Published online Jun 15, 2010. doi: 10.4251/wjgo.v2.i6.282
Revised: February 20, 2010
Accepted: February 27, 2010
Published online: June 15, 2010
This report presents a case of highly advanced gastric cancer that achieved a histologically complete response (CR) to preoperative chemoradiotherapy with S-1 plus low-dose Cisplatin. A 60-year-old male patient underwent FDG positron emission tomography (PET) during a routine health examination. The patient was found to have swollen paraaortic lymph nodes. Shortly thereafter, he was diagnosed with gastric carcinoma with a type 2 tumor in the antrum with paraaortic lymph node metastases based on FDG-PET, endoscopic examination and abdominal computed tomography. After the completion of chemoradiation therapy (CRT), the tumor and the paraaortic lymph node metastases disappeared. The patient underwent surgery 5 wk after the completion of CRT, including a subtotal gastrectomy with Roux-en-Y reconstruction, D3 lymph node dissection and a left adrenalectomy. No cancer cells were detected in the resected specimen either in the primary lesion or lymph nodes, thus confirming a pathologically CR to CRT (CR grade 3). The patient has been stable and well without any evidence of recurrence for 48 mo after surgery. Such a preoperative CRT regimen might therefore be very effective for treatment of some advanced gastric cancers.
- Citation: Shigeoka H, Imamoto H, Nishimura Y, Shimono T, Furukawa H, Imamura H, Yasuda T, Shiozaki H. Complete response to preoperative chemoradiotherapy in highly advanced gastric adenocarcinoma. World J Gastrointest Oncol 2010; 2(6): 282-286
- URL: https://www.wjgnet.com/1948-5204/full/v2/i6/282.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v2.i6.282
Surgical therapy and endoscopic resection is the primary treatment for gastric carcinoma. However, for patients with stage IV advanced gastric cancer the prognosis is unfavorable even if macroscopically curative resection is performed.
Several new perioperative adjunctive approaches (neoadjuvant and/or adjuvant) for highly advanced gastric cancer have been explored[1-4]. Although a high incidence of partial response by chemotherapy with S-1 plus Cisplatin has been reported, a pathologically complete response (CR) is seldom observed with this combination. Therefore, chemoradiation therapy (CRT) has attracted considerable attention as a breakthrough for treating cases of highly advanced gastric cancer.
This report presents the case of a patient initially diagnosed with an unresectable advanced gastric cancer who was successfully treated by preoperative chemoradiotherapy with S-1 plus low-dose Cisplatin. The patient achieved a histologically CR that continued to a long-term survival of more than four years without any recurrence.
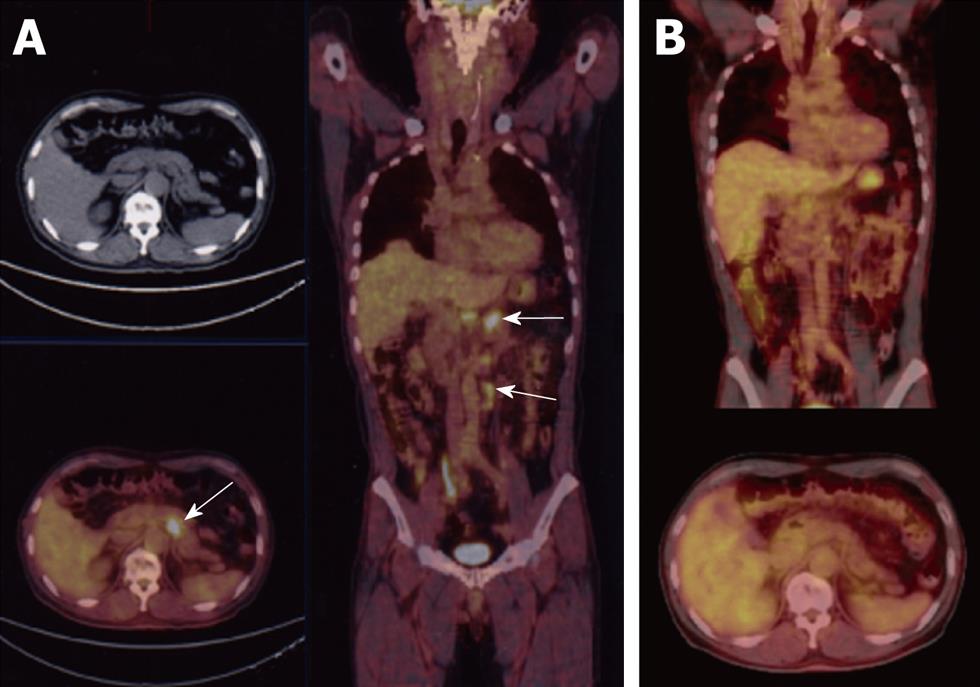
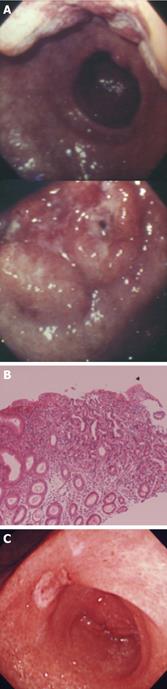
A 60-year-old male patient was found to have swollen paraaortic lymph nodes by FDG positron emission tomography (PET) (Figure 1A) during a routine health examination. The patient had no complaints and no palpable mass was found by an abdominal physical examination. The serum carcinoembryonic antigen and carbohydrate antigen (CA) 19-9 levels were negative, 2.0 mg/mL and 9 U/mL respectively. The serum sIL-2R level was 669 U/mL which was slightly increased over normal levels. The blood chemistry findings were all normal and the hemoglobin level was 15.9 g/dL. The chest X-ray was also normal. Gastrointestinal fiberscopy showed a type 2 gastric carcinoma in the antrum (Figure 2A). An endoscopic biopsy revealed an intestinal type adenocarcinoma (moderately differentiated tubular adenocarcinoma; Figure 2B). Previously, the patient had undergone a gastrointestinal endoscopic examination almost every year. Unfortunately, he did not have an endoscopic examination in the year prior to the FDG-PET since there had been no symptoms such as stomach pain.
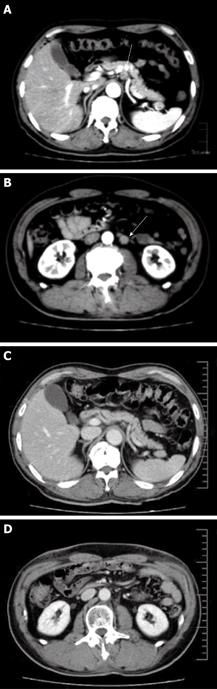
Abdominal computed tomography (CT) also revealed lymph node metastases in the paraaortic region (Figure 3A and B). Therefore, this case was diagnosed to have stage IV advanced gastric carcinoma using the Japanese classification of gastric carcinoma (cT2, cN3, cH0, cP0, cM0). Stage IV gastric cancer was also indicated by the UICC TNM classification because of the paraaortic lymph node metastasis.
Preoperative CRT was administered since the tumor was apparently too advanced to be curatively resected. A 10 MV X-ray was used. The daily fractional dose of radiation therapy was 1.8 or 2 Gy, administered 5 d a week. The radiation treatment was delivered through the anterior and orthogonal lateral portals with 45-degree wedges. The radiation fields included the body and antrum of the stomach, the perigastric lymph nodes and the lower paraaortic lymph nodes. Concurrent chemotherapy was combined with radiation therapy of 40 Gy over 22 fractions for 5 wk. S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) was administered orally at a dose of 120 mg/d on days 1-14 and at a dose of 80 mg/d on days 21-34 and 17 doses of CDDP (7 mg/d) were infused for 1 h prior to radiation therapy. The dose of S-1 in the latter half was reduced to 80 mg/d due to adverse reactions (grade 2 leukocytopenia and grade 2 fatigue). The tumor and the paraaortic lymph node metastases completely disappeared at the completion of CRT (Figure 3C and D) thus leaving a tiny area of erosion on the mucosa of the antrum (Figure 2C). Grade 3 leukocytopenia and grade 2 thrombocytopenia were the only adverse effects observed after CRT.
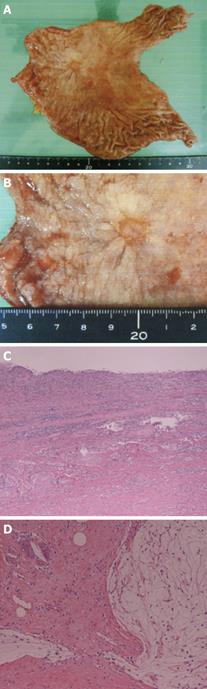
Five weeks after the completion of CRT, the patient underwent surgery, including a subtotal gastrectomy with Roux-en-Y reconstruction, D3 lymph node dissection and a left adrenalectomy (Figure 4A and B). No cancer cells were detected in the resected specimens in the primary lesion (Figure 4C) or in the lymph nodes (Figure 4D), confirming a pathologically CR (CR grade 3).
The patient had no surgical complications and was discharged from the hospital 10 d after surgery. The patient received no adjuvant chemotherapy and is presently alive and well at 48 mo after surgery with no evidence of recurrence.
Gastric cancer is one of the most frequent malignant tumors in the world. A gastric cancer screening program was introduced in the 1960s in Japan as a public health service. Since that time, the proportion of early stage gastric cancer has been increasing. However, highly advanced gastric cancer patients such as the current patient are still frequently diagnosed. The patient described in this case report was initially found to have swollen paraaortic lymph nodes by FDG-PET during a routine health examination. He subsequently underwent gastrointestinal fiberscopy which revealed a type 2 tumor in the antrum. Thereafter, an endoscopic biopsy revealed an intestinal type adenocarcinoma. FDG-PET is usually not used to detect or stage gastric cancer. Chen et al[5] reported that FDG-PET demonstrated an increased uptake in 64 of 68 patients (sensitivity 94%) and also improved the preoperative TNM staging of adenocarcinoma. FDG-PET was therefore found to be very useful in this case. In addition, it may also be complementary to CT scans for the preoperative staging of gastric cancer.
Because this case was diagnosed to have stage IV gastric cancer using the Japanese classification of gastric carcinoma due to paraaortic lymph node metastases, the prognosis was unfavorable even if an R0 resection (complete local-regional tumor removal with negative resection margins) could be performed. A successful preoperative therapeutic strategy consisting of either chemotherapy or chemoradiotherapy may improve R0 resection and reduce recurrence, although the efficacy of neoadjuvant therapy for advanced gastric cancer is still controversial[6,7]. Adjuvant therapy may also be useful. The MAGIC trial demonstrated that pre and postoperative ECF regimens (a combination of Epirubicin, CDDP and a continuous infusion of 5-FU) decreased the tumor size and stage and significantly improved the rates of progression-free survival[8].
S-1 is an effective anticancer therapy. Even if given alone, the response rate is approximately 40%-50%[9,10]. Combination chemotherapy with S-1 and CDDP has demonstrated a favorable antitumor activity[11-14]. There are some case reports with a CR of gastric cancer by S-1 monotherapy[15,16] and chemotherapy with S-1 plus low-dose CDDP[17]. Preoperative CRT using S-1 and low-dose CDDP (4 mg/m2 per day) was administered to the current patient.
Ajani et al[18] reported the overall survival of patients who achieved a pathologically CR (pathCR) to be significantly longer than that of patients who did not have a pathCR. The frequency of pathCR by preoperative chemotherapy is much less than that by preoperative CRT which was the reasoning for administering preoperative CRT in the current case. A phase II multi-institutional trial by the Radiation Therapy Oncology Group (RTOG 99-04) of pre-operative chemoradiation for localized gastric adenocarcinoma demonstrated the pathologic CR and R0 resection rates to be 26% and 77% respectively[19].
Fortunately, preoperative CRT was very effective for this patient and the paraaortic lymph node metastases disappeared after the completion of CRT, confirmed both by FDG-PET and CT scans. A complete pathologic response of advanced gastric adenocarcinoma has been achieved with several regimens[20,21]. A curative resection could not have been performed if the preoperative CRT had not been effective in this case. Radiation was very effective in this case. There are very few pathCR case reports of highly advanced gastric cancer with neoadjuvant CRT which describe a large radiation field including the paraaortic area. Advances in conformal radiation and chemotherapy-based treatment planning now allow for the treatment of such a large radiation field and for it to be combined with chemotherapy.
Neoadjuvant approaches are very attractive because the pathologic response can be precisely assessed in the treated tumor. Pre-operative CRT does have potential risks. The RTOG 99-04 reported Grade 4 toxicity in 21% of all patients. Although preoperative CRT has been used to treat patients with potentially resectable localized gastric adenocarcinomas in some countries, preoperative CRT is usually applied for unresectable cases in Japan. Preoperative CRT might be useful as a standard procedure for advanced gastric cancer after the completion of the phase III trial.
The radiation doses of 31 to 50 Gy have been applied for preoperative treatment[7]. The radiation dose was 40 Gy in the present case and it yielded a pathologic CR.
Although the role of chemotherapy as an adjuvant treatment remains controversial, several randomized trials have shown the advantages of adjuvant chemotherapy. No adjuvant chemotherapy was administered in the current case because no cancer cells were detected in any of the resected specimens.
This report presented the case of a successfully treated patient who had highly advanced gastric carcinoma with paraaortic lymph node metastases.
Peer reviewer: Marius Raica, Professor, Department of Histology and Cytology, “Victor Babes” University of Medicine and Pharmacy, Pta Eftimie Murgu 2, 300041, Timisoara, Romania
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C
| 1. | Kodera Y, Fujiwara M, Koike M, Nakao A. Chemotherapy as a component of multimodal therapy for gastric carcinoma. World J Gastroenterol. 2006;12:2000-2005. |
| 2. | Sano T. Adjuvant and neoadjuvant therapy of gastric cancer: a comparison of three pivotal studies. Curr Oncol Rep. 2008;10:191-198. |
| 3. | Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774-2780. |
| 4. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. |
| 5. | Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383-2390. |
| 6. | Sasako M. Role of surgery in multidisciplinary treatment for solid cancers. Int J Clin Oncol. 2004;9:346-351. |
| 7. | Hazard L, O'Connor J, Scaife C. Role of radiation therapy in gastric adenocarcinoma. World J Gastroenterol. 2006;12:1511-1520. |
| 8. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. |
| 9. | Sugimachi K, Maehara Y, Horikoshi N, Shimada Y, Sakata Y, Mitachi Y, Taguchi T. An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. The S-1 Gastrointestinal Cancer Study Group. Oncology. 1999;57:202-210. |
| 10. | Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715-1720. |
| 11. | Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207-2212. |
| 12. | Tsujitani S, Fukuda K, Kaibara N. Combination chemotherapy of S-1 and low-dose cisplatin for advanced gastric cancer. Gastric Cancer. 2003;6 Suppl 1:50-57. |
| 13. | Saikawa Y, Akasaka Y, Kanai T, Otani Y, Kumai K, Kubota T, Kitajima M. Preoperative combination chemotherapy with S-1 and low dose cisplatin against highly advanced gastric carcinoma. Oncol Rep. 2003;10:381-386. |
| 14. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. |
| 15. | Mitomi H, Kishimoto I, Amemiya A, Kaneda G, Adachi K, Shimoda T, Takigawa M, Fukui N, Ohkura Y. Advanced gastric cancer showing long-term complete remission in response to S-1 monotherapy: two case reports. Cases J. 2008;1:405. |
| 16. | Schöffski P, Chollet P, Ganser A, Wiese KH, Rambusch E, de Vries MJ, Hanauske A. Complete response of a gastric primary after a short but toxic course of 'S-1' EORTC Early Clinical Studies Group. Ann Oncol. 1999;10:1117-1120. |
| 17. | Iwahashi M, Nakamori M, Tani M, Yamaue H, Sakaguchi S, Nakamura M, Ueda K, Ichiro M, Nishino E, Tanimura H. Complete response of highly advanced gastric cancer with peritoneal dissemination after new combined chemotherapy of S-1 and low-dose cisplatin: report of a case. Oncology. 2001;61:16-22. |
| 18. | Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237-1244. |
| 19. | Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958. |
| 20. | Yoshimizu N, Saikawa Y, Kubota T, Akiba Y, Yoshida M, Otani Y, Kumai K, Hibi T, Kitajima M. Complete response of a highly advanced gastric carcinoma to preoperative chemoradiotherapy with S-1 and low-dose cisplatin. Gastric Cancer. 2003;6:185-190. |
| 21. | Takahashi T, Saikawa Y, Kubota T, Akiba Y, Shigematsu N, Yoshida M, Otani Y, Kumai K, Hibi T, Kitajima M. Histological complete response in a case of advanced gastric cancer treated by chemotherapy with S-1 plus low-dose cisplatin and radiation. Jpn J Clin Oncol. 2003;33:584-588. |