Published online Mar 15, 2014. doi: 10.4251/wjgo.v6.i3.74
Revised: January 11, 2014
Accepted: January 15, 2014
Published online: March 15, 2014
Processing time: 331 Days and 16.7 Hours
AIM: To investigate whether the inhibition of autophagy by chloroquine (CQ) sensitizes rectal tumors to radiation therapy (RT) or concurrent chemoradiation (chemoRT).
METHODS: In vitro, HCT-116 and HT-29 colorectal cancer (CRC) cell lines were treated as following: (1) PBS; (2) CQ; (3) 5-fluorouracil (5-FU); (4) RT; (5) CQ and RT; (6) 5-FU and RT; (7) CQ and 5-FU; and (8) 5-FU and CQ and RT. Each group was then exposed to various doses of radiation (0-8 Gy) depending on the experiment. Cell viability and proliferative capacity were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and clonogenic assays. Clonogenic survival curves were constructed and compared across treatment groups. Autophagy status was determined by assessing the LC3-II to LC3-I ratio on western blot analysis, autophagosome formation on electron microscopy and identification of a perinuclear punctate pattern with GFP-labeled LC3 on fluorescence microscopy. Cell cycle arrest and cell death were evaluated by FACS and Annexin V analysis. All experiments were performed in triplicate and statistical analysis was performed by the student’s t test to compare means between treatment groups.
RESULTS: RT (2-8 Gy) induced autophagy in HCT-116 and HT-29 CRC cell lines at 4 and 6 h post-radiation, respectively, as measured by increasing LC3-II to LC3-I ratio on western blot. Additionally, electron microscopy demonstrated autophagy induction in HT-29 cells 24 h following irradiation at a dose of 8 Gy. Drug treatment with 5-FU (25 μmol/L) induced autophagy and the combination of 5-FU and RT demonstrated synergism in autophagy induction. CQ (10 μmol/L) alone and in combination with RT effectively inhibited autophagy and sensitized both HCT-116 and HT-29 cells to treatment with radiation (8 Gy; P < 0.001 and 0.00001, respectively). Significant decrease in clonogenic survival was seen only in the HT-29 cell line, when CQ was combined with RT at doses of 2 and 8 Gy (P < 0.5 and P = 0.05, respectively). There were no differences in cell cycle progression or Annexin V staining upon CQ addition to RT.
CONCLUSION: Autophagy inhibition by CQ increases CRC cell sensitivity to concurrent treatment with 5-FU and RT in vitro, suggesting that addition of CQ to chemoRT improves CRC treatment response.
Core tip: Autophagy is implicated as a mechanism of resistance to cancer treatment. We hypothesized that chloroquine, a lysosomotropic autophagy inhibitor, would sensitize colorectal cancer (CRC) cell lines to both radiation therapy (RT) alone and concurrent chemoradiation. Our results showed that chloroquine decreased clonogenic survival of CRC cells when given in combination with RT or concurrent 5-fluorouracil and RT. Radiosensitization by chloroquine represents a novel therapeutic approach to enhance treatment efficacy in rectal cancer.
- Citation: Schonewolf CA, Mehta M, Schiff D, Wu H, Haffty BG, Karantza V, Jabbour SK. Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World J Gastrointest Oncol 2014; 6(3): 74-82
- URL: https://www.wjgnet.com/1948-5204/full/v6/i3/74.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i3.74
In 2013 it is estimated that 40340 new cases of rectal cancer will be diagnosed in the United States[1]. The standard of care for patients with locally advanced rectal cancer consists of pre-operative 5-fluorouracil (5-FU) and radiation therapy (RT), followed by surgical resection. Five-year survival rates vary drastically depending on pathologic response after neoadjuvant treatment, from 85%-90% in patients with a pathologic complete response (pCR) to 66% in patients without pCR[2,3]. Therefore, improvements in the efficacy of pre-operative treatment for locally advanced rectal cancer have great potential to significantly impact patient survival.
Autophagy, a lysosomal degradation process of cellular organelle and protein recycling under stressful conditions, has been implicated as a cancer cell survival mechanism. Increased levels of autophagy have been observed in nutrient- and oxygen-poor tumor regions as compared to highly vascularized, nutrient-enriched areas[4]. Autophagy induction in metabolically stressed tumor regions allows cancer cells to generate new substrates for growth through recycling of “self” material. Autophagy also supports the increased metabolic needs of Ras-mutant cancer cells by providing substrates for oxidative phosphorylation[5] and several studies have suggested that the tumorigenic potential of Ras-transformed tumor cells is highly dependent on autophagy[5]. Autophagy induction in hypoxic tumor cores has been proposed as a mechanism of resistance to chemotherapy and radiation. During RT, intermittent hypoxia occurs in association with a significant increase in the level of reactive oxygen species and concomitant stabilization of HIF-1α under aerobic conditions[6-8]. By targeting the compensatory and prosurvival mechanism induced in response to tumor hypoxia in RT-treated neoplasms, autophagy inhibition may improve the efficacy of treatment.
Given that autophagy is a mechanism of resistance to both chemotherapy and radiotherapy, the addition of chloroquine (CQ), an inhibitor of autophagy, may allow for improvements in tumor responsiveness. An earlier study demonstrated the anti-cancer effect of CQ with 5-FU in CRC cells[9]. As expected, 5-FU inhibited CRC cell proliferation through cell cycle arrest and, to a lesser degree, apoptosis. This effect was potentiated by CQ and autophagy induction was demonstrated by increased acidic vesicles and increased LC3-II expression. While CQ demonstrated synergism with chemotherapy, its actions with respect to RT in CRC require exploration. Understanding autophagy’s role in CRC radioresistance is critical and has the potential to create new opportunities for therapeutic intervention.
The purpose of this study is to provide data and rationale for the application of autophagy inhibition in the treatment of localized rectal cancer by adding hydroxychloroquine to routine 5-FU and RT. We hypothesized that autophagy inhibition by CQ with standard chemoRT for rectal cancer will enhance radiosensitization. We tested our hypothesis by characterizing the effects of radiation on autophagy in CRC cells and evaluating the efficacy of combination treatment with CQ, 5-FU and radiation.
HCT-116 and HT-29 CRC cell lines (ATCC) were maintained in McCoy’s 5A (GIBCO, Invitrogen, New York, United States) medium containing 10% fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (GIBCO). Cells were incubated at 37 °C with 5% CO2.
Drug treatments included the following groups: (1) Control group, vehicle; (2) CQ (Sigma Aldrich); (3) 5-FU (Sigma Aldrich); (4) RT (Gammacell 40 Exactor, Best Theratronics); (5) CQ and RT; (6) 5-FU and RT; (7) CQ and 5-FU; and (8) 5-FU and CQ and RT.
Cells were harvested by trypsinization, fixed in 2.5% gluteraldehyde/4% paraformaldehyde in 0.1 mol/L cacodylate buffer, then post-fixed in 1% osmium tetroxide buffer. After acetone dehydration, cells were embedded in spur resin. Thin sections (90 nm) were cut on a Reichert Ultracut E microtome and stained with saturated uranyl acetate and lead citrate solution. Sections were examined at 80 kV with a JEOL 1200EX transmission electron microscope (TEM).
GFP-labeled LC3 plasmid and a GFP-expressing control plasmid were transiently transfected into HCT-116 and HT-29 cell lines using Lipofectamine 200 (Invitrogen). Cells attached overnight, and were treated with Rapamycin (200 nmol/L), or RT (2-8 Gy) ± 5-FU (15 μmol/L for HT-29 cells, 25 μmol/L for HCT-116 cells). Six hours later, cells were washed with PBS and fixed with 10% Formalde-Fresh Solution (Fisher Scientific). Cells were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and GFP fluorescence was examined at 60 × magnification. Autophagy was quantified by percentage of cells exhibiting a perinuclear punctate pattern per 50 cells.
Cells were plated into 96-well plates (2.5 × 103 cells/well). After overnight incubation, cells were incubated with either media without drug or media containing CQ (10 μmol/L), 5-FU (25 μmol/L) or both. Each group of drug-treated cells was irradiated (0-8 Gy) within 1 h of drug exposure. Following irradiation, cells were incubated for 72 h and cell viability was measured using 50 μL of 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution. Medium was removed after 4 h of incubation at 37 °C and 250 μL of DMSO was used to dissolve the blue MTT formazan precipitate. Absorbance was measured at 560 nm on a Victor plate reader. Cell survival was calculated relative to untreated cells.
HT-29 cells were plated at concentrations of 150, 350, 600, and 20 × 103 cells per 10-cm plate for irradiation at 0, 2, 4 and 8 Gy, respectively. HCT-116 cells were plated at concentrations of 400, 2.5 × 103, 5 × 103 and 250 × 103 cells per 10-cm plate for irradiation at 0, 2, 4 and 8 Gy, respectively. After plating and overnight incubation, cells were treated with drug combinations followed by radiation within 1 h. Then HT-29 and HCT-116 cells were incubated for 14 and 7 d, respectively. Cells were washed with PBS and stained with 50% methylene blue for 30 min. Colonies were counted positive if they contained > 50 cells.
After plating and overnight incubation, cells were treated with CQ, 5-FU or both, and then irradiated within 1 h at 2-8 Gy. At time points of 30 min, 1 h, 2 h, 4 h, 6 h and 24 h after irradiation, cells were harvested by scraping technique and stored as a pellet at -80 °C. Cell lysis buffer with protease inhibitor cocktail was added to each sample, followed by sonication. Protein concentrations were quantified using Bio-Rad Protein Assay. Equal amounts of protein (12.5 μg) were loaded onto 4%-20% Tris-Glycine PAGE gels (Invitrogen, New York, United States) and run using the Invitrogen XCell SureLock system. Proteins were transferred onto PVDF paper and blots were blocked with 0.25% Milk in TBST using Milipore SnapID. Primary antibodies to LC3 (rabbit, Novus Biologicals, dilution 1:10000) and p62 (mouse, MBL, dilution 1:10000 ) were incubated overnight at 4 °C. Blots were washed with 1X TBS with 0.1% Tween using Millipore SnapID and then incubated at room temperature for 1 h with secondary antibody, goat anti-rabbit (CalBio, 1:10000 ) or goat anti-mouse (CalBio, dilution 1:10000 ) in 0.25% milk in TBST. Pierce ECL Western Blotting Substrate was used to visualize proteins and expression was quantified using ImageJ software.
Cells were treated according to the treatment groups previously described. Within 1 h of drug treatment, cells were irradiated at 8 Gy. After incubation for 24 and 48 h, floating and adherent cells were collected, washed with PBS, fixed with ice-cold 70% ethanol and stained with propidium iodide (PI, 50 μg/mL). Cell cycle progression was analyzed using a Cytomics FC500 Analyzer (Beckman Coulter, Brea, Ca). Gating was used to remove cellular debris and fixation artifacts.
At 48 h after drug and RT, floating cells and trypsinized adherent cells were collected. Cells were stained with PI and Annexin V, per manufacturer’s instructions. Cells, which stained positive for Annexin V, but negative for PI, were considered early apoptotic.
Experimental results were reported as a mean of at least three independent experiments conducted in triplicate. Statistical analysis was performed by a two-tailed Student’s t test and a P value < 0.05 was considered statistical significant.
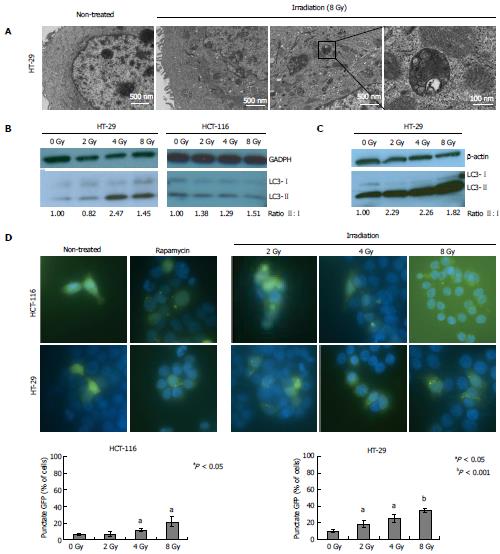
Irradiation induced autophagy in both HCT116 and HT-29 cell lines (Figure 1). TEM demonstrated autophagy induction in HT-29 cells 24 h following irradiation at a dose of 8 Gy (Figure 1A). Compared to non-irradiated controls, RT-treated cells exhibited increased autophagosome formation, as illustrated by increased numbers of double membrane vesicles (Figure 1A, inset).
Western blotting for the autophagosome-associated protein light chain 3 (LC3) confirmed autophagy induction at multiple time points following irradiation (Figure 1). Conversion of cytosolic LC3-I to the proteolytically cleaved and phosphatidyl-ethanolamine (PE)-conjugated, membrane bound form LC3-II occurs during autophagosome formation and increased LC3-II:I ratio is considered a marker of autophagy induction[10]. Increased LC3-II:I ratios were seen in HT-29 and HCT-116 cell lines at early time points, namely at 4 and 6 h post-RT, respectively (Figure 1B). RT doses of 4 and 8 Gy induced autophagy in HT-29 cells, while HCT-116 cells showed autophagy induction at 2, 4 and 8 Gy. By 24 h following RT, autophagy induction, as indicated by increased LC3-II:I ratio, was only seen in HT-29 cell lines and occurred across all radiation doses, from 2 to 8 Gy, (Figure 1C).
To further investigate the effects of radiation on autophagic response, HCT-116 and HT-29 cell lines were transiently transfected with GFP-labeled LC3 plasmid and examined for green fluorescent LC3 puncta, representing autophagosomes. Increasing RT doses significantly increased the number of cells with GFP punctate pattern compared to untreated controls for both HCT-116 and HT-29 cell lines (P < 0.05) (Figure 1D).
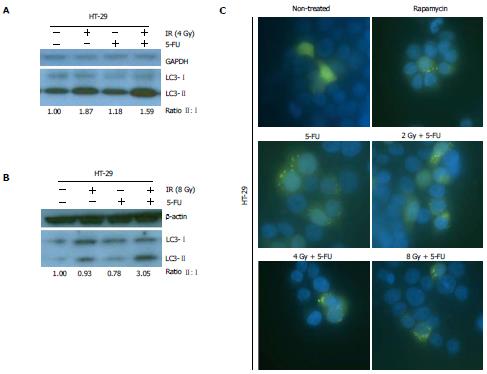
Previous studies demonstrated autophagy induction in HCT-116 and HT-29 cells following treatment with 5-FU alone[9,11,12]. We now examined the impact of concurrent treatment with 5-FU and RT on autophagy functional status in CRC cells. ChemoRT resulted in increased autophagy induction in HT-29 cells compared to 5-FU alone (Figure 2A and B) and may have had a synergistic effect at higher RT doses (8 Gy) (Figure 2B). Autophagy induction in HT-29 cells following chemoRT was also qualitatively assessed by fluorescence microscopy (Figure 2C). GFP-fluorescent puncta formation confirmed 5-FU induced autophagy as previously reported[9,11,12] and demonstrated that chemoRT resulted in more robust autophagy upregulation.
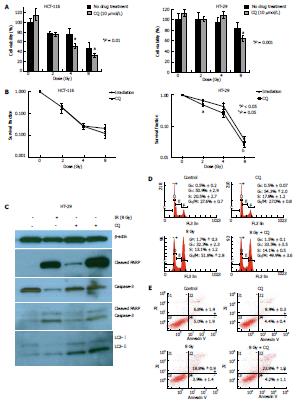
To investigate whether autophagy inhibition by CQ increases the radiosensitivity of CRC lines, we first used MTT assays (Figure 3A). Cell viability of HCT-116 cells at 72 h post-RT was significantly decreased upon addition of CQ (10 μmol/L) just prior to irradiation at 4 and 8 Gy (P < 0.001). Significant decreases in cell viability in the presence of CQ (10 μmol/L) in HT-29 cells were seen at 8 Gy (P < 0.001).
Cancer cell proliferation after treatment was examined by clonogenic survival assays. For HCT-116 cells, clonogenic survival was similar under RT alone or RT and CQ (Figure 3B), whereas HT-29 cells showed decreased survival after combination treatment with RT and CQ (0.5 μmol/L) compared to RT alone at doses of 2 and 8 Gy (P < 0.05 and P = 0.05, respectively), further supporting the MTT assay results that showed radiosensitization of CRC cell lines by CQ.
Chloroquine inhibits the last phase of autophagy by changing the pH of lysosomes, thus rendering them nonfunctional and unable to process proteins[10]. Effective autophagy inhibition by CQ is manifested as LC3-II accumulation due to failure to re-process LC3-II back into LC3-I[10]. As shown in Figure 3C, single agent CQ increased LC3-II levels in HT-29 cells compared to vehicle treatment, demonstrating that CQ effectively blocked autophagic flux at the concentration used. Furthermore, HT-29 cells irradiated at 8 Gy after exposure to CQ showed increased LC3-II accumulation compared to cells treated with CQ alone (Figure 3C), indicating that RT induced autophagy.
To investigate the mechanism underlying radiosensitization of HT-29 cells by CQ, cell death by apoptosis and cell cycle progression were assessed. CQ addition to RT (8 Gy) increased PARP cleavage, but had little effect on cleaved caspase-3 levels, compared to RT alone (Figure 3C), suggesting that concurrent use of CQ likely increased the RT-induced DNA damage response through PARP, but alternative cell death pathways other than apoptosis were responsible for decreased HT-29 cell survival upon radiosensitization by CQ. Cell cycle analysis demonstrated that CQ did not alter the proportion of cells in any phase of the cell cycle (Figure 3D). Flow cytometry for Annexin V and PI at 48 h after treatment (Figure 3E) confirmed that CQ did not affect early apoptosis in irradiated HT-29 cells, as the AnnexinV+/PI- cell population remained stable. Of note, addition of CQ to RT significantly increased the number of cells staining positive for both Annexin V and PI, as compared to RT alone (P < 0.05). Since Annexin V+/PI+ cells are necrotic or in late apoptosis, the radiosensitization of HT-29 cells by CQ may result from increased necrosis rather than apoptosis or cell cycle arrest.
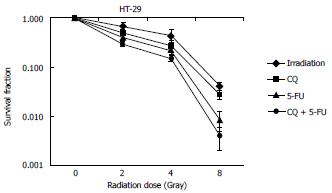
To investigate whether autophagy inhibition by CQ increased the therapeutic efficacy of chemoRT in rectal cancer, we examined the clonogenic survival of HT-29 cells treated with CQ (0.5 μmol/L) in combination with 5-FU (0.5 μmol/L) and/or radiation (2-8 Gy) (Figure 4). CQ addition to chemoradiation at 2 and 4 Gy significantly sensitized HT-29 cells to treatment and decreased their clonogenic survival (P < 0.05), whereas at 8 Gy, CQ resulted in decreased clonogenic survival showing a trend toward statistical significance compared to chemoRT alone (P = 0.12).
Autophagy is an evolutionarily conserved, self-digestive process in which proteins and other cytoplasmic material are recycled to support cell survival under stressful conditions (i.e., cancer therapy)[10]. Autophagy has been proposed as a mechanism of resistance to RT and chemotherapy[13]. Autophagy inhibition using siRNAs against AuTophaGy (ATG)-related genes sensitizes human breast, pharyngeal, cervical, lung, and rectal carcinoma cells to RT[14]. Chloroquine, as an indirect autophagy inhibitor, renders CRC cells more sensitive to 5-FU[9], but its effects on chemoRT had not been previously explored. We hypothesized that autophagy inhibition by CQ may deprive CRC cells of an essential survival mechanism and radiosensitize treatment-resistant regions of rectal tumors.
We examined autophagic flux in HT-29 and HCT-116 cells following RT. Autophagy induction occurred in both cell lines post-radiation at early time points (Figure 1B), but was sustained at 24 h only in HT-29 cells (Figure 1C). Earlier reports of 5-FU-induced autophagy were confirmed in our study, and treatment of HT-29 cells with the combination of 5-FU and RT upregulated autophagy more than either treatment alone (Figure 2B). These results demonstrate that 5-FU and radiation, both individually and potentially synergistically when in combination, induce the prosurvival autophagic pathway in CRC cell lines, particularly HT-29 cells.
We also examined whether autophagy inhibition by CQ sensitized CRC cells to 5-FU alone, radiation alone and combined chemoRT. Increased LC3-II values confirmed that CQ effectively inhibited RT-induced autophagy in HT-29 cells (Figure 3C), and, thus, the radiosensitization of these cells by CQ can be attributed to the CQ-mediated autophagy inhibition. Furthermore, inhibition of autophagy by CQ sensitized HT-29 cells to concurrent chemoradiotherapy (Figure 4), thus indicating that addition of CQ to chemoRT enhances treatment efficacy in CRC.
In our study, we also examined cell death mechanisms possibly underlying the decreased survival of HT-29 cells, following treatment with CQ and radiation. Although significant differences in cell cycle progression or apoptosis were not observed (Figure 3D), FACS analysis indicated increased necrosis upon addition of CQ to RT. Further studies will be needed to determine whether programmed necrosis (necroptosis) plays a role in the decreased survival of CQ- and RT-treated HT-29 cells.
Our results showed radiosensitization by CQ in HT-29 (p53-mutant) cells, but not HCT-116 (p53-wild type) cells. While these cell lines cannot be directly compared solely on the basis of their p53 status, the differences in sensitization is an observation needing further investigation. Autophagy is regulated by several signal transduction pathways, including mTOR[10] and p53[15]. In particular, p53 inhibits autophagy through various mechanisms in CRC cells[16,17], and its ablation in HCT-116 cells results in autophagy induction and resistance to irinotecan[18]. Further investigation into the relationship between p53 and the autophagic pathway following RT needs to be conducted, as p53 status may play a role in susceptibility to radiosensitization by CQ.
In conclusion, autophagy inhibition by CQ enhanced the radiosensitivity of CRC cells and improved the therapeutic efficacy of chemoRT in CRC in vitro, strongly suggesting that adding hydroxychloroquine to the pre-operative regimen of 5-FU and radiation in locally advanced rectal cancer may improve treatment response. Further studies examining the anti-tumor effects of CQ-mediated autophagy inhibition should be performed in xenograft tumor models to elucidate the impact of autophagy on chemoRT responsiveness in vivo. Additional studies are needed to elucidate the molecular mechanisms responsible for the radiosensitizing effects of autophagy inhibition, as these results could form the basis for rationally selecting patients who may benefit most from pharmacologic autophagy modulation.
Standard of care for patients diagnosed with locally advanced rectal tumors consists of a pre-operative regimen of 5-fluorouracil (FU) and radiation therapy (RT). Five-year survival rates vary drastically depending on pathologic response after neoadjuvant chemoradiation; 66% survival in patients without a pathologic complete response and 85%-90% in patients with a pathologic complete response. The aim of this study was to evaluate whether the addition of an autophagy inhibitor such as chloroquine to the preoperative regimen of chemoradiation (chemoRT) could improve the efficacy of treatment.
Autophagy has been proposed as a mechanism of resistance to RT and chemotherapy and autophagy inhibition using siRNAs against AuTophaGy (ATG)-related genes sensitizes human breast, pharyngeal, cervical, lung, and rectal carcinoma cells to radiation therapy. Chloroquine, as an indirect autophagy inhibitor, renders colorectal cancer cells more sensitive to 5-FU, but its effects on radiation and chemoRT had not been previously explored.
Autophagy inhibition by chloroquine increases HT-29 colorectal cancer cell sensitivity to concurrent treatment with 5-FU and RT in vitro.
The results of this study provide data and rationale for the clinical application of autophagy inhibition in the treatment of locally advanced rectal cancer by adding hydroxychloroquine to the standard preoperative regimen of 5-FU and radiation therapy.
Autophagy is an evolutionarily conserved, self-digestive process in which proteins and other cytoplasmic material are recycled to support cell survival under stressful conditions (i.e., cancer therapy).
This manuscript addresses an important research question - Whether autophagy inhibitor can enhance the radio-sensitivity in treating locally advanced rectal cancer. The experimental design regarding outcome measures chosen seems well considered.
P- Reviewer: Shi Q S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1460] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 3. | Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621-1635. [PubMed] |
| 5. | Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1053] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 6. | Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429-441. [PubMed] |
| 7. | Dewhirst MW. Intermittent hypoxia furthers the rationale for hypoxia-inducible factor-1 targeting. Cancer Res. 2007;67:854-855. [PubMed] |
| 8. | Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99-110. [PubMed] |
| 9. | Sasaki K, Tsuno NH, Sunami E, Tsurita G, Kawai K, Okaji Y, Nishikawa T, Shuno Y, Hongo K, Hiyoshi M. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev. 2011;25:1999-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 11. | Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol. 2009;16:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46:1900-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 718] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 14. | Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Sui X, Jin L, Huang X, Geng S, He C, Hu X. p53 signaling and autophagy in cancer: a revolutionary strategy could be developed for cancer treatment. Autophagy. 2011;7:565-571. [PubMed] |
| 16. | Morselli E, Shen S, Ruckenstuhl C, Bauer MA, Mariño G, Galluzzi L, Criollo A, Michaud M, Maiuri MC, Chano T. p53 inhibits autophagy by interacting with the human ortholog of yeast Atg17, RB1CC1/FIP200. Cell Cycle. 2011;10:2763-2769. [PubMed] |
| 17. | Livesey KM, Kang R, Zeh HJ, Lotze MT, Tang D. Direct molecular interactions between HMGB1 and TP53 in colorectal cancer. Autophagy. 2012;8:846-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Paillas S, Causse A, Marzi L, de Medina P, Poirot M, Denis V, Vezzio-Vie N, Espert L, Arzouk H, Coquelle A. MAPK14/p38α confers irinotecan resistance to TP53-defective cells by inducing survival autophagy. Autophagy. 2012;8:1098-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |