MSCT/MRI EXAMINATION OF SMALL INTESTINAL TUMORS
MSCT
CT has been used for inspection of digestive tract tumors since the 1980’s with spiral CT becoming available in 1989. With fast speed, large volume data acquisition and contrast-enhanced scanning, CT observation of small intestinal tumors has progressed. The development of MSCT technology in 1998 allowed data acquisition over the entire abdomen in thin slices within one breath-hold, leading to fewer peristaltic and breathing artifacts. In addition, MSCT also has many other advantages, such as high spatial resolution and powerful post-processing of the images. It is widely accepted that MSCT can be used for the investigation of intestinal pathologies. Computed tomography enteroclysis (CTE) is used to perform enhanced MSCT scanning and image post-processing after the small intestine is distended by administrating a high volume of contrast medium orally or via a nasojejunal catheter.
Oral ingestion of contrast medium is easy to perform and is tolerated by most patients. However, a high volume of contrast medium (1500-2000 mL) is required to make the small intestine sufficiently distended. Administration via a nasojejunal catheter is efficient for small intestinal distension, but this procedure produces additional discomfort to patients. In addition, the speed of pumping the contrast medium via the nasojejunal catheter needs to be strictly controlled. Contrast agents used for small intestine distension can be divided into low-density contrast medium (e.g. water, methyl cellulose solution and air) and high-density contrast medium (e.g. 2% meglucamine diatrizoate solution)[4-6]. Previous studies[7] showed that low-density contrast agent can efficiently display small intestine wall enhancement that is located between the hypodensity of the intraluminal fluid and the hypodensity of the extraluminal fat tissues. Furthermore, a low-density contrast agent does not show interference with 3D angiography-like reconstructions. Our experience has shown that continuous and steady administration of 2.5% mannitol solution (2000 mL) orally within 30 min can ensure adequate distension of the entire small intestine. Mannitol solution (2.5%) is one type of isotonic solution that is not easily absorbed by the small intestine. Application of anticholinergic drugs before scanning can boost the distension of the small intestine. 16-MSCT and 64-MSCT can acquire data in the arterial and venous phases of contrast-enhanced scanning in thin slices after plain scanning. These data are used for multiplanar reconstruction to obtain isotropic images in sectional, coronal and sagittal directions, which is helpful to depict intestinal wall and small lesions. At the same time, maximum intensity projection and volume rendering technique reconstruction can be performed to identify blood vessel occlusion and stenosis[8-12].
CTE, which is easy to perform and produces less complications, can display the cavity and wall of small intestine, parenteral lymph nodes, mesentery, mesenteric vessels and the adjacent structures. It can be applied to observe a variety of intestinal pathological changes. In addition, CTE can accurately display the mucosal lesions, thickening of the wall and parenteral complications. CTE can also differentiate between outward growth and inward growth tumors, indicate whether the tumor is lobulated or whether there is internal necrosis, depict the depth of infiltration, and determine the types of pathological changes based on the general morphology of the tumors. Furthermore, it can also be used to identify metastasis over time using the entire abdominal scan, to make accurate preoperative staging, to design an appropriate treatment plan and to make a prognostic evaluation[8-14]. Conventional CT scans can show large intestinal tumors, however, they cannot provide accurate information about tumor infiltration in the intestinal wall. CTE can accurately determine the number of small intestinal tumors and can be used to make early diagnosis of small intestinal tumors. Thus, CTE is the primary choice for the detection and localization of small intestinal tumors[13]. Boudiaf’s study[14] showed that CTE has a high sensitivity (100%) and specificity (95%) in the diagnosis of small intestinal tumors. CTE can even detect tumors that are only 5 mm in diameter.
MRI
MRI has the power to produce excellent soft tissue contrast and multiplanar imaging without radiation exposure. With the introduction of fast imaging techniques and improvement of contrast agents, magnetic resonance enteroclysis (MRE) has been widely applied for the visualization of small intestinal diseases.
Adequate cleansing of the bowel and optimal bowel distension are also required for the performance of MRI. Therefore, it is important to select a contrast agent that is not harmful to humans and has the capability of maximal bowel distension and good contrast with the intestine wall. Furthermore, the contrast agents should not introduce artifacts that may interfere with diagnosis. Based on the bowel cavity signal of T1WI, MRI contrast agents can be divided into negative contrast agents (e.g. air, diluted barium sulfate solution and methyl cellulose) that can reduce the intestine cavity signal and positive contrast agents (e.g. diluted solution of superparamagnetic iron oxide, gadolinium agent with the mixture of methyl cellulose solution) that can increase the signal in the intestine cavity. Previous studies showed that application of 2.5% isotonic mannitol solution in the MRE can achieve optimal distension and efficient contrast within the intestine wall[15]. In T1WI sequences, mannitol solution shows low signal while the intestinal wall shows high signal after enhancement, which clearly depicts the structure of the wall. In T2WI sequences, mannitol solution shows high signal occupying the entire intestine and cavity filling defects with low signals can be fully displayed at this time. Similar to CTE, contrast agents for MRI can be administered orally or by using a nasojejunal catheter. Before scanning, an anticholinergic drug is applied to inhibit peristalsis and reduce artifacts caused by bowel movement.
Coronal and transversal fast spoiled gradient echo (FSPGR) sequence and SE sequence (breath hold) plain scans can be performed after the application of a gas contrast agent. In addition, the scan can be repeated after Gadolinium is administered. The disadvantage of the gas contrast agent is there are obvious magnetic susceptibility artifacts on FSPGR sequences. However, there are few artifacts caused by the gas on SE sequences, except for breathing artifacts. Liquid contrast agents do not have magnetic susceptibility artifacts and performance of SE sequences is not required. The modality consists of T2-weighted coronal single-shot fast spin echo (SSFSE) sequence and T1-weighted coronal FSPGR sequence (breath hold) in the plain scan. Furthermore, an enhancement scan with a coronal and transversal FSPGR sequence (breath hold) can be performed after Gadolinium administration. Fat saturation technology can be included with SE, SSFSE and FSPGR sequences, which are described above. With its maximal luminal distension and high spatial resolution, conventional small intestinal double contrast can depict small mucosal pathological changes, but it is difficult to observe intramural or extramural lesions and parenteral complications. Moreover, it brings additional radiation exposures. MRE is a radiation-free technology, which has good soft-tissue contrast and three-dimensional imaging capability. It can not only be used to observe the mucous membrane but also reveals the pathological changes around the intestine. MRE can easily display small intestinal structures, especially in tumors with intestinal obstruction based on the signal difference generated by intestinal wall and luminal contrast agents. Studies have showed that MRE is superior to capsule endoscopy in the diagnosis of small intestinal tumors[16].
MSCT AND MRI PERFORMANCE OF SMALL INTESTINAL TUMORS
Both primary and secondary tumors exist in the small intestine. However, the incidence of primary tumors is low, accounting for approximately 1%-3% of all gastrointestinal tumors[2]. Secondary intestinal tumors, which originate from other parts of the body and translocate to the small intestine, are common clinically and may cause symptoms similar to primary intestinal neoplasms. When a small intestinal tumor is suspected, multiple diagnostic technologies including enteroclysis, endoscopy, MSCT, MRI or other inspections can be performed. Among those technologies, MSCT and MRI can clearly display the structure of the tumor and determine the relationship between the tumor and surrounding tissues. In addition, MSCT and MRI can reveal if there are metastases in the mesenteric lymph nodes and liver, which is important for tumor grading and treatment selection.
Adenoma
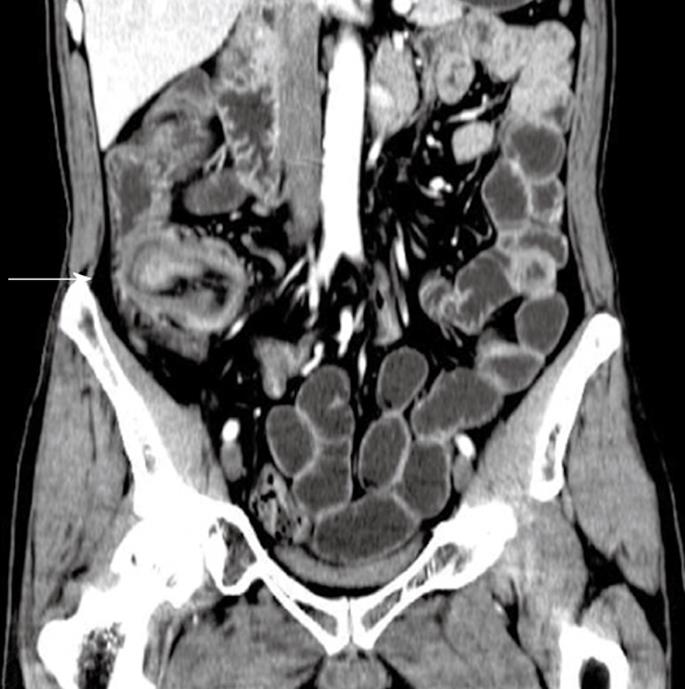
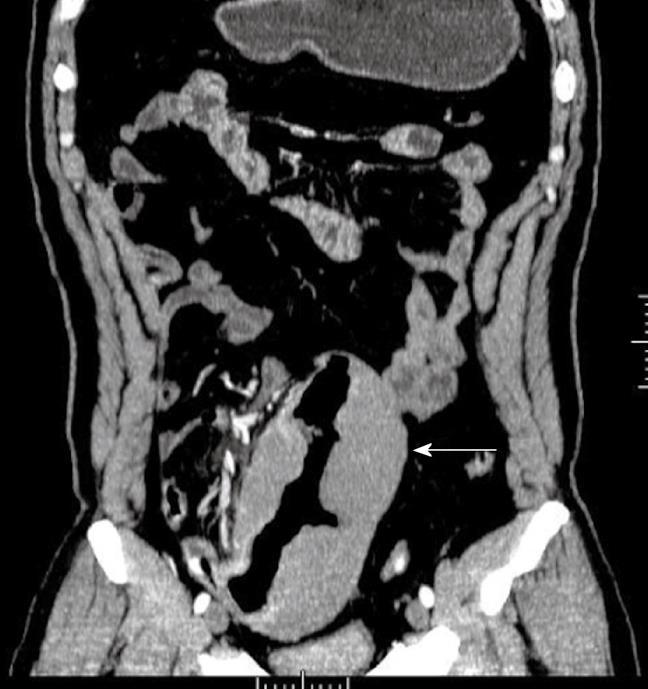
Adenoma, the most common benign tumor, is usually asymptomatic and diagnosed incidentally. Adenoma is predominantly observed in the duodenum, especially in the periampullary regions. Adenoma is usually single, small (1-3 cm), smooth or a lobulated polypoid lesion. Multiple lesions can be observed in familial adenomatous polyposis. The typical radiologic abnormalities are the sharply defined polyp-like masses and homogenous tissue density/signal and uniform contrast enhancement on CT/MR examination (Figure 1). In some cases, it is difficult to differentiate between adenoma and intestinal polyps.
Figure 1 Adenoma of the ileum in a 54-year-old man who presented with a 1-year history of abdominal fullness and pain with hematochezia.
Contrast-enhanced computed tomography (CT) scan shows a homogenous, moderate enhanced mass and ileocolic intussusceptions with thickening of the terminal ileum (white arrow).
Stromal tumor
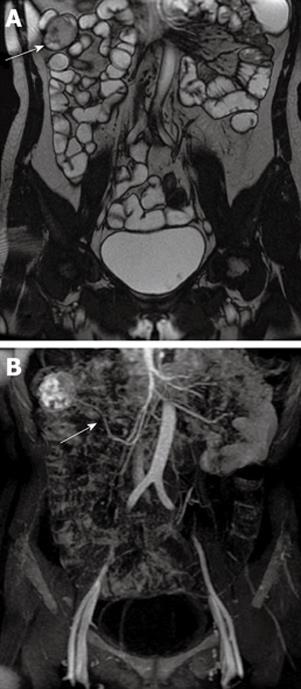
Small intestinal stromal tumor accounts for approximately 20%-30% of all gastrointestinal stromal tumors. Small intestinal stromal tumor occurs primarily in elderly patients and is usually located in the jejunum, followed by the duodenum and ileum[17]. It is thought that stromal tumor originates from the intestinal cell of Cajal, and can be large or small in size, single or multiple in number, intraluminal, mural, or extraserosal in location. Stromal tumors are typically shown as well-circumscribed masses. Hemorrhage, cystic degeneration and necrosis may occur in the center of the tumor. CT imaging shows a round soft tissue density mass with an adjacent thickened wall. Following intravenous administration of contrast media, it is typically seen as an enhanced mass with areas of low attenuation from hemorrhage, necrosis, or cyst formation. Similar to CT scanning, MRI can depict tumors and obtain information about surrounding structures. The signal of a tumor is heterogeneous on MRI, mainly due to internal hemorrhage and necrosis. On T1-weighted images, it has low or intermediate signal intensity. On T2-weighted images, it has heterogeneous high signal intensity[18-20] (Figure 2).
Figure 2 Gastrointestinal stromal tumor of the ileum in a 52-year-old woman who presented with persistent abdominal pain and hematochezia.
A: Gadolinium-enhanced T2-weighted image shows a well-circumscribed mass with heterogeneous intermediate signal intensity (white arrow); B: MIP image shows the tumor was supplied by the superior mesenteric artery (white arrow). MIP: Maximum intensity projection.
Lipoma
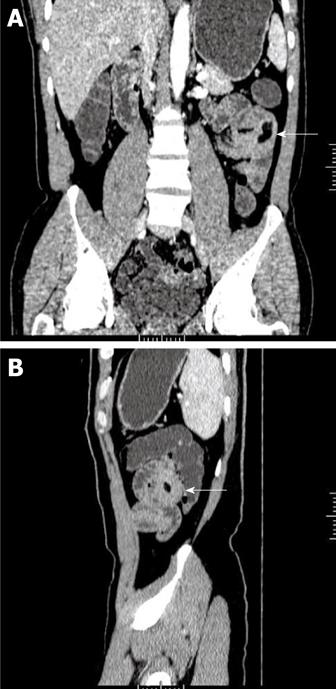
Lipoma, which is also one of the common benign tumors of the small intestine, accounts for about 15% of small intestinal tumors. It originates from adipose tissue in the intestinal submucosa and usually locates in the remote small bowel. Lipoma is usually observed as a single tumor that is 1-6 cm in diameter. Lipoma can cause intussusception, which is the most frequent triggering factor of intussusception in adults. Intussusception is usually the first symptom of lipoma. The typical performance of lipoma on CT/MR images is the sharply demarcated, homogeneous fat-tissue density/signal mass closely related to the slightly thickened intestinal wall. Hounsfield units range between -80 and -120[21] (Figure 3). Fat suppression MR sequences can provide additional assistance for a clear diagnosis.
Figure 3 Lipoma of the jejunum in a 56-year-old man who presented with a 3-year history of hematochezia.
A: Image (Coronal MPR) of contrast-enhanced CT scan shows a homogeneous fat-tissue density mass in jejunal smooth muscle (white arrow); B: Image (Sagittal MPR) shows obviously thickened intestinal wall (white arrow). MPR: Multiplanar reconstruction.
