INTRODUCTION
Infliximab, a mouse-human chimeric monoclonal antibody to tumor necrosis factor α (TNF-α), appears to reduce disease activity in Crohn’s disease[1,2]. Fistula drainage can also be reduced or terminated, but the role of biological agents in causing complete resolution of fistulous tracts remains controversial[3,4]. Adalimumab, a more “humanized” monoclonal antibody to TNF-α, has also been used in Crohn’s disease. Soon after the introduction of these agents, reports of serious suspected adverse effects began to appear[5,6]. Concern has been expressed for the potential to increase risk for malignant disease possibly reflecting, in part, in vivo biological effects of anti-tumor necrosis factor agents[7]. Hepatosplenic lymphoma, a rare T-lymphocyte malignant disorder, has been reported in children, after treatment with infliximab[8-10].
Cohort and population-based studies have also described an increased intestinal cancer risk in Crohn’s disease[11-13]. Other neoplasms may also occur more frequently in Crohn’s disease, including myeloid and lymphoid malignancies as well as carcinoid tumors[14-16]. Characteristics of the colorectal cancers in Crohn’s disease appear to include: long-standing and extensive colonic disease, young age at cancer diagnosis, tendency to localize in the distal colorectum and mucinous type histopathogical features[12]. In Crohn’s disease, concern has been expressed regarding a potential for superimposed cancer risk associated with use of biological agents, particularly with fistula closure[17].
CASE REPORT
An 18-year old male was first seen in November 1995 with abdominal pain and diarrhea, present for 3 years. Physical exam was normal. Fecal studies for occult blood, bacteria and parasites were negative, but fecal leukocytes were present. Lab studies showed an anemia (hemoglobin, 110 g/L vs normal, 130-172 g/L) with an elevated sedimentation rate to 38 mm/h and a reduced serum albumin of 30 g/L (normal, 35 to 50). Upper gastrointestinal barium series, including a small bowel follow through, revealed 4 short segments of narrowing from the distal jejunum to ileum, including terminal ileum, consistent with Crohn’s disease. Treatment with a 5-aminosalicylate (5-ASA) alone led to symptom resolution.
Two years later, diarrhea recurred with weight loss of 4 kg, in spite of 5-ASA. Physical exam showed an anal fissure. Hemoglobin was 98 g/L and sedimentation rate was 72 mm/h. Serum albumin was 21 g/L. Flexible sigmoidoscopy and biopsy showed focal aphthoid ulcers with inflammatory changes but no granulomas. The patient was treated with a 6-wk course of prednisone. As his symptoms resolved, he discontinued 5-ASA.
In November 1997, a perianal abscess developed and required surgical drainage. Because of diarrhea and further weight loss of 5 kg, parenteral nutrition was given. A colonoscopy and biopsies were normal. Although the patient’s diarrhea resolved and weight improved with 5-ASA and oral budesonide, anorectal pain and drainage persisted so ciprofloxacin and metronidazole were added. Examination under anesthesia revealed that a fistulous tract extended 6 to 10 cm cephalad and a sinogram showed extension into the ischiorectal space. After further surgical drainage and packing, he was treated with metronidazole for 3 mo. His symptoms resolved and blood tests normalized. A small bowel barium study was reported to be normal.
One year later, abdominal pain recurred and a small bowel barium study showed numerous strictures. The patient was unable to eat because of pain from obstructive symptoms, so parenteral nutrition was provided. At laparotomy, 30 cm of the mid-jejunum and 35 cm of the ileocecum were resected and 7 stricturoplasties performed. Resected small bowel showed transmural inflammation, focal acute and chronic inflammation, ulceration and fibrosis consistent with Crohn’s disease. After surgery, his appetite and bowel function became normal and he regained his weight.
In March 1999, anorectal pain and drainage recurred that failed to respond to ciprofloxacin and metronidazole. An ischiorectal abscess was drained. Despite ongoing ciprofloxacin and metronidazole, the incisional site continued to drain intermittently. In March 2000, anorectal pain recurred along with rectal bleeding. Colonoscopy revealed limited patchy areas of focal aphthoid ulceration in the sigmoid and descending colon. Biopsies showed inflammatory change with a single well-formed granuloma. Because of paraesthesiae in his feet, metronidazole was discontinued. The possibility of ileostomy or diverting colostomy was offered, but he declined further surgery. The patient requested referral for infliximab treatment.
Over the next year, the patient was treated with 5-ASA, budesonide, ciprofloxacin and metronidazole. In addition, azathioprine and prednisone were given. In spite of these medications, his perianal fistula continued to drain. Infliximab infusions were initiated and provided up to 2007, along with 5-ASA and azathioprine. In October 2006, despite infliximab therapy, another ischiorectal abscess was drained. Unfortunately, persistent drainage occurred while left lower quadrant and perianal pain developed with radiation into his right lower limb. Endoscopic examination showed no rectosigmoid abnormality. In November 2007, subcutaneous adalimumab was tried but his pain worsened and drainage continued.
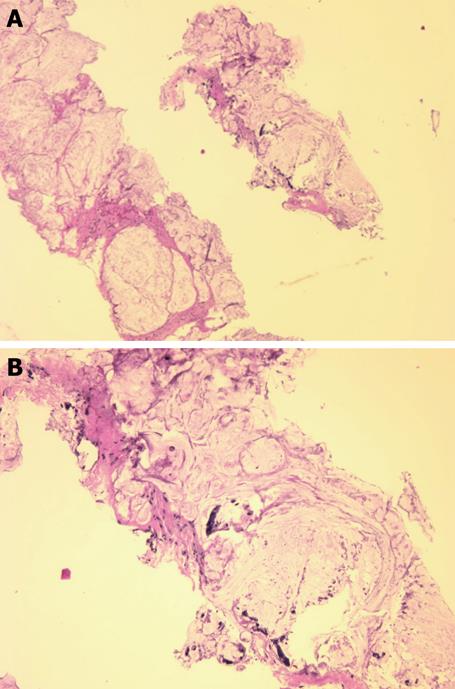
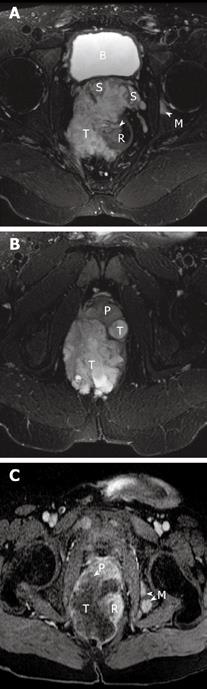
Magnetic resonance imaging (MRI) revealed a large right-sided perirectal mass and CT guided core biopsies confirmed the presence of mucinous adenocarcinoma from the fistulous tract (Figure 1). The lesion also extended inferiorly into the pelvic floor and perineum, anteriorly into the prostate gland and seminal vesicles, and posteriorly into the lumbar spine (Figure 2). The patient underwent a diverting loop colostomy, followed by local radio-therapy. Post-operatively, he required palliative treatment for ongoing severe pain and, in July 2008, he succumbed to the extensive malignancy at age 28 years.
Figure 1 Needle biopsy fragments showing mucinous adenocarcinoma (HE stain).
A: Low power photomicrograph above shows fragments of infiltrating cancer; B: High power photomicrograph below shows individual cancer cells or clusters of cancer cells within mucus.
Figure 2 Magnetic resonance images of the patient.
A and B show the axial T2-weighted fat suppressed MR images of the pelvis. A heterogeneous but predominantly high signal intensity tumor mass (T) is seen arising from the right lateral wall (arrow) of the rectum (R). This mass extends anteriorly to invade both seminal vesicles (S) and abuts and displaces the prostate gland (P). A high signal focus in the left acetabulum represents a bone metastasis (M). The bladder is noted as B. C is the MR image about 5 wk later, now repeated with contrast. T1-weighted post-contrast fat saturation MR image correlating to image in Figure 2b shows rim enhancement of the mass with a large central low signal area consistent with central necrosis. There is now invasion of the tumor into the prostate gland (P) and multiple new osseous metastases (M).
DISCUSSION
This report describes Crohn’s disease in a young man involving the small and large intestine, complicated by recurrent peri-anal and peri-rectal fistulous disease. Both drug and surgical treatment of the fistula were attempted; eventually, biological agents were used. Although symptomatic improvement temporarily occurred, his compliance to therapy, at times, was limited and may have played a role in control of his disease. Later, in his clinical course, an aggressive anorectal carcinoma developed, appearing to originate from the fistulous tract. Malignant change in fistulous tracts has previously been reported to occur rarely in Crohn’s disease[18-24], but development after treatment with biological agents has not been previously recorded.
Malignant complications occur in Crohn’s disease. Indeed, several characteristics of colorectal cancer known to complicate the clinical course of Crohn’s disease were all confirmed here. These include: duration over a decade, young age at onset of malignancy, and definition of the malignant lesion as a mucinous type carcinoma[12]. This fatal carcinoma could be attributed to the underlying Crohn’s disease, possibly related to “adenomatous epithelialization” of the fistula tract[19] with progression from dysplastic epithelium to carcinoma. Neoplastic change could be a consequence of long term exposure to drugs such as metronidazole or azathioprine. However, the potential long-term effects of biological agents in this setting also need to be considered. Recently, advanced colon cancer has been recognized not long after commencement of infliximab therapy[25,26].
Two patients with recent cancer-negative colonoscopies developed advanced and extensive colon cancer within 2 years after initiation of infliximab therapy for their Crohn’s colitis[25]. A multicenter study found no increase in the short-term incidence of newly diagnosed neoplasia in infliximab-treated Crohn’s. However, the investigators felt that longer term studies with larger patient groups were needed to learn the ultimate effect of these agents[26]. Biological agents could play a role in the initiation of cancers, but in long-standing and extensive Crohn’s disease where the risk of neoplasia is already increased, such agents could also affect progression of this process or influence its aggressiveness.
Clinicians who care for Crohn’s disease complicated by long-standing and persistently draining fistulas need to be especially wary of the potential for malignancy as a complication. This is especially important for patients most likely to be considered candidates for biological therapy, such as people with persistently draining fistulae that have not responded to other forms of treatment. Modern imaging methods, such as MRI, may be very helpful in detection, especially if biological agents are contemplated for treatment. Published clinical trial data on use of biological agents in Crohn’s disease and other disorders are for relatively short term use and do not provide insight into the longer term effects of treatment. The US FDA has appealed to clinicians using approved TNF blockers and caring for these patients to be alert to this issue[27]. While the FDA has required that future studies supply such data, long-term results will not be available for at least another decade. In the meantime, clinicians are encouraged to report suspected or possible adverse reactions or unexpected outcomes during therapy since this may be the only practical means to identify serious problems with such new therapies.