Published online Nov 15, 2013. doi: 10.4251/wjgo.v5.i11.198
Revised: October 9, 2013
Accepted: November 2, 2013
Published online: November 15, 2013
Processing time: 84 Days and 0.3 Hours
We are reporting on a colorectal cancer patient with the longest disease-free interval ever published, where chromosomal microarray analysis was used to confirm the link between the primary and metastatic lesions. This rare case reports on a patient with late recurrence of colorectal cancer in the lung 19 years after its initial diagnosis. We used high-resolution array CGH (aCGH) to analyze the genetic aberrations of both the primary rectal and the recurrent metastatic lung lesions. Interestingly, we found striking similarities between the two lesions, despite the 19 years disease-free interval. In addition, most of the genes that were previously reported to be associated with a high recurrence score showed copy number gains by aCGH in one or both lesions. Our findings suggest that aCGH may be a helpful tool in analyzing the origin of metastases and underline the need for a better understanding of the characteristics of rectal tumors that have a late recurrence potential.
Core tip: The role of genetic profiling in determining the risk of recurrence in colorectal cancer has been under serious investigation. This case report not only represents the longest rectal cancer disease-free interval in the literature, but also applies genetic analysis as a tool to confirm the similarity of the original and metastatic tumor and to predict the risk of recurrence.
- Citation: Rahma OE, Burotto M, Do Canto LM, Germanos AA, Haddad BR, Marshall JL. Striking similarities in genetic aberrations between a rectal tumor and its lung recurrence. World J Gastrointest Oncol 2013; 5(11): 198-203
- URL: https://www.wjgnet.com/1948-5204/full/v5/i11/198.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i11.198
Colorectal cancer (CRC) has a high incidence worldwide with more than 1.2 million new cases diagnosed in 2008[1]. The 5-year overall survival in the United States for all stages is 61%[2]. Rectal cancer accounts for approximately 30% of CRC cases[3]. The treatment of localized rectal tumors differs from colon tumors in that it involves a multidisciplinary approach that includes surgery, radiation and chemotherapy[4]. The goal of neoadjuvant or adjuvant treatment is to decrease local and distant recurrence of the disease[5]. As of today, we do not have predictive biomarkers that indicate when a particular patient will benefit from systemic chemotherapy or, more importantly, in which cases the tumor will recur. Preliminary molecular tools have been developed to help predict which patient is more likely to experience disease recurrence and eventually die from the disease[6,7]. Despite these efforts we only partially understand the complexity of rectal cancer, clonal evolution and dormancy of micro-metastatic disease[8,9]. In this report we present the case of a long term survivor of CRC with a delayed recurrence almost two decades later.
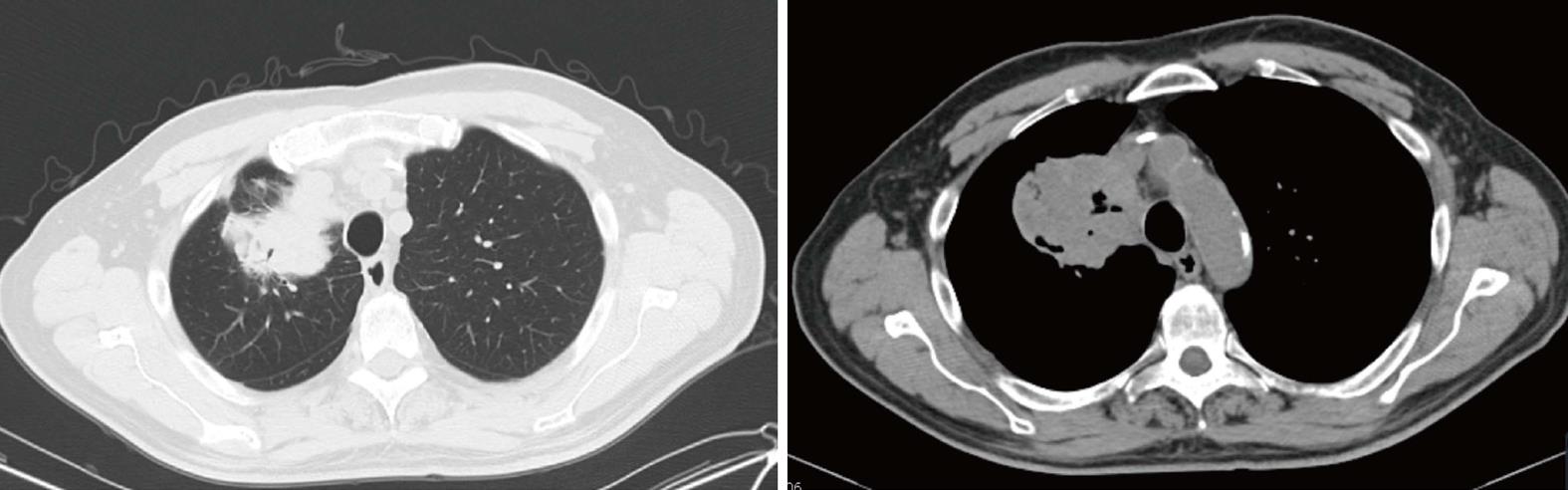
An 81-years-old Caucasian male initially presented with rectal bleeding in 1991. He underwent a colonoscopy with biopsy that was later tested and revealed a wild type K-ras moderately differentiated adenocarcinoma. The patient was diagnosed with stage IIIB (T3N1M0) rectal cancer and treated with surgical resection and colostomy followed by chemoradiation with fluorouracil (5-FU) and leucovorin. He subsequently underwent a colostomy reversal and remained in remission with no evidence of disease until 2011 when he developed a cough and was found to have a lung mass in the right upper lobe (RUL) and right sided mediastinal and hilar lymphadenopathy (Figure 1). A PET scan showed the RUL mass to be 8.2 cm × 7.3 cm with SUV of 14.5, and confirmed the right-sided mediastinal and hilar lymphadenopathy, in addition to a 1.2 cm nodule in the left costophrenic angle with no FDG activity. He underwent bronchoscopy and biopsy that revealed a wild type K-ras adenocarcinoma of colonic primary, CDX2/CK20 positive and TTF1/CK7/CD58 negative. The tumor characteristics were consistent with the primary tumor. The patient had a colonoscopy that only showed friable rectal mucosa with no evidence of malignancy. Accordingly, the patient was treated with FOLFOX (5-FU, oxaliplatin and leucovorin) in combination with bevacizumab for his recurrent metastatic rectal cancer. He received 10 cycles of FOLFOX/bevacizumab. The oxaliplatin was stopped due to cumulative neuropathy and he was switched to capecitabine and bevacizumab. The patient had a good response to chemotherapy by PET scan that showed a decrease in the RUL mass size (from 8.2 to 6.6 cm) and SUV (from 14.5 to 4.6), in addition to a decrease in the bilateral hilar and subcarinal lymph nodes’ uptake. Given the patient good response to chemotherapy he subsequently underwent right upper and middle lobectomies in July 2012. His pathology showed metastatic adenocarcinoma with extensive necrosis consistent with his known primary colorectal carcinoma. Given the dormancy of his disease for so many years, the options were presented to the patient including watchful waiting versus maintenance chemotherapy with capecitabine for 1-2 years. The patient opted not to proceed with more treatment and to be monitored with regular CT-scans.
In order to compare the DNA copy number changes in the metastatic lung lesion to the changes in the primary tumor, we evaluated both lesions by high-resolution aCGH analysis using an Agilent® platform (SurePrint G3 Human CGH Microarray Kit 8x60K, Agilent, Santa Clara, CA). Genomic DNA was isolated from formalin-fixed paraffin embedded tumor tissues using a standard phenol-chloroform laboratory protocol and cleaned with a MinElute® Reaction Cleanup Kit (Qiagen, Valencia, CA). Commercially available, pooled, normal control DNA (Promega, Madison, WI) was used as a reference DNA. aCGH experiments were performed according to the manufacturer’s protocol with minor modifications. In brief, tumor and reference DNA were labeled using enzymatic labeling (Agilent, Santa Clara, CA), hybridized for 40 h at 65 °C, washed, and immediately scanned using Agilent Scanner (G2505C). Data were extracted using Agilent Feature Extraction 10.7.3.1 software, and analyzed with Agilent Workbench 7.0 software. High-resolution aCGH analysis showed that both lesions share a large number of similar aberrations (Table 1 and Figure 2). Review of these aberrations revealed that many of them have been reported to be very common in colorectal cancers (e.g., segments with copy number increase on chromosomes 13, 7, 8q, and 20q)[9,10], thus supporting the conclusion that the lung lesion is a recurrent metastasis from the primary rectal lesion.
| Chr | Cytoband | Base pair | Aber | ||
| Start | Stop | ||||
| Recurrent metastatic lung lesion | chr1 | p34.2-p34.1 | 40022181 | 45250726 | G |
| chr1 | q21.1- q44 | 143700072 | 245804497 | G | |
| chr2 | p25.3-p11.2 | 32444 | 89387655 | G | |
| chr2 | q11.2-q37.3 | 96143358 | 241478888 | G | |
| chr4 | q32.1- q35.1 | 156452014 | 186681608 | L | |
| chr6 | p25.3-p11.1 | 200350 | 58722020 | G | |
| chr7 | p12.3-p11.1 | 49282714 | 57498383 | G | |
| chr7 | q11.21-q22.1 | 62291739 | 99905860 | G | |
| chr7 | q36.1-q36.3 | 150049339 | 158781397 | G | |
| chr8 | p23.1- p12 | 12627630 | 32499834 | L | |
| chr8 | p12-p11.21 | 32882718 | 42971936 | G | |
| chr8 | q11.21-q24.3 | 48549253 | 146250824 | G | |
| chr9 | q33.2-q34.3 | 124984647 | 139633014 | G | |
| chr10 | q22.3-q24.2 | 80370579 | 101360302 | L | |
| chr10 | q26.3 | 131868597 | 134682710 | L | |
| chr11 | p15.5-p11.12 | 974637 | 48986659 | G | |
| chr13 | q12.11-q34 | 18556982 | 113766081 | G | |
| chr15 | q25.3-q26.3 | 83411251 | 96875147 | G | |
| chr19 | p13.3-p13.11 | 318892 | 19154766 | G | |
| chr20 | q11.21-q13.33 | 29436537 | 62320720 | G | |
| chrX | p11.23-p11.1 | 48639378 | 57116899 | G | |
| chrX | q11.1-q28 | 61980262 | 154886101 | G | |
| chrY | p11.31-p11.2 | 2716461 | 8521949 | L | |
| chrY | q11.21-q11.221 | 13208776 | 17558012 | L | |
| Primary rectal lesion | chr1 | q21.1-q44 | 143700072 | 243198779 | G |
| chr2 | p25.3-p11.2 | 698239 | 89387655 | G | |
| chr2 | q11.1-q37.3 | 95562654 | 241301905 | G | |
| chr3 | p26.3-p11.1 | 134711 | 90336752 | G | |
| chr3 | q11.2-q29 | 95063426 | 197289184 | G | |
| chr6 | q11.1-q27 | 63002508 | 170700061 | G | |
| chr7 | p22.3-p11.2 | 524935 | 55936992 | G | |
| chr7 | q11.21-q36.3 | 62291739 | 158602499 | G | |
| chr8 | p23.3-p12 | 369418 | 32621998 | L | |
| chr8 | p12-p11.21 | 32705506 | 42971936 | G | |
| chr8 | q11.1-q24.3 | 47800500 | 146024209 | G | |
| chr9 | p24.3-p13.2 | 319684 | 37451026 | G | |
| chr11 | p15.5-p11.2 | 2121540 | 46490960 | G | |
| chr13 | q11-q34 | 18361637 | 113964366 | G | |
| chr20 | q11.21-q13.33 | 29352138 | 62343283 | G | |
| chrX | p22.33-p11.1 | 2719027 | 58068490 | G | |
| chrX | q11.1-q28 | 61848414 | 154561665 | G | |
| chrY | p11.31-p11.2 | 2716461 | 10511314 | L | |
| chrY | q11.21-q11.23 | 12593244 | 27176992 | L | |
| Common aberrations between the two lesions | chr1 | q21.1-q44 | 143700072 | 243198779 | G |
| chr2 | p25.3-p11.2 | 698239 | 89387655 | G | |
| chr2 | q11.2-q37.3 | 96143358 | 241301905 | G | |
| chr7 | p12.3-p11.2 | 49282714 | 55936992 | G | |
| chr7 | q11.21-q22.1 | 62291739 | 99905860 | G | |
| chr7 | q36.1- q36.3 | 150049339 | 158602499 | G | |
| chr8 | p23.1- p12 | 12627630 | 32499834 | L | |
| chr8 | p12-p11.21 | 32882718 | 42971936 | G | |
| chr8 | q11.21-q24.3 | 48549253 | 146024209 | G | |
| chr11 | p15.5-p11.2 | 2121540 | 46490960 | G | |
| chr13 | q12.11-q34 | 18556982 | 113766081 | G | |
| chr20 | q11.21-q13.33 | 29436537 | 62320720 | G | |
| chrX | p11.23-p11.1 | 48639378 | 57116899 | G | |
| chrX | q11.1-q28 | 61980262 | 154561665 | G | |
| chrY | p11.31-p11.2 | 2716461 | 8521949 | L | |
| chrY | q11.21-q11.221 | 13208776 | 17558012 | L | |
This case represents an atypical course for rectal cancer, with prolonged disease-free survival of about 19 years prior to the manifestation of disease recurrence in the form of metastatic disease to the lung.
Our aCGH results are consistent with other studies showing similar patterns of chromosomal imbalances in primary colorectal tumors and their corresponding pulmonary metastasis[11,12]. While we realize that aCGH analysis reveals the DNA copy number changes in tumor cells and not the exact origin of these cells, specific trends and patterns of genetic aberrations have been reported to be associated with specific tumor sites and types[10,13].
O’Connell et al[14] identified a recurrence risk score based on the expression of 12 genes (seven cancer-related genes and five reference genes). Six of the seven cancer-related genes were grouped into two biological pathways: cell cycle control (KI-67, C-MYC, MYBL2) and stromal response (BGN, FAP, INHBA), and the seventh gene (GADD45B) may regulate the activity of the stromal response genes. Interestingly, while we have not evaluated the expression of these genes in the primary rectal tumor or the recurrent lung metastatic lesion, we have noticed that most of those genes show copy number gains by aCGH in one or both lesions. Specifically, BGN (Xq28), FAP (2q24.2), C-MYC (8q24.21), and MYBL2 (20q13.12) have copy number gain in both the rectal and lung lesions; INHBA (7p14.1) has copy number gain in the rectal lesion; GADD45B (19p13.3) has copy number gain in the lung lesion; and KI-67 (10q26.2) has no changes in the copy number in either lesions.
Approximately 30% of patients with colorectal carcinoma who undergo primary curative surgical resection experience recurrent disease[15,16]. Several predictive factors for recurrence have been reported including: primary site (rectum vs colon), advanced stage, invasion of contiguous organs, and presence of perforation[16]. The most frequent sites of recurrence are liver and lungs (33% and 22%, respectively), with the majority of these recurrences occurring in the first two years after surgery[17]. In a retrospective study by Galandiuk et al[18], the median time to recurrence for patients who had undergone curative resection for stage III colorectal cancer was 16.7 mo. Likewise, another retrospective study by Obrand et al[17] reported an average time for distant recurrence of 22.9 mo.
It was established in the early 90’s that adjuvant therapy with fluorouracil and radiation in rectal cancer patients with locally invasive or regional nodal involvement reduces the risk of cancer recurrence and improves the overall survival[18]. More recently the German Rectal Cancer Trial established preoperative chemoradiotherapy as the standard of care in locally advanced rectal cancer showing a lower pelvic relapse rate (6% vs 13%) with no change in 10-years disease-free survival (68%) or overall survival (60%) compared to postoperative chemoradiation treatment[19]. Our patient was treated prior to the era of preoperative chemoradiation therapy and therefore received postoperative chemoradiation therapy. It would be difficult to determine whether the prolonged remission time in this case is due to the administration of adjuvant chemoradiation therapy or simply due to this patient’s unique tumor biology.
Late recurrence of colorectal cancer has been reported in small series. Recently, Ishii et al[21] reviewed 16 cases of colorectal cancer recurrence after a disease-free interval of 5 years or more. The median disease-free interval was 10 years with a range of 5-16 years. Shimoda et al[22] reported the longest recurrence interval in the literature of 16 years in a rectal cancer patient who had recurrent solitary metastatic ileal cancer. To our knowledge, the case we are reporting here represents the longest disease-free survival of 19 years in recurrent colorectal cancer after surgical resection.
The 5-years survival of patients with untreated metastatic disease is less than 5%[22]. Pulmonary metastasectomy in a select group of patients has a positive effect on survival (5-year survival rate of up to 50%)[23]. Accordingly, recurrent disease in this case was treated with preoperative chemotherapy followed by surgical resection. Whether patients with metastasectomies should receive perioperative chemotherapy remains controversial[24].
This case identified striking similarities in genetic aberrations between a primary rectal tumor and its lung recurrence after long disease-free survival. Indeed, it reflects a lack of our full understanding of the tumor microenvironment. The mechanism responsible for recurrence following years of “dormancy” of the cancer cells deserves further investigation, in order to identify a subgroup of colorectal cancer patients that should be treated differently and, perhaps, should have prolonged surveillance. Focusing research efforts on outliers such as this case may help identify fundamental biological patterns that would also help in the treatment of more traditional patients.
COMMENTS
An 81-years-old male with a history of resected rectal cancer presented with cough.
Dullness to percussion and decrease breath sounds over the upper lobe of the right lung.
Lung mass, lung abscess, pneumonia.
WBC 8.20 k/uL; HGB 12.10 gm/dL; CEA 1.20 ng/mL; metabolic panel and liver function test were within normal limits.
CT/PET scan showed right upper lobe mass (8.2 cm × 7.3 cm) with SUV of 14.5, and right-sided mediastinal and hilar lymphadenopathy, in addition to a 1.2 cm nodule in the left costophrenic angle with no FDG activity.
Bronchoscopy and biopsy revealed a wild type K-ras adenocarcinoma of colonic primary, CDX2/CK20 positive and TTF1/CK7/CD58 negative.
The patient was treated with FOLFOX (5-FU, oxaliplatin and leucovorin) in combination with bevacizumab.
The tumor biology of colorectal cancer of is not very well understood and we do not have predictive biomarkers that indicate when a particular patient tumor will recur.
High-resolution array CGH is a molecular cytogenetic method that is used for analyzing DNA copy number aberrations which is applied to detect genomic abnormalities in cancer.
This case report not only represents the longest rectal cancer disease-free interval in the literature, but also applies genetic analysis as a tool to confirm the similarity of the original and metastatic tumor and to predict the risk of recurrence.
This article applies genetic analysis to confirm the origin of a recurrent rectal tumor and to predict the risk of recurrence.
P- Reviewer: Chetty R, Cardinale V, Wongkham S, Zoli W S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 2. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1178] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 3. | Kosinski L, Habr-Gama A, Ludwig K, Perez R. Shifting concepts in rectal cancer management: a review of contemporary primary rectal cancer treatment strategies. CA Cancer J Clin. 2012;62:173-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg. 2013;100:E1-33. [PubMed] |
| 5. | Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, Jones J, Glass A, Lerner H, Lawrence W. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst. 1988;80:30-36. [PubMed] |
| 6. | Roth AD, Delorenzi M, Tejpar S, Yan P, Klingbiel D, Fiocca R, d’Ario G, Cisar L, Labianca R, Cunningham D. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104:1635-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 917] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 8. | Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA. 2008;105:4283-4288. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 635] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 9. | Wells A, Griffith L, Wells JZ, Taylor DP. The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res. 2013;73:3811-3816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Vivanco Calderón F, Martín Duce A, Badía de Yébenes A, Domingo García P. [Risk factors in the closure of lateral colostomies]. Rev Esp Enferm Apar Dig. 1987;72:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Heim SMF. Cancer cytogenetics. New York: Wiley-Liss 1995; . |
| 12. | Danner BC, Gerdes JS, Jung K, Sander B, Enders C, Liersch T, Seipelt R, Gutenberg A, Gunawan B, Schöndube FA. Comparison of chromosomal aberrations in primary colorectal carcinomas to their pulmonary metastases. Cancer Genet. 2011;204:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Jiang JK, Chen YJ, Lin CH, Yu IT, Lin JK. Genetic changes and clonality relationship between primary colorectal cancers and their pulmonary metastases--an analysis by comparative genomic hybridization. Genes Chromosomes Cancer. 2005;43:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | O’Connell MJ, Lavery I, Yothers G, Paik S, Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol. 2010;28:3937-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Camps J, Grade M, Nguyen QT, Hörmann P, Becker S, Hummon AB, Rodriguez V, Chandrasekharappa S, Chen Y, Difilippantonio MJ. Chromosomal breakpoints in primary colon cancer cluster at sites of structural variants in the genome. Cancer Res. 2008;68:1284-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, André T, Haller DG, Sargent DJ. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008;26:2336-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15-24. [PubMed] |
| 18. | Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ, Pemberton JH, Wolff BG. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27-32. [PubMed] |
| 19. | NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444-1450. [PubMed] |
| 20. | Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-1933. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1489] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 21. | Ishii M, Chiba N, Ono D, Nakamura T, Ishikawa S, Arisawa Y, Hashimoto M. Lymph node metastasis from colon carcinoma at 11 years after the initial operation managed by lymph node resection and chemoradiation: A case report and a review of the literature. Int J Surg Case Rep. 2012;3:358-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Shimoda M, Saitou M, Ueda N, Maeura Y, Matsunaga S, Okamoto S. A case of solitary metastatic ileal tumor occurred after 16 years from the resection of rectal carcinoma. J Clin Surg. 2002;57:549-552. |
| 23. | Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531-535. [PubMed] |
| 24. | Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 411] [Article Influence: 22.8] [Reference Citation Analysis (0)] |