Adenocarcinoma
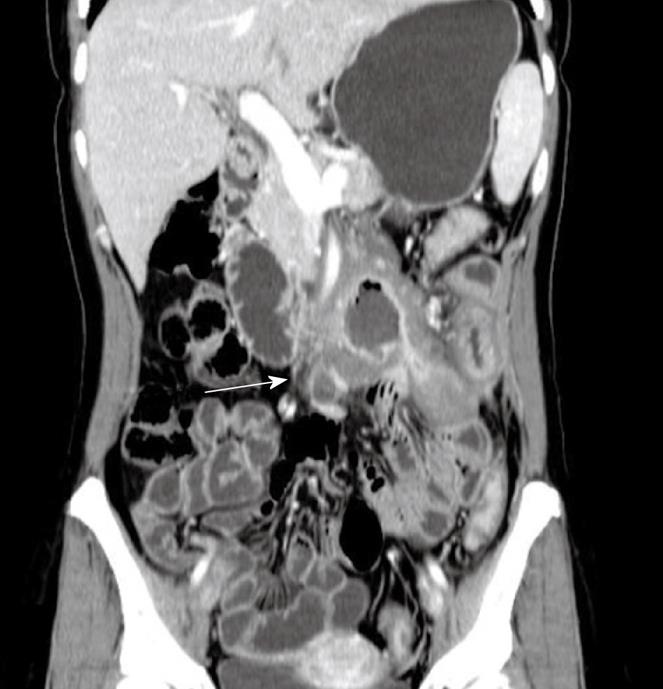
Adenocarcinoma is the most common primary malignant tumor in the small intestine. It occurs in the junction areas between the duodenum and the jejunum. Mucosal damage and disruption, irregular bowel wall thickening and concentric mesocaval narrowing are typical observations. Sometimes, ring-like invasion, tumor-like nodules as well as ulcers can also be observed. On CT scanning, adenocarcinoma is typically manifested as an annular lesion in the proximal small intestine without a clear edge. In addition, moderate enhancement can be observed after intravenous administration with contrast medium. However, the intensity of the enhancement is less than that of stromal tumors. MRI shows asymmetric wall thickening of the small intestine, luminal narrowing, and significant heterogeneous enhancement[19]. MRI and MSCT can observe early small tumors under the mucous membrane, can evaluate the depth of invasion of the bowel wall as well as extension outside the wall, and can display tumor metastasis in mesentery, omentum, retroperitoneal and other organs. In particular, MRI and MSCT can clearly show a tumor in the horizontal part of the duodenum and determine its relationship with the pancreas, aorta, and mesenteric vessels[10] (Figure 4).
Figure 4 Adenocarcinoma of the jejunum in a 38-year-old woman who presented with weight loss, abdominal pain and an abdominal mass.
Contrast-enhanced CT scan shows a large, moderate enhanced soft tissue mass without a clear edge and infiltration of mesenteric vessels (white arrow).
Carcinoid
Carcinoid is a slow-growing malignant tumor, originating from enterochromaffin cells of Kulchitsky and accounting for 1.5% of all gastrointestinal tumors. Approximately 95% of the gastrointestinal carcinoids occur in the appendix, rectum and small intestine. Besides the appendix, the small intestine is the most common site of gastrointestinal carcinoids. The small intestinal carcinoid is also the most common gastrointestinal carcinoid with clinical symptoms and often occurs in the distal ileum. Most patients with carcinoid syndrome have liver metastases, although in rare cases, the humoral load from a primary tumor may overwhelm the liver and the capacity of the lungs to metabolize serotonin. Local connective tissue proliferation stimulated by tumor secretion of 5-hydroxytryptamine is the typical appearance on CT scanning, including stellate soft tissue density mass[21] calcification of some tumors. Linear strands within the mesenteric fat are probably thickened and the vascular bundles are retracted, which are indications of peritumoral desmoplastic reaction. Small tumors may be best visualized on gadolinium-enhanced T1-weighted MR images obtained with fat suppression, where they are manifested as nodules or focal areas of mural thickening with moderately intense gadolinium enhancement[22].
Lymphoma
Primary lymphoma of the intestine occurs most commonly in the ileum, especially in the terminal ileum. Intestinal lymphomas may be polypoid, ulcerative, constrictive or aneurismal, with the polypoid growth being the most common[21]. Small intestinal lymphoma may appear as a circumferential bulky mass in the intestinal wall or an exophytic mass associated with thickening of intestinal walls, dilatation of the lumen and regional lymph nodes. CT and MRI imaging are useful to observe the dilated intestinal loops, regular and diffuse thickening of the intestinal walls, perforation, fistula, mesenteric edema, lymph node enlargement and other complications[23]. Bowel-wall thickening combined with mesenteric and/or retroperitoneal lymph node enlargement, shown as “sandwich sign,” are the main features of performance of the primary small intestine lymphoma on CT images. Aneurysmal dilatation of the lumen and mesenteric lymph node enlargement may be indications of non-Hodgkin’s lymphoma (Figure 5). Diffuse thickening of the intestinal wall with multiple lesions, multiple site involvement with the same performance and multiple lymph node enlargement are suggestive of lymphoma.
Figure 5 Diffuse large B-cell lymphoma of the ileum in a 46-year-old man who presented with a 2-year history of abdominal pain and intermittent diarrhea and was previously suspected as having Crohn’s Disease.
Contrast-enhanced CT scan shows diffuse, homogeneous wall thickening of the ileum, aneurysmal dilatation of the lumen and no bowel obstruction (white arrow).
Metastatic tumors
Tumor cells can spread to the small intestine through extension, hematogenous dissemination, lymphatic channels and intraperitoneal seeding. Metastatic tumors can cause symptoms similar to primary small intestinal tumors. Melanoma, carcinoma of the cervix, and lung, breast and soft tissue tumors are the common primary tumors that may spread to the small intestine. Obstruction and bleeding are common symptoms of small bowel metastases. In patients with a known history of malignancy, obstructive symptoms or bleeding from the gastrointestinal tract are indications of metastatic tumors[21].
CT and MR play an important role in the diagnosis of metastatic tumors in the small intestine. Metastatic lesions in mesentery, peritoneal surfaces, lymph nodes and the intestinal wall can be identified by CT/MR scanning. The performance of metastatic tumors through extension results as irregular masses of intestinal wall linked to the primary tumor. Metastatic lesions in the intestinal wall can be submucosal nodules. Infiltrating lesions with diffuse thickening of the intestinal wall may be observed as well, which may result in irregular lumen narrowing. The formation of multiple metastatic nodules in the serosa, omenta and mesentery may be seen if the primary tumors contain a lot of mucus. Infiltration in mesentery and fat inside the abdominal cavity can also be seen, which may result in the increased density and thickening of the mesenteric vascular bundle. Omental fat infiltration is termed as “omental cake”. The shift and adhesion of the small intestine can be formed by pressure due to the large metastatic mass in the mesentery. Gadolinium-enhanced MR images with fat suppression can be helpful for the detection of metastases.
PROBLEMS AND PROSPECTS OF MSCT AND MRI EXAMINATION IN SMALL INTESTINE NEOPLASMS
The diagnosis of small intestine tumors, especially early detection and differential diagnosis of tumors, is still difficult although many sensitive, direct and indirect techniques have been applied. MSCT is widely used in the diagnosis of small intestine tumors, but the images obtained by MSCT are static, instantaneous and are unable to observe the details of the small intestinal mucosa. More importantly, these images cannot display the functional changes of small intestine[13,24,25]. Meanwhile, MSCT technology with thin slices, large-scale scanning, high-resolution and multi-phase data acquisition brings increased radiation exposure, which must be given sufficient attention. Measures must be taken to optimize the scanning program to reduce radiation exposure to the patients, especially with children[26]. Though there are still many shortcomings, such as relatively higher cost, longer time required for scanning, more mobile artifacts compared to MSCT scanning, MRI scanning is able to obtain high soft tissue contrast resolution and multi-dimensional imaging. In addition, it is non-invasive and requires no radiation exposure, which is helpful to popularize the MRI examination for small intestines[27-32]. New technologies, such as functional cine MR imaging, have been applied to observe dynamic images of the small intestine[31,32]. With new introductions of fast imaging techniques and shorter examination times, MRI scanning in the detection of small intestine diseases will be widely applied.
In conclusion, with the rapid development and advancement of imaging technologies, MSCT and MRI techniques will become main approaches in the diagnosis of small intestinal tumors. CTE or MRE may be the first choice for examination and diagnosis of small intestinal tumors, and in addition, combinational use of new techniques (MSCT or MRI enterography) with traditional X-ray and endoscopy (capsule endoscopy and balloon-assisted enteroscopy) will provide comprehensive information on localization, scope and features of the small intestinal neoplasms, leading to earlier diagnosis and treatment of small intestine neoplasms.