant compared to HCT-116 wild-type cells. Tumor xenografts derived from the same cells demonstrated a similar effect[28]. These results have been mirrored in models of colorectal cancer in vitro and in vivo[101,102], but in disagreement with others[103-105]. Other studies have suggested that p53 mutations may render cells more radiosensitive owing to a reduction in p53-dependent DNA repair mechanisms[106]. Thus, in vitro and in vivo studies with regards to p53 have shown mixed results. In vitro, data indicates that lack of p53 leads to radioresistance. However, the mutational status of p53 is important to consider in all analyses examining p53[22].
Ex vivo studies have demonstrated a number of heterogeneous findings as well. Some studies have shown that mutated p53 leads to radioresistance in rectal cancer tissues[107]. Nuclear expression of p53 in rectal cancers predicted treatment failure and signified resistance to preoperative IR[96]. Other studies have demonstrated no usefulness of p53 as a marker of a response to IR[108,109]. To date, ex vivo studies have failed to provide usefulness as a marker of a response to IR. This might be the result of the low number of subjects included in the studies, the wide range of techniques utilized to detect p53, or the ability of the antibody to recognize the mutated vs the wild-type form of p53[22].
Cell cycle factors such as p53 and the cyclin dependent kinase inhibitors (CDKIs) (p21 and p27) have been studied as possible candidates to predict a response to ionizing radiation in rectal cancer. p21 is the classical CDKI and is activated by p53[110,111]. Irradiated colon cancer DLD-1 cells expressed low levels of p21[112]. The expected response to IR in cells and tumors deficient of p21 would be a radioresistant phenotype. Recent studies have shown that HCT-116 cell deficient of p21 are, in fact, more sensitive to ionizing radiation compared to wild-type HCT-116 cells[28,113]. Tumor xenografts deficient of p21 demonstrated more tumor regression compared to the wild-type genotype treated with the same dose of ionizing radiation[28].
p21:Ex vivo studies demonstrated the p21 positive tumors had a good response to IR[114]. Another study showed that p21 expression correlated with good pathological response and tumor radiosensitivity[115]. Similarly, a reduction by 50% in post-irradiated rectal tissue compared to pre-irradiated one was associated with radio-resistance[116]. Another study did not find p21 useful as a predictor of a response to IR[117].
p27: This study found that p27 positive tumors had a better response to IR with an OR of 3.3[117]. Similarly, the absence of p53 and p27 prior to treatment was associated with poor response to IR in rectal tumors[118].
Bax: Bax is a pro-apoptotic protein that leads to the release of cytochrome c from the intermitochondrial membrane[100]. It may be anticipated that Bax deficiency would be associated with radioresistance. In vitro and in vivo studies have demonstrated the opposite phenotype to IR (Figure 10)[28]. While a few studies demonstrate that Bax deficient cells are resistant to chemotherapeutic agents[119-121], evidence indicating the response of Bax deficient colorectal cancer cells to IR in pre-clinical studies is lacking. Limited ex vivo studies have shown that Bax tumor expression had a positive response to chemoradiation in patients treated for rectal cancer[122,123].
Bcl-2 inhibits cellular apoptosis and is overexpressed in many colorectal tumors[124]. BAX is the apoptogenic counter part of Bcl-2. Current studies have failed to demonstrate the association of Bcl-2 as a marker of response to IR[22,123,125].
Survivin: Survivin is one of eight inhibitors of apoptosis (IAPs) that are generated via induction of NFκB[100]. Survivin binds and inactivates caspases 3, 7 and 9[100]. In vitro and in vivo data showed that the NFκB-IAPs axis is a predictor of a poor response to IR when over expressed[22]. Ex vivo data supports the role of survivin in raidoresistance[93]. Furthermore, the five year survival of patients with survivin positive stage II colon cancer tumors was 41% lower than patients with survivin negative tumors[126]. The role of other r IAPs (i.e., XIAP, cIAP, etc.) and a response to IR remains at large.
The role of the IAPs in response to IR has been further interrogated by directly inhibiting the inhibition of the IAPs via augmentation of an antagonistic factor to the IAPs: SMAC/Diablo.
SMAC/Diablo: Pro-apoptotic molecules with the ability to reduce the functional activity of the inhibitors of apoptosis might have potential therapeutic applications. Compounds that mimic the action of SMAC/Diablo (Smac-mimetics) are under study for their ability to chemo- and radiosensitize tumor cells[127]. The Smac mimetic JP-1201 radiosensitized HT-29 colorectal cancer cells and xenografts by a marked augmentation in apoptosis, which was associated with a reduction in the levels of the IAP XIAP[94].
Proliferation markers and mitotic index as markers: A few studies have reported high Ki-67 staining correlated with a positive response to IR[128,129]. In contrast, most studies have demonstrated that proliferating nuclear antigen labeling index does not correlate with response to IR[115,125,130].
Apoptotic index: Evaluation of apoptosis in cancer cells has shown that patients with higher pre-radiation level of apoptosis (apoptotic index) had lower rate of recurrence and longer disease free period after radiation[131].
Logically, tumors that have an intact machinery to undergo apoptosis should respond better to ionizing radiation rather that those with mutation of one or more pro-apoptotic factors or activation of anti-apoptotic factors. Caspase mediated apoptosis has been shown to play a promising role in predicting a response to IR. A high spontaneous apoptotic index in pretreated tumor tissue was associated with a superior rate of response to radiation[132]. Furthermore, in a large study including 465 pre-irradiated biopsies tumors underwent immunohistochemistry staining against the active form of caspase 3. This study showed that tumors with a high apoptotic index had less recurrence and a higher disease free survival[131].
While these results seem promising, uniformity across studies has not been established nor substantial reproducibility or adoption to clinical practice. The practical usefulness of this approach is limited by the dynamic process of apoptosis and by the wide variety of measurements and laboratory standardizations. The individual evaluation of specific molecules in the process of apoptosis either as a single factor or in combination with others seems to suffer from the same issues.
Hypoxia: Lack of oxygen supply to cancer cells has been linked to poor response to radiation. This premise was tested in patients undergoing neoadjuvant therapy for rectal cancer with the assistance of positron emission tomography using the copper-60-diacetyl-bis (N4-methylthiosemicarbazone (60Cu-ATSM), an agent that accumulates in tissues lacking adequate oxygenation. Tumors with higher baseline tumor-muscle activity ratios (suggesting hypoxia) in the pre-treatment PET scan were shown to have a poor response to radiation[133]. Other agents tested in different studies have been less useful probably as a result of technical limitations[134].
Further evidence of the role of hypoxia in response to IR was demonstrated by the fact that higher levels of HIF-1 (hypoxia inducible protein factor 1, a protein that increases in oxygen deprived tissues) predicts poor response to neoadjuvant chemotherapy in patients with rectal cancer[135]. Additionally, HIF-1 correlates with increased levels of pro-angiogenic vascular endothelial growth factor (VEGF), a marker of angiogenesis for tumor growth[136].
VEGF: Low levels of VEGF have been associated with improved response to radiation[135,137,138], and vice versa[135,137-139]. Therefore, VEGF inhibition with the antibody bevacizumab has shown beneficial effects in treating cancers with neoadjuvant chemoradiotherapy[137,140,141]. Various mechanisms by which VEGF inhibition causes this effect may include reducing vascular density within a tumor, decreasing interstitial tumor pressures, improving global oxygenation status, vascular normalization and thus increasing responsiveness of endothelial cells to radiation[137,141,142]. It seems logical that if bevacizumab were to be used as a neoadjuvant agent in combination with IR for the treatment of patients with rectal cancer, these should have a higher rate of pCR compared to standard treatments. However, this observation has not been validated in clinical trials[143].
EGFR signaling: Initial reports revealed that combination of radiation and EGFR inhibition exerted a synergistic cytotoxic effect and hence raised interest in developing EGFR inhibitors. Hence, multiple EGFR inhibitors (e.g., cetuximab and panitumumab) were developed and tested and have demonstrated promise in patients with KRAS wild-type tumors. However, with regards to the usefulness in EGFR signaling as a predictor of a response to IR, the data is lacking. Similarly, data pertaining to the usefulness of inhibiting the EGFR signaling pathway as a radiosensitizing modality has also demonstrated disappointing results[144].
Microarray analysis: Single molecules as independent factors or in combination with other molecules of specific pathways (i.e., apoptosis or angiogenesis) have not provided to be clinically useful to date. A major limitation of examining a specific pathway had to do with the dynamics of the process and the particular point in time at which it is being measured. Further, many tumors are heterogeneous in terms of mutations and alterations. Thus, interrogating several genes or proteins simultaneously is a logical approach in terms of elucidating origins of radioresistance in rectal cancer. In the era of personalized care, these tumor “fingerprints” not only make sense, but is the direction of the future.
Unfortunately, as appealing as it might seem, current efforts have been unsuccessful. Two studies have independently performed RNA arrays to analyze radioresistant and radiosensitive tumors. These studies have had limited genes and have had different results[145,146].
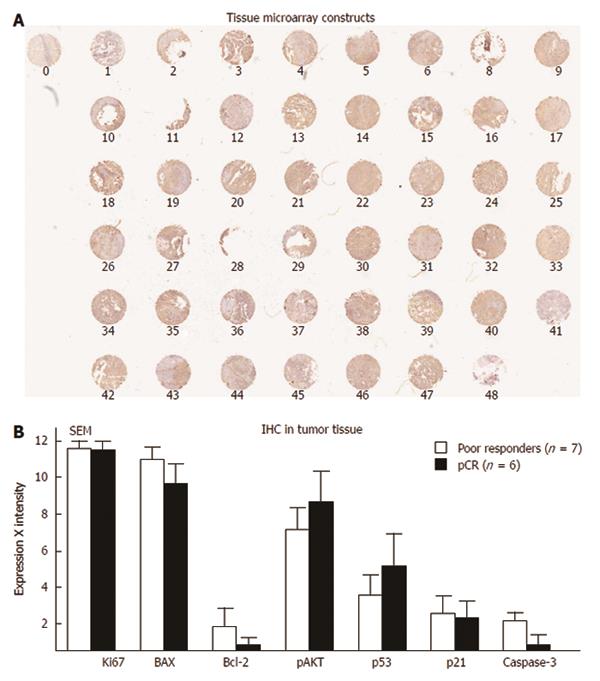
Tissue microarray: Tissue microarray is another technique to assess multiple proteins with a single experiment with tissues handled in a similar fashion. In one study, tissue microarray was performed with the goal of predicting survival and recurrence in patients treated with chemoradiation. In this study, Cox-2 emerged as a potential predictor of survival[147]. In a second study, our group subjected rectal cancer tissue to tissue microarray and tested eight different antibodies. MIB was the only independent predictor of a response to chemoradiation[8]. In our analysis, we examined tissue microarray in 48 patients who were treated with preoperative IR. We then divided all of these patients in two groups: patients who achieved a pCR (n = 6) compared to those who did not respond to IR or patients who experienced tumor growth (n = 7) in spite of pre-operative chemoradiation. We stained the tissue microarrays with seven antibodies and demonstrated no particular protein that could be used to differentiate these groups (Figure 11)[8].
Rectal cancer is the ideal clinical problem where personalized treatment could be investigated. This theory stems from the fact that a select patient population obtains an excellent response from the same form of chemo-radiation, while others do not. Despite putting forward multiple mechanisms of tumor death from ionizing radiation and various possible causes of radioresistance, there has not been a unifying pathway that can reliably predict a response to IR in vitro, in vivo or ex vivo. It is difficult to explain the reasons behind a clear discrepancy in the current observations in the literature. However, differences in tumor biology, genotypic profiling or phenotypic characteristics are some of these factors. There are currently good in vitro, in vivo, and ex vivo models for the study of rectal cancer and the trend seems optimistic in developing a predictive finger print for patients with rectal cancer that might respond well to IR. Recent data has shown that DNA-PKcs and Ku proteins (as vital players in NHEJ pathway allowing DSB repair) may have a central role in radiation induced cell death. Nevertheless many facets of its function in conjunction with the complex and intricate details of the pathway are still under investigation. More data is required before we can formulate one unified explanation for the heterogeneity noted in therapeutic effect of ionizing radiation. Until then, the hope of developing novel therapies for rectal cancer and improving the therapeutic yield of ionizing radiation with radiosensitizers remains a challenging clinical problem. The findings so far should not be viewed in a pessimistic fashion. There are several pathways that have provided potential targets for chemoradiotherapeutic interventions. We need to continue to investigate potential molecules predictive of a response to IR. As we dwell into the future, we need to remember that markers predictive of an aggressive behavior are currently in clinical practice such as testing for BRCA or RET proto-oncogene mutations. A view into the future also includes investigating base line characteristics of patient’s genotypic background in normal tissue compared to tumor tissue after IR. It is important to determine if a patient starts with high levels at base line, but a particular gene is not activated then the base line levels are not as predictive. In the opposite scenario, we might have a patient with a molecule that at base line is low, but it is activated substantially with IR. In that scenario, we might consider those features as more predictive. The future, therefore, should be viewed with optimism.
P- Reviewers: Ogino S, Toth K S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9855] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Morino M, Parini U, Allaix ME, Monasterolo G, Brachet Contul R, Garrone C. Male sexual and urinary function after laparoscopic total mesorectal excision. Surg Endosc. 2009;23:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Chari RS, Tyler DS, Anscher MS, Russell L, Clary BM, Hathorn J, Seigler HF. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg. 1995;221:778-786; discussion 786-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Wanebo HJ, Koness RJ, Vezeridis MP, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer. Ann Surg. 1994;220:586-595; discussion 595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 153] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Huerta S, Murray B, Olson C, Patel P, Anthony T. Current evidence-based opinions in the management of adenocarcionoma of the rectum. Indian J Surg. 2009;71:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Carraro S, Roca EL, Cartelli C, Rafailovici L, Castillo Odena S, Wasserman E, Gualdrini U, Huertas E, Barugel M, Ballarino G. Radiochemotherapy with short daily infusion of low-dose oxaliplatin, leucovorin, and 5-FU in T3-T4 unresectable rectal cancer: a phase II IATTGI study. Int J Radiat Oncol Biol Phys. 2002;54:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Gérard A, Buyse M, Nordlinger B, Loygue J, Pène F, Kempf P, Bosset JF, Gignoux M, Arnaud JP, Desaive C. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg. 1988;208:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 499] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Huerta S, Hrom J, Gao X, Saha D, Anthony T, Reinhart H, Kapur P. Tissue microarray constructs to predict a response to chemoradiation in rectal cancer. Dig Liver Dis. 2010;42:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Minsky BD, Röedel C, Valentini V. Combined modality therapy for rectal cancer. Cancer J. 2010;16:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Willett CG, Hagan M, Daley W, Warland G, Shellito PC, Compton CC. Changes in tumor proliferation of rectal cancer induced by preoperative 5-fluorouracil and irradiation. Dis Colon Rectum. 1998;41:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1449] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 12. | Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS, Daniels IR. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Glynne-Jones R, Wallace M, Livingstone JI, Meyrick-Thomas J. Complete clinical response after preoperative chemoradiation in rectal cancer: is a “wait and see” policy justified? Dis Colon Rectum. 2008;51:10-19; discussion 19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-717; discussion 717-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1353] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 15. | Habr-Gama A, Perez RO. Non-operative management of rectal cancer after neoadjuvant chemoradiation. Br J Surg. 2009;96:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633-4640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 773] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 17. | Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1231] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 19. | Simsek D, Brunet E, Wong SY, Katyal S, Gao Y, McKinnon PJ, Lou J, Zhang L, Li J, Rebar EJ. DNA ligase III promotes alternative nonhomologous end-joining during chromosomal translocation formation. PLoS Genet. 2011;7:e1002080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 20. | DeFazio LG, Stansel RM, Griffith JD, Chu G. Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J. 2002;21:3192-3200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Roberts SA, Strande N, Burkhalter MD, Strom C, Havener JM, Hasty P, Ramsden DA. Ku is a 5’-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Huerta S, Gao X, Saha D. Mechanisms of resistance to ionizing radiation in rectal cancer. Expert Rev Mol Diagn. 2009;9:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Auckley DH, Crowell RE, Heaphy ER, Stidley CA, Lechner JF, Gilliland FD, Belinsky SA. Reduced DNA-dependent protein kinase activity is associated with lung cancer. Carcinogenesis. 2001;22:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Harima Y, Sawada S, Miyazaki Y, Kin K, Ishihara H, Imamura M, Sougawa M, Shikata N, Ohnishi T. Expression of Ku80 in cervical cancer correlates with response to radiotherapy and survival. Am J Clin Oncol. 2003;26:e80-e85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Muller C, Christodoulopoulos G, Salles B, Panasci L. DNA-Dependent protein kinase activity correlates with clinical and in vitro sensitivity of chronic lymphocytic leukemia lymphocytes to nitrogen mustards. Blood. 1998;92:2213-2219. [PubMed] |
| 26. | Townsend DM, Shen H, Staros AL, Gaté L, Tew KD. Efficacy of a glutathione S-transferase pi-activated prodrug in platinum-resistant ovarian cancer cells. Mol Cancer Ther. 2002;1:1089-1095. [PubMed] |
| 27. | Zhao Y, Thomas HD, Batey MA, Cowell IG, Richardson CJ, Griffin RJ, Calvert AH, Newell DR, Smith GC, Curtin NJ. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66:5354-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 334] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Huerta S, Gao X, Dineen S, Kapur P, Saha D, Meyer J. Role of p53, Bax, p21, and DNA-PKcs in radiation sensitivity of HCT-116 cells and xenografts. Surgery. 2013;154:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Ruis BL, Fattah KR, Hendrickson EA. The catalytic subunit of DNA-dependent protein kinase regulates proliferation, telomere length, and genomic stability in human somatic cells. Mol Cell Biol. 2008;28:6182-6195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki N. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol. 2004;25:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Lü Y, Zhang HL, Li YZ, Zhao P. [Clinicopathological significance of expressions of DNA dependent protein kinase catalytic subunit and P16 in colorectal carcinoma]. Zhonghua Yixue Zazhi. 2008;88:2025-2029. [PubMed] |
| 32. | Tonotsuka N, Hosoi Y, Miyazaki S, Miyata G, Sugawara K, Mori T, Ouchi N, Satomi S, Matsumoto Y, Nakagawa K. Heterogeneous expression of DNA-dependent protein kinase in esophageal cancer and normal epithelium. Int J Mol Med. 2006;18:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Yu S, Xiong Y, Tian S. [The expression of DNA-PKcs in non-small cell lung cancer and its relationship with apoptosis associated proteins]. Zhongguo Feiai Zazhi. 2003;6:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Lee HS, Yang HK, Kim WH, Choe G. Loss of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) expression in gastric cancers. Cancer Res Treat. 2005;37:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Lee HS, Choe G, Park KU, Park do J, Yang HK, Lee BL, Kim WH. Altered expression of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) during gastric carcinogenesis and its clinical implications on gastric cancer. Int J Oncol. 2007;31:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Noguchi T, Shibata T, Fumoto S, Uchida Y, Mueller W, Takeno S. DNA-PKcs expression in esophageal cancer as a predictor for chemoradiation therapeutic sensitivity. Ann Surg Oncol. 2002;9:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Pan H, Zuo C, Mao N, Chen J, Cao J, Tang B. [Expression and clinical significance of Ku70, Ku80 and DNA-PKcs proteins in patients with stageI-II non-small cell lung cancer by tissue microarray]. Zhongguo Feiai Zazhi. 2007;10:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Söderlund Leifler K, Queseth S, Fornander T, Askmalm MS. Low expression of Ku70/80, but high expression of DNA-PKcs, predict good response to radiotherapy in early breast cancer. Int J Oncol. 2010;37:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Shintani S, Mihara M, Li C, Nakahara Y, Hino S, Nakashiro K, Hamakawa H. Up-regulation of DNA-dependent protein kinase correlates with radiation resistance in oral squamous cell carcinoma. Cancer Sci. 2003;94:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Mitchell J, Smith GC, Curtin NJ. Poly(ADP-Ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys. 2009;75:1520-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Shaheen FS, Znojek P, Fisher A, Webster M, Plummer R, Gaughan L, Smith GC, Leung HY, Curtin NJ, Robson CN. Targeting the DNA double strand break repair machinery in prostate cancer. PLoS One. 2011;6:e20311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Tavecchio M, Munck JM, Cano C, Newell DR, Curtin NJ. Further characterisation of the cellular activity of the DNA-PK inhibitor, NU7441, reveals potential cross-talk with homologous recombination. Cancer Chemother Pharmacol. 2012;69:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Azad A, Jackson S, Cullinane C, Natoli A, Neilsen PM, Callen DF, Maira SM, Hackl W, McArthur GA, Solomon B. Inhibition of DNA-dependent protein kinase induces accelerated senescence in irradiated human cancer cells. Mol Cancer Res. 2011;9:1696-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Du L, Zhou LJ, Pan XJ, Wang YX, Xu QZ, Yang ZH, Wang Y, Liu XD, Zhu MX, Zhou PK. Radiosensitization and growth inhibition of cancer cells mediated by an scFv antibody gene against DNA-PKcs in vitro and in vivo. Radiat Oncol. 2010;5:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Davidson D, Grenier J, Martinez-Marignac V, Amrein L, Shawi M, Tokars M, Aloyz R, Panasci L. Effects of the novel DNA dependent protein kinase inhibitor, IC486241, on the DNA damage response to doxorubicin and cisplatin in breast cancer cells. Invest New Drugs. 2012;30:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Davidson D, Coulombe Y, Martinez-Marignac VL, Amrein L, Grenier J, Hodkinson K, Masson JY, Aloyz R, Panasci L. Irinotecan and DNA-PKcs inhibitors synergize in killing of colon cancer cells. Invest New Drugs. 2012;30:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Durant S, Karran P. Vanillins--a novel family of DNA-PK inhibitors. Nucleic Acids Res. 2003;31:5501-5512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 323] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Malewicz M, Kadkhodaei B, Kee N, Volakakis N, Hellman U, Viktorsson K, Leung CY, Chen B, Lewensohn R, van Gent DC. Essential role for DNA-PK-mediated phosphorylation of NR4A nuclear orphan receptors in DNA double-strand break repair. Genes Dev. 2011;25:2031-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Quanz M, Herbette A, Sayarath M, de Koning L, Dubois T, Sun JS, Dutreix M. Heat shock protein 90α (Hsp90α) is phosphorylated in response to DNA damage and accumulates in repair foci. J Biol Chem. 2012;287:8803-8815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Solier S, Kohn KW, Scroggins B, Xu W, Trepel J, Neckers L, Pommier Y. Heat shock protein 90α (HSP90α), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proc Natl Acad Sci USA. 2012;109:12866-12872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Toulany M, Lee KJ, Fattah KR, Lin YF, Fehrenbacher B, Schaller M, Chen BP, Chen DJ, Rodemann HP. Akt promotes post-irradiation survival of human tumor cells through initiation, progression, and termination of DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Res. 2012;10:945-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 53. | Li S, Takeda Y, Wragg S, Barrett J, Phillips A, Dynan WS. Modification of the ionizing radiation response in living cells by an scFv against the DNA-dependent protein kinase. Nucleic Acids Res. 2003;31:5848-5857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Xiong H, Li S, Yang Z, Burgess RR, Dynan WS. E. coli expression of a soluble, active single-chain antibody variable fragment containing a nuclear localization signal. Protein Expr Purif. 2009;66:172-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Xiong H, Lee RJ, Haura EB, Edwards JG, Dynan WS, Li S. Intranuclear delivery of a novel antibody-derived radiosensitizer targeting the DNA-dependent protein kinase catalytic subunit. Int J Radiat Oncol Biol Phys. 2012;83:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Collis SJ, Swartz MJ, Nelson WG, DeWeese TL. Enhanced radiation and chemotherapy-mediated cell killing of human cancer cells by small inhibitory RNA silencing of DNA repair factors. Cancer Res. 2003;63:1550-1554. [PubMed] |
| 57. | Ni X, Zhang Y, Ribas J, Chowdhury WH, Castanares M, Zhang Z, Laiho M, DeWeese TL, Lupold SE. Prostate-targeted radiosensitization via aptamer-shRNA chimeras in human tumor xenografts. J Clin Invest. 2011;121:2383-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | Sak A, Stuschke M, Wurm R, Schroeder G, Sinn B, Wolf G, Budach V. Selective inactivation of DNA-dependent protein kinase with antisense oligodeoxynucleotides: consequences for the rejoining of radiation-induced DNA double-strand breaks and radiosensitivity of human cancer cell lines. Cancer Res. 2002;62:6621-6624. [PubMed] |
| 59. | Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, Nirodi CS. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007;67:5267-5274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 61. | Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005;76:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 62. | Friedmann BJ, Caplin M, Savic B, Shah T, Lord CJ, Ashworth A, Hartley JA, Hochhauser D. Interaction of the epidermal growth factor receptor and the DNA-dependent protein kinase pathway following gefitinib treatment. Mol Cancer Ther. 2006;5:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Leahy JJ, Golding BT, Griffin RJ, Hardcastle IR, Richardson C, Rigoreau L, Smith GC. Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg Med Chem Lett. 2004;14:6083-6087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 64. | Kashishian A, Douangpanya H, Clark D, Schlachter ST, Eary CT, Schiro JG, Huang H, Burgess LE, Kesicki EA, Halbrook J. DNA-dependent protein kinase inhibitors as drug candidates for the treatment of cancer. Mol Cancer Ther. 2003;2:1257-1264. [PubMed] |
| 65. | Shinohara ET, Geng L, Tan J, Chen H, Shir Y, Edwards E, Halbrook J, Kesicki EA, Kashishian A, Hallahan DE. DNA-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res. 2005;65:4987-4992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Shang ZF, Huang B, Xu QZ, Zhang SM, Fan R, Liu XD, Wang Y, Zhou PK. Inactivation of DNA-dependent protein kinase leads to spindle disruption and mitotic catastrophe with attenuated checkpoint protein 2 Phosphorylation in response to DNA damage. Cancer Res. 2010;70:3657-3666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Zhuang W, Li B, Long L, Chen L, Huang Q, Liang ZQ. Knockdown of the DNA-dependent protein kinase catalytic subunit radiosensitizes glioma-initiating cells by inducing autophagy. Brain Res. 2011;1371:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Someya M, Sakata K, Matsumoto Y, Yamamoto H, Monobe M, Ikeda H, Ando K, Hosoi Y, Suzuki N, Hareyama M. The association of DNA-dependent protein kinase activity with chromosomal instability and risk of cancer. Carcinogenesis. 2006;27:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Someya M, Sakata KI, Matsumoto Y, Kamdar RP, Kai M, Toyota M, Hareyama M. The association of DNA-dependent protein kinase activity of peripheral blood lymphocytes with prognosis of cancer. Br J Cancer. 2011;104:1724-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Rajagopalan S, Moyle MW, Joosten I, Long EO. DNA-PKcs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells. Sci Signal. 2010;3:ra14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Becknell B, Caligiuri MA. Natural killer cells in innate immunity and cancer. J Immunother. 2008;31:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Eriksson A, Lewensoh R, Larsson R, Nilsson A. DNA-dependent protein kinase in leukaemia cells and correlation with drug sensitivity. Anticancer Res. 2002;22:1787-1793. [PubMed] |
| 73. | Shao CJ, Fu J, Shi HL, Mu YG, Chen ZP. Activities of DNA-PK and Ku86, but not Ku70, may predict sensitivity to cisplatin in human gliomas. J Neurooncol. 2008;89:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Dejmek J, Iglehart JD, Lazaro JB. DNA-dependent protein kinase (DNA-PK)-dependent cisplatin-induced loss of nucleolar facilitator of chromatin transcription (FACT) and regulation of cisplatin sensitivity by DNA-PK and FACT. Mol Cancer Res. 2009;7:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Stronach EA, Chen M, Maginn EN, Agarwal R, Mills GB, Wasan H, Gabra H. DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia. 2011;13:1069-1080. [PubMed] |
| 76. | Bouchaert P, Guerif S, Debiais C, Irani J, Fromont G. DNA-PKcs expression predicts response to radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Gu J, Lieber MR. Mechanistic flexibility as a conserved theme across 3 billion years of nonhomologous DNA end-joining. Genes Dev. 2008;22:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Uematsu N, Weterings E, Yano K, Morotomi-Yano K, Jakob B, Taucher-Scholz G, Mari PO, van Gent DC, Chen BP, Chen DJ. Autophosphorylation of DNA-PKCS regulates its dynamics at DNA double-strand breaks. J Cell Biol. 2007;177:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 79. | Yoo S, Dynan WS. Geometry of a complex formed by double strand break repair proteins at a single DNA end: recruitment of DNA-PKcs induces inward translocation of Ku protein. Nucleic Acids Res. 1999;27:4679-4686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Costantini S, Woodbine L, Andreoli L, Jeggo PA, Vindigni A. Interaction of the Ku heterodimer with the DNA ligase IV/Xrcc4 complex and its regulation by DNA-PK. DNA Repair (Amst). 2007;6:712-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Mari PO, Florea BI, Persengiev SP, Verkaik NS, Brüggenwirth HT, Modesti M, Giglia-Mari G, Bezstarosti K, Demmers JA, Luider TM. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci USA. 2006;103:18597-18602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 82. | Yano K, Morotomi-Yano K, Wang SY, Uematsu N, Lee KJ, Asaithamby A, Weterings E, Chen DJ. Ku recruits XLF to DNA double-strand breaks. EMBO Rep. 2008;9:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 83. | Grundy GJ, Rulten SL, Zeng Z, Arribas-Bosacoma R, Iles N, Manley K, Oliver A, Caldecott KW. APLF promotes the assembly and activity of non-homologous end joining protein complexes. EMBO J. 2013;32:112-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Wilson CR, Davidson SE, Margison GP, Jackson SP, Hendry JH, West CM. Expression of Ku70 correlates with survival in carcinoma of the cervix. Br J Cancer. 2000;83:1702-1706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Moeller BJ, Yordy JS, Williams MD, Giri U, Raju U, Molkentine DP, Byers LA, Heymach JV, Story MD, Lee JJ. DNA repair biomarker profiling of head and neck cancer: Ku80 expression predicts locoregional failure and death following radiotherapy. Clin Cancer Res. 2011;17:2035-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Komuro Y, Watanabe T, Hosoi Y, Matsumoto Y, Nakagawa K, Tsuno N, Kazama S, Kitayama J, Suzuki N, Nagawa H. The expression pattern of Ku correlates with tumor radiosensitivity and disease free survival in patients with rectal carcinoma. Cancer. 2002;95:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Holgersson A, Erdal H, Nilsson A, Lewensohn R, Kanter L. Expression of DNA-PKcs and Ku86, but not Ku70, differs between lymphoid malignancies. Exp Mol Pathol. 2004;77:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Deriano L, Guipaud O, Merle-Béral H, Binet JL, Ricoul M, Potocki-Veronese G, Favaudon V, Maciorowski Z, Muller C, Salles B. Human chronic lymphocytic leukemia B cells can escape DNA damage-induced apoptosis through the nonhomologous end-joining DNA repair pathway. Blood. 2005;105:4776-4783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 89. | Beskow C, Skikuniene J, Holgersson A, Nilsson B, Lewensohn R, Kanter L, Viktorsson K. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer. 2009;101:816-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 90. | Jensen R, Glazer PM. Cell-interdependent cisplatin killing by Ku/DNA-dependent protein kinase signaling transduced through gap junctions. Proc Natl Acad Sci USA. 2004;101:6134-6139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Reynolds P, Anderson JA, Harper JV, Hill MA, Botchway SW, Parker AW, O’Neill P. The dynamics of Ku70/80 and DNA-PKcs at DSBs induced by ionizing radiation is dependent on the complexity of damage. Nucleic Acids Res. 2012;40:10821-10831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 92. | Gao X, Saha D, Kapur P, Anthony T, Livingston EH, Huerta S. Radiosensitization of HT-29 cells and xenografts by the nitric oxide donor DETANONOate. J Surg Oncol. 2009;100:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Rödel F, Hoffmann J, Distel L, Herrmann M, Noisternig T, Papadopoulos T, Sauer R, Rödel C. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005;65:4881-4887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 94. | Huerta S, Gao X, Livingston EH, Kapur P, Sun H, Anthony T. In vitro and in vivo radiosensitization of colorectal cancer HT-29 cells by the smac mimetic JP-1201. Surgery. 2010;148:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Gao X, Meyer J, Huerta S. Role of DNA-PKcs, Ku80 and Bax in Radioresistance of HT-29 Cells and Xenografts. J Surg Res. 2014;186:683. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Adell G, Sun XF, Stål O, Klintenberg C, Sjödahl R, Nordenskjöld B. p53 status: an indicator for the effect of preoperative radiotherapy of rectal cancer. Radiother Oncol. 1999;51:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Huerta S, Gao X, Saha D. Murine orthotopic model for the assessment of chemoradiotherapeutic interventions in rectal cancer. Anticancer Drugs. 2011;22:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 98. | Millan A, Huerta S. Apoptosis-inducing factor and colon cancer. J Surg Res. 2009;151:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 99. | Meyer J, Huerta S. Origins of Radioresistance and Molecular Predictors of Rectal Adenocarcinoma Response to Chemoradiation. CML-Colorectal Cancer. 2010;4:1-8. |
| 100. | Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res. 2007;139:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 101. | Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614-617. [PubMed] |
| 102. | Spitz FR, Nguyen D, Skibber JM, Meyn RE, Cristiano RJ, Roth JA. Adenoviral-mediated wild-type p53 gene expression sensitizes colorectal cancer cells to ionizing radiation. Clin Cancer Res. 1996;2:1665-1671. [PubMed] |
| 103. | Hendry JH, Cai WB, Roberts SA, Potten CS. p53 deficiency sensitizes clonogenic cells to irradiation in the large but not the small intestine. Radiat Res. 1997;148:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Slichenmyer WJ, Nelson WG, Slebos RJ, Kastan MB. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993;53:4164-4168. [PubMed] |
| 105. | Cook T, Wang Z, Alber S, Liu K, Watkins SC, Vodovotz Y, Billiar TR, Blumberg D. Nitric oxide and ionizing radiation synergistically promote apoptosis and growth inhibition of cancer by activating p53. Cancer Res. 2004;64:8015-8021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 106. | Ribeiro JC, Barnetson AR, Fisher RJ, Mameghan H, Russell PJ. Relationship between radiation response and p53 status in human bladder cancer cells. Int J Radiat Biol. 1997;72:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N, Takahashi K, Roth JA, Tanaka N, Orita K. The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers. J Cancer Res Clin Oncol. 1996;122:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Elsaleh H, Robbins P, Joseph D, Powell B, Grieu F, Menso L, Iacopetta B. Can p53 alterations be used to predict tumour response to pre-operative chemo-radiotherapy in locally advanced rectal cancer? Radiother Oncol. 2000;56:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Nehls O, Klump B, Holzmann K, Lammering G, Borchard F, Gruenagel HH, Gaco V, Gregor M, Porschen R. Influence of p53 status on prognosis in preoperatively irradiated rectal carcinoma. Cancer. 1999;85:2541-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 110. | el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6088] [Cited by in RCA: 6302] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 111. | Namba H, Hara T, Tukazaki T, Migita K, Ishikawa N, Ito K, Nagataki S, Yamashita S. Radiation-induced G1 arrest is selectively mediated by the p53-WAF1/Cip1 pathway in human thyroid cells. Cancer Res. 1995;55:2075-2080. [PubMed] |
| 112. | Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 530] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 113. | Tian H, Wittmack EK, Jorgensen TJ. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679-684. [PubMed] |
| 114. | Fu CG, Tominaga O, Nagawa H, Nita ME, Masaki T, Ishimaru G, Higuchi Y, Tsuruo T, Muto T. Role of p53 and p21/WAF1 detection in patient selection for preoperative radiotherapy in rectal cancer patients. Dis Colon Rectum. 1998;41:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Qiu H, Sirivongs P, Rothenberger M, Rothenberger DA, Garciá-Aguilar J. Molecular prognostic factors in rectal cancer treated by radiation and surgery. Dis Colon Rectum. 2000;43:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Palazzo JP, Kafka NJ, Grasso L, Chakrani F, Hanau C, Cuesta KH, Mercer WE. The role of p53, p21WAF1/C1PI, and bcl-2 in radioresistant colorectal carcinoma. Hum Pathol. 1997;28:1189-1195. [PubMed] |
| 117. | Lin LC, Lee HH, Hwang WS, Li CF, Huang CT, Que J, Lin KL, Lin FC, Lu CL. p53 and p27 as predictors of clinical outcome for rectal-cancer patients receiving neoadjuvant therapy. Surg Oncol. 2006;15:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Esposito G, Pucciarelli S, Alaggio R, Giacomelli L, Marchiori E, Iaderosa GA, Friso ML, Toppan P, Chieco-Bianchi L, Lise M. P27kip1 expression is associated with tumor response to preoperative chemoradiotherapy in rectal cancer. Ann Surg Oncol. 2001;8:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Wagener C, Bargou RC, Daniel PT, Bommert K, Mapara MY, Royer HD, Dörken B. Induction of the death-promoting gene bax-alpha sensitizes cultured breast-cancer cells to drug-induced apoptosis. Int J Cancer. 1996;67:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Yamaguchi H, Bhalla K, Wang HG. Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res. 2003;63:1483-1489. [PubMed] |
| 121. | Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 674] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 122. | Chang HJ, Jung KH, Kim DY, Jeong SY, Choi HS, Kim YH, Sohn DK, Yoo BC, Lim SB, Kim DH. Bax, a predictive marker for therapeutic response to preoperative chemoradiotherapy in patients with rectal carcinoma. Hum Pathol. 2005;36:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 123. | Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 124. | Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg. 2006;191:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 125. | Tannapfel A, Nüsslein S, Fietkau R, Katalinic A, Köckerling F, Wittekind C. Apoptosis, proliferation, bax, bcl-2 and p53 status prior to and after preoperative radiochemotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 1998;41:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Sarela AI, Scott N, Ramsdale J, Markham AF, Guillou PJ. Immunohistochemical detection of the anti-apoptosis protein, survivin, predicts survival after curative resection of stage II colorectal carcinomas. Ann Surg Oncol. 2001;8:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Chen DJ, Huerta S. Smac mimetics as new cancer therapeutics. Anticancer Drugs. 2009;20:646-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 128. | Rödel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Günther K, Schick C, Peters A, Sauer R, Rödel F. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 129. | Willett CG, Warland G, Cheek R, Coen J, Efird J, Shellito PC, Compton CC. Proliferating cell nuclear antigen and mitotic activity in rectal cancer: predictor of response to preoperative irradiation. J Clin Oncol. 1994;12:679-682. [PubMed] |
| 130. | Desai GR, Myerson RJ, Higashikubo R, Birnbaum E, Fleshman J, Fry R, Kodner I, Kucik N, Lacey D, Ribeiro M. Carcinoma of the rectum. Possible cellular predictors of metastatic potential and response to radiation therapy. Dis Colon Rectum. 1996;39:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | de Bruin EC, van de Velde CJ, van de Pas S, Nagtegaal ID, van Krieken JH, Gosens MJ, Peltenburg LT, Medema JP, Marijnen CA. Prognostic value of apoptosis in rectal cancer patients of the dutch total mesorectal excision trial: radiotherapy is redundant in intrinsically high-apoptotic tumors. Clin Cancer Res. 2006;12:6432-6436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 132. | Scott N, Hale A, Deakin M, Hand P, Adab FA, Hall C, Williams GT, Elder JB. A histopathological assessment of the response of rectal adenocarcinoma to combination chemo-radiotherapy: relationship to apoptotic activity, p53 and bcl-2 expression. Eur J Surg Oncol. 1998;24:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Dietz DW, Dehdashti F, Grigsby PW, Malyapa RS, Myerson RJ, Picus J, Ritter J, Lewis JS, Welch MJ, Siegel BA. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum. 2008;51:1641-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 134. | Roels S, Slagmolen P, Nuyts J, Lee JA, Loeckx D, Maes F, Stroobants S, Penninckx F, Haustermans K. Biological image-guided radiotherapy in rectal cancer: is there a role for FMISO or FLT, next to FDG? Acta Oncol. 2008;47:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | Toiyama Y, Inoue Y, Saigusa S, Okugawa Y, Yokoe T, Tanaka K, Miki C, Kusunoki M. Gene expression profiles of epidermal growth factor receptor, vascular endothelial growth factor and hypoxia-inducible factor-1 with special reference to local responsiveness to neoadjuvant chemoradiotherapy and disease recurrence after rectal cancer surgery. Clin Oncol (R Coll Radiol). 2010;22:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, Papatheodosiou K, Gazouli M, Bramis J, Patsouris E, Panoussopoulos D. Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis. 2006;21:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 137. | Gupta VK, Jaskowiak NT, Beckett MA, Mauceri HJ, Grunstein J, Johnson RS, Calvin DA, Nodzenski E, Pejovic M, Kufe DW. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 2002;8:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 138. | Zlobec I, Steele R, Compton CC. VEGF as a predictive marker of rectal tumor response to preoperative radiotherapy. Cancer. 2005;104:2517-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 139. | Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207-1225. [PubMed] |
| 140. | Crane CH, Eng C, Feig BW, Das P, Skibber JM, Chang GJ, Wolff RA, Krishnan S, Hamilton S, Janjan NA. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 141. | Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020-3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 142. | Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4319] [Cited by in RCA: 3951] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 143. | Huerta S. Radiosensitizing agents for the management of rectal cancer. Anticancer Drugs. 2011;22:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 144. | Glynne-Jones R, Mawdsley S, Harrison M. Cetuximab and chemoradiation for rectal cancer--is the water getting muddy? Acta Oncol. 2010;49:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 145. | Ghadimi BM, Grade M, Difilippantonio MJ, Varma S, Simon R, Montagna C, Füzesi L, Langer C, Becker H, Liersch T. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol. 2005;23:1826-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 146. | Rimkus C, Friederichs J, Boulesteix AL, Theisen J, Mages J, Becker K, Nekarda H, Rosenberg R, Janssen KP, Siewert JR. Microarray-based prediction of tumor response to neoadjuvant radiochemotherapy of patients with locally advanced rectal cancer. Clin Gastroenterol Hepatol. 2008;6:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 147. | Debucquoy A, Goethals L, Libbrecht L, Perneel C, Geboes K, Ectors N, McBride WH, Haustermans K. Molecular and clinico-pathological markers in rectal cancer: a tissue micro-array study. Int J Colorectal Dis. 2009;24:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 148. | Hsu FM, Zhang S, Chen BP. Role of DNA-dependent protein kinase catalytic subunit in cancer development and treatment. Transl Cancer Res. 2012;1:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 149. | Wang C, Lees-Miller SP. Detection and repair of ionizing radiation-induced DNA double strand breaks: new developments in nonhomologous end joining. Int J Radiat Oncol Biol Phys. 2013;86:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |