Published online May 15, 2011. doi: 10.4251/wjgo.v3.i5.75
Revised: April 26, 2011
Accepted: May 3, 2011
Published online: May 15, 2011
Gastric carcinoma is one of the malignancies that are most frequently associated with esophageal carcinoma. We describe herein our device for advanced esophageal cancer associated with early gastric cancer in the antrum. A 57-year-old man presenting with dysphagia and upper abdominal pain was admitted to our hospital. Preoperative examinations revealed locally advanced squamous cell carcinoma (SCC) of the middle thoracic esophagus (T3N0M0 Stage IIA) and mucosal signet-ring cell carcinoma of the gastric antrum (T1N0M0 Stage IA). Although the gastric tumor appeared to be an intramucosal carcinoma, its margin was obscure, so endoscopic en-bloc resection was considered inadequate. We chose surgical resection of the gastric tumor as well as the esophageal SCC after neoadjuvant chemotherapy with 5-fluorouracil and cisplatin for advanced esophageal cancer. Following transthoracic esophagectomy with three-field lymph node dissection, the gastric carcinoma was removed by gastric antrectomy, which preserved the right gastroepiploic vessels, and a pedunculated short gastric tube was used as the esophageal substitute. Twenty-eight months after the surgery, the patient is well with no evidence of cancer recurrence. Because it minimizes surgical stress and organ sacrifice, gastric tube interposition is a potentially useful technique for esophageal cancer associated with localized early gastric cancer.
- Citation: Kanda T, Sato Y, Yajima K, Kosugi SI, Matsuki A, Ishikawa T, Bamba T, Umezu H, Suzuki T, Hatakeyama K. Pedunculated gastric tube interposition in an esophageal cancer patient with prepyloric adenocarcinoma. World J Gastrointest Oncol 2011; 3(5): 75-78
- URL: https://www.wjgnet.com/1948-5204/full/v3/i5/75.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v3.i5.75
Esophageal reconstruction with a gastric tube is a well-established surgical procedure following radical esophagectomy for carcinoma of the thoracic esophagus. In cases where the stomach is unavailable, for example, patients with a history of gastrectomy, concurrent gastric diseases, or tumor involvement of the stomach, a colonic conduit is preferentially selected as an esophageal substitute because a long segment can be harvested and brought up to the neck without microvascular surgery[1]. However, this procedure is more surgically invasive than gastric pull-up and the functional loss of both stomach and colon may potentially deteriorate patient’s nutritional status.
Gastric adenocarcinoma is the second greatest cause of cancer death in the world[2]. Furthermore, it is second most common malignancy associated with esophageal carcinoma[3,4]. Thus, esophageal cancer associated with gastric adenocarcinoma is not exceptionally rare, particularly in East Asia where both malignancies are more common than in western countries[5].
We present herein details of surgical treatment of a patient with locally advanced squamous cell carcinoma (SCC) of the mid-thoracic esophagus and early gastric adenocarcinoma of the prepyloric region. The patient underwent radical esophagectomy with three-field lymph node dissection and the stomach tumor was excised by gastric antrectomy, which preserved the right gastroepiploic artery and vein. The remaining pedunculated short gastric tube was used as the esophageal substitute in conjunction with Roux-en-Y gastrojejunostomy.
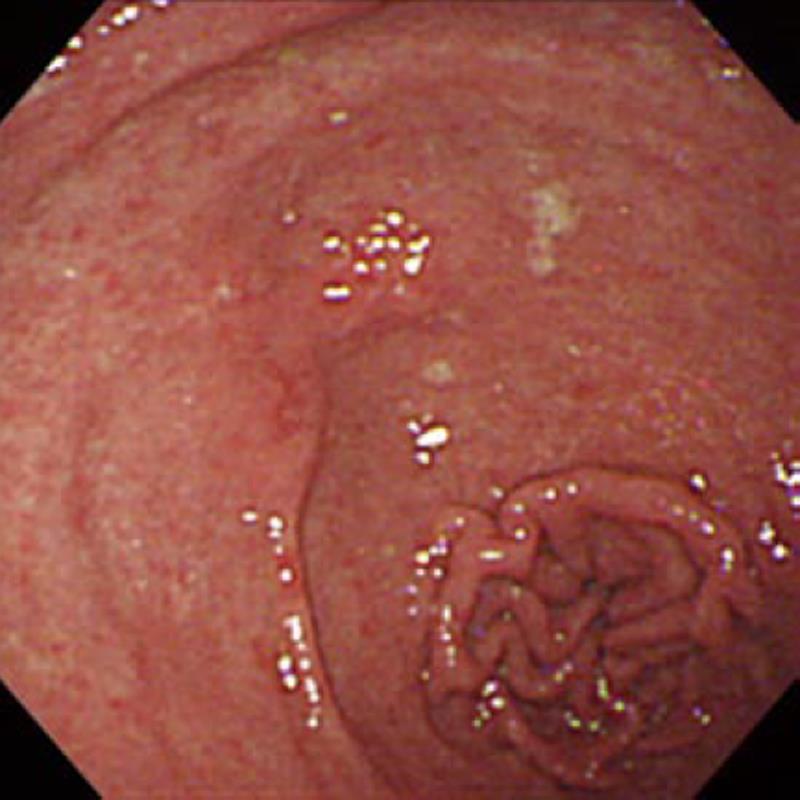
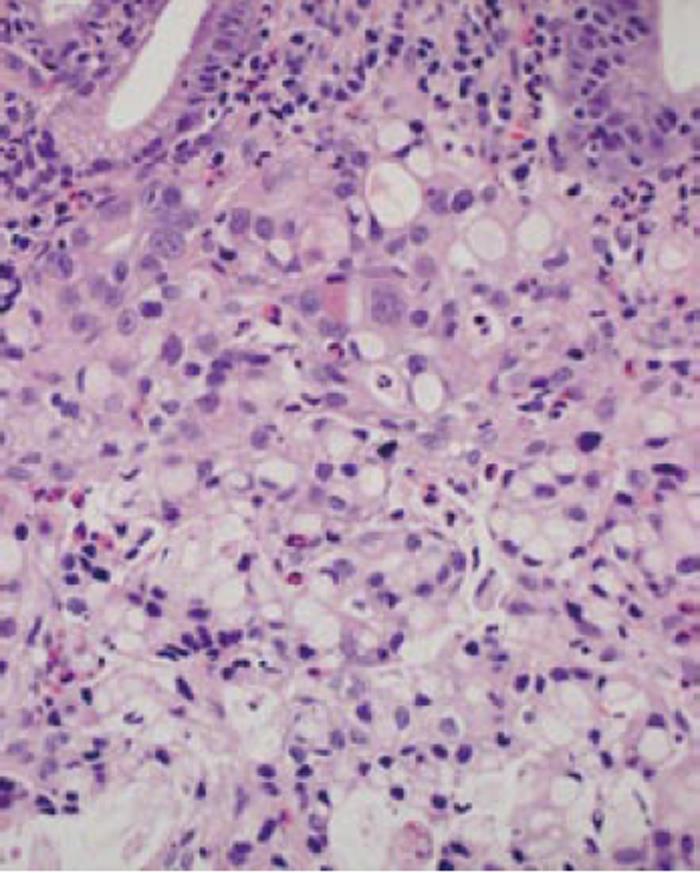
In November 2008, a 57-year-old man presenting with dysphagia was diagnosed with locally advanced SCC of the mid-thoracic esophagus by endoscopic examination of the upper gastrointestinal tract. The endoscopic examination simultaneously revealed the coexistence of an irregular depressed lesion in the prepylorus (Figure 1). Biopsy specimens taken from the gastric lesion showed poorly differentiated adenocarcinoma with signet-ring carcinoma cells (Figure 2). Computed tomography showed neither distant metastasis nor lymph node metastasis to the mediastinum or the perigastric area. A clinical diagnosis of double cancer was made - locally advanced esophageal SCC (T3N0M0 Stage IIA) and early gastric adenocarcinoma of the prepylorus (T1N0M0 Stage IA). Further endoscopic examination was carried out to look into the possibility of endoscopic treatment of the gastric tumor. Although the gastric tumor was endoscopically estimated to be an intramucosal carcinoma unassociated with ulcer scar, it was histologically a signet-ring cell carcinoma and the tumor boundary was poorly defined. As wide resection with the endoscopic submucosal dissection (ESD) technique would cause stenosis of the gastric outlet[6,7], we selected surgical resection of the gastric tumor.
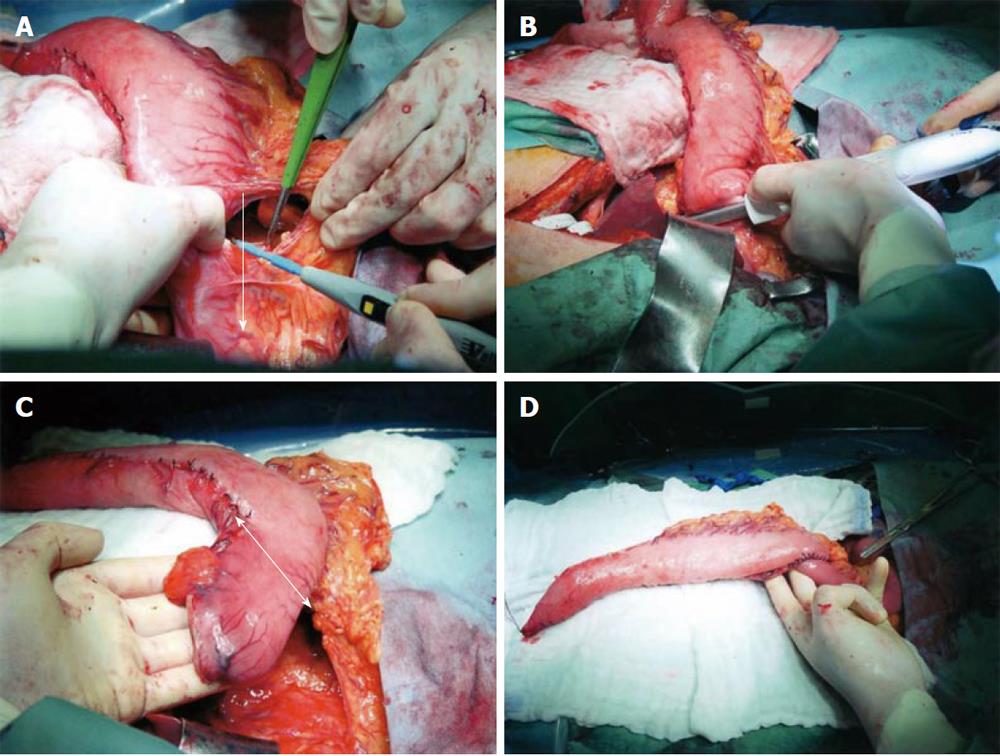
The surgery consisted of transthoracic radical esophagectomy with three-field dissection and resection of the pyloroantral region of the stomach. As an esophageal substitute, we selected a short gastric tube using the right gastroepiploic artery as a vascular pedicle, considering the low incidence of lymph node metastasis from gastric carcinoma. The surgery was performed 26 d after neoadjuvant chemotherapy with 5-fluorouracil and cisplatin for advanced esophageal cancer. After completion of the radical esophagectomy, the stomach was mobilized as for the construction of a conventional gastric tube. No lymph node metastasis was found macroscopically along the gastroepiploic arteries. The branches of the right gastroepiploic vessels were carefully cut at 7 cm distal from the pylorus (Figure 3A). This procedure was extended downward to the pylorus. The duodenum was divided immediately distal to the pyloric ring and the duodenal stump was closed (Figure 3B). The distal part of the gastric tube was resected along with suprapyloric lymph nodes (Figure 3C). The distal stump of the gastric tube was anastomosed to the upper jejunum for a Roux-en-Y reconstruction (Figure 3D). The gastric tube was pulled up through the posterior mediastinum and anastomosed to the cervical esophagus. The cervical anastomosis was easy because separation from the duodenum increased the flexibility of the pedunculated short gastric tube. The operation time was 630 min and blood loss was 635 mL.
Pathological examination of surgical specimens revealed that the esophageal tumor was a well-differentiated SCC that had spread to the esophageal adventitia in depth (pT3) and was associated with no lymph node metastasis. The gastric tumor was a moderately differentiated adenocarcinoma with a mucinous component and was localized in the mucosa. No vascular or lymphatic invasion was found. Pathological examination revealed a gastric tumor measuring 1.5 cm × 1.0 cm in size and complete tumor-free margins. No lymph node metastasis was found in the suprapyloric lymph nodes dissected.
The patient suffered from minor leakage from the esophagogastrostomy but soon recovered non-surgically. He was discharged on the 39th postoperative day. At the time of writing, i.e. 28 mo after the surgery, the patient is well with no evidence of cancer recurrence.
It is not uncommon that patients with esophageal carcinoma suffer from other malignancies synchronously and/or metachronously. According to the Comprehensive Registry of Esophageal Cancer in Japan[8], 18.5% of patients with esophageal carcinoma simultaneously had multiple primary cancers, in which gastric carcinoma was the most common (4.7%), followed by head and neck cancer (2.7%), colorectal carcinoma (1.2%), and lung cancer (0.7%). These data suggest the need to devise an adequate treatment plan for patients having double cancer. In the case of combination with gastric carcinoma, reconstruction following radical esophagectomy is problematic because the conventionally used gastric pull-up is unavailable. Recently, endoscopic mucosal resection has become the standard treatment for gastric intramucosal carcinoma with a differentiated morphology. The development of ESD, in which the mucosa containing tumors is dissected along the submucosal layer, allows for en-bloc resection of large tumors and extends the application of endoscopic treatment to early gastric cancer with a very low risk of regional lymph node metastasis[9]. Endoscopic resection would potentially have been suitable for our case. However, the tumor in the present case was determined to be a small intramucosal carcinoma whose boundary could not be identified. Furthermore, it was located in the prepyloric region. Based on these conditions, the gastrointestinal cancer board in our hospital, which consists of esophagogastric surgeons, gastrointestinal endoscopists, and radiologists, determined that endoscopic resection should be avoided because it had a high risk of incomplete resection and/or stenosis following wide resection.
Interposition with a pedunculated gastric tube in a Roux-en-Y fashion was reported for the first time by Yamagishi et al[10] in 1970. They used this technique originally for the bypass surgery of advanced esophageal cancer, and then extended it to normal esophageal reconstruction following radical esophagectomy, noticing that this technique improved gastric tube flexibility and enabled safe anastomosis at the lower level of the gastric tube where blood flow is rich. Hanyu et al[11] applied the gastric tube interposition to patients with esophageal carcinoma associated with early carcinoma that was located in the lesser curvature of the stomach. They presented this as a procedure that keeps the radicality of the regional lymph node dissection for coexisting early gastric cancer. In their procedure, tumors at the gastric angle were excised in the process of making a short gastric tube using a part of the greater curvature of the stomach. In 2006, Motoyama et al[12] reported distal gastrectomy with preservation of the gastroepiploic artery in two cases of adenocarcinoma that metachronously occurred in a gastric tube following esophagectomy. The surgery in the present case is essentially the synchronous counterpart of that of Motoyama et al[12].
In the present case, we omitted the dissection of lymph nodes along the right gastroepiploic artery, station nos. 4d and 6 in the Japanese Classification of Gastric Carcinoma[13], in which lymph node metastasis is frequently found in the carcinoma of the lower third of the stomach. However, there are data showing that the incidence of lymph node metastasis from an intramucosal carcinoma without ulcer scar was approximately 4% even if the tumor was morphologically classified as the diffuse type[14]. The esophageal cancer in the present case was diagnosed, both clinically and pathologically, as Stage IIA. The 5-year survival rate of patients with esophageal cancer at Stage IIA is reported to be 52%-84%[8,15-17] and approximately one third of the patients show recurrence despite undergoing radical esophagectomy with extended lymphadenectomy. Thus, the risk of lymph node recurrence after local resection of early gastric cancer is acceptable, compared with the prognosis of patients with advanced esophageal cancer. Our treatment choice offers certain benefits to the patient, including low invasiveness and good nutritional status although we need to conduct continuous follow-up to determine whether or not our treatment choice is indeed warranted.
The indication of the technique presented here is limited to tumors located in the distal stomach, early stage carcinomas with a low risk of lymph node metastasis, and widely spreading tumors unmanageable by endoscopic resection. However, pedunculated gastric tube interposition combined with gastric antrectomy is a minimally invasive surgical technique that minimizes organ sacrifice. Clinicians involved in esophagogastric cancer treatment should consider this technique as a possible alternative to colonic interposition following radical gastrectomy for gastric carcinoma associated with advanced esophageal cancer.
Peer reviewer: Markku Voutilainen, MD, Department of Internal Medicine, Central Hospital Central Finland, Keskusairaalantie 19, Jyväskylä FIN-40620, Finland
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Thomas P, Fuentes P, Giudicelli R, Reboud E. Colon interposition for esophageal replacement: current indications and long-term function. Ann Thorac Surg. 1997;64:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13556] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 3. | Nagasawa S, Onda M, Sasajima K, Takubo K, Miyashita M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Kumagai Y, Kawano T, Nakajima Y, Nagai K, Inoue H, Nara S, Iwai T. Multiple primary cancers associated with esophageal carcinoma. Surg Today. 2001;31:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kato H, Tachimori Y, Watanabe H, Mizobuchi S, Igaki H, Yamaguchi H, Ochiai A. Esophageal carcinoma simultaneously associated with gastric carcinoma: analysis of clinicopathologic features and treatments. J Surg Oncol. 1994;56:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Tsunada S, Ogata S, Mannen K, Arima S, Sakata Y, Shiraishi R, Shimoda R, Ootani H, Yamaguchi K, Fujise T. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc. 2008;67:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy. 2009;41:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Comprehensive registry of esophageal cancer in Japan (1998, 1999) and long-term results of esophagoectomy in Japan (1988-1997). 3rd ed. Tokyo: Japanese Society for Esophageal Diseases 2002; . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 10. | Yamagishi M, Ikeda N, Yonemoto T. An isoperistaltic gastric tube. New method of esophageal replacement. Arch Surg. 1970;100:689-692. [PubMed] |
| 11. | Hanyu F, Hayashi T, Kinoshita Y, Takada T, Yoshida M, Hukushima Y, Ide H, Endo M. Esophageal reconstruction by means of pedunculated small gastric tube for the esophageal cancer with localized gastric cancer (In Japanese). Rinsyou Geka. 1981;36:993-997. [PubMed] |
| 12. | Motoyama S, Saito R, Okuyama M, Maruyama K, Ogawa J. Treating gastric tube cancer with distal gastrectomy preserving the gastroepiploic artery. Ann Thorac Surg. 2006;81:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association . Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [PubMed] |
| 14. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 15. | Nishimaki T, Suzuki T, Suzuki S, Kuwabara S, Hatakeyama K. Outcomes of extended radical esophagectomy for thoracic esophageal cancer. J Am Coll Surg. 1998;186:306-312. [PubMed] |
| 16. | Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177-183. [PubMed] |
| 17. | Ferahköşe Z, Anadol AZ, Gökbayir H, Dursun A, Oztürk E. Three-field lymph node dissection in the treatment of thoracic esophageal carcinoma: the Turkish experience. Dis Esophagus. 2006;19:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |