Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.110206
Revised: June 23, 2025
Accepted: July 14, 2025
Published online: August 15, 2025
Processing time: 75 Days and 3.3 Hours
Esophageal squamous cell carcinoma (ESCC) is a prevalent gastrointestinal ma
Case 1: In this patient, the submucosal tumor-like lesion was initially thought to be a gastrointestinal stromal tumor. T1-stage ESCC was then diagnosed by gastro-endoscopy during subsequent evaluation. The lesion was finally confirmed to be submucosal metastasis of ESCC following pathological analysis. The patient has been receiving treatment for over seven months, with an encouraging outcome. Case 2: In this patient, the initial diagnostic phase showed esophageal and gastric lesions which were classified as two distinct conditions: Early-stage ESCC and a gastrointestinal stromal tumor, respectively. However, postoperative pathology revealed ESCC with IGM. The patient received adjuvant chemotherapy following surgical intervention. Regrettably, the patient was lost to follow-up shortly after surgery.
The occurrence of IGM in T1-stage ESCC is rare. IGM should be taken into consi
Core Tip: This case report presents the diagnosis and management of two rare cases of T1-stage esophageal squamous cell carcinoma with intramural gastric metastasis, which were initially considered to be gastrointestinal stromal tumors. In clinical practice, it is necessary to pay attention to this uncommon condition in the diagnosis of gastric submucosal tumor-like lesions. We hope that this study will provide guidance for the diagnosis and therapeutic strategies of this disease.
- Citation: Wang HY, Song C, Ma J, Sun HQ, Yuan P, Liu ZX, Dou WJ. Challenges in the diagnosis of esophageal cancer with intramural gastric metastasis: Two case reports. World J Gastrointest Oncol 2025; 17(8): 110206
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/110206.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.110206
Esophageal cancer is the seventh most prevalent malignant tumor globally and is categorized into two histological types: Esophageal squamous cell carcinoma (ESCC), which constitutes approximately 90% of cases, and adenocarcinoma, which represents about 10% of cases[1]. ESCC is predominantly observed in Asia, South America, and Africa. Cancer cells infiltrating the mucosa or submucosa, without invasion into the muscularis propria, is regarded as T1-stage ESCC[2]. Meta
SMTs are masses or elevated lesions protruding into the gastrointestinal lumen, that originate from the submucosa, muscularis propria or even serosa of the gastrointestinal wall[6]. These lesions, encompassing a diverse range of histological types, are challenging to diagnose in clinical practice. Although gastrointestinal stromal tumors (GISTs) are the predominant type of gastrointestinal SMTs, uncommon lesions, such as tumor metastases, should also be considered during diagnosis[7]. Gastro-endoscopy, ultrasound (EUS)-guided fine needle aspiration (FNA), contrast-enhanced computed tomography (CT)/magnetic resonance imaging and positron emission tomography (PET)-CT are valuable tools for diagnosing gastric SMTs[7].
In this report, we present two rare cases of T1-stage ESCC accompanied by submucosal metastasis to the stomach, which were initially thought to be GISTs. By integrating the treatment processes of these two patients, we conducted a literature review on the advancements in the diagnosis and management of ESCC with intramural gastric metastasis (IGM), and provide novel insights for the clinical diagnosis and treatment of this condition.
Case 1: The patient had a submucosal gastric lesion for over one year, accompanied by dysphagia persisting for more than four months.
Case 2: The patient complained of right upper abdominal pain persisting for over two months.
Case 1: In 2023, the 76-year-old male presented with a submucosal lesion measuring approximately 2 cm in diameter in the gastric cardia-fundus during a physical examination at regional medical facilities. Considering his advanced age and small lesion size, the patient did not undergo further diagnosis and treatment. One year later, the patient exhibited symptoms of dysphagia for more than four months.
Case 2: In June 2019, the 66-year-old male presented to our hospital with complaints of right upper abdominal pain persisting for over two months.
Case 1: The patient had no relevant past illness.
Case 2: The patient had no relevant past illness.
Case 1: The patient had a 40-year smoking history, with 10 cigarettes daily and infrequent alcohol consumption.
Case 2: The patient had no smoking history or drinking history.
Cases 1 and 2: No abnormalities were found during physical examination.
Cases 1 and 2: No laboratory findings necessitated special attention.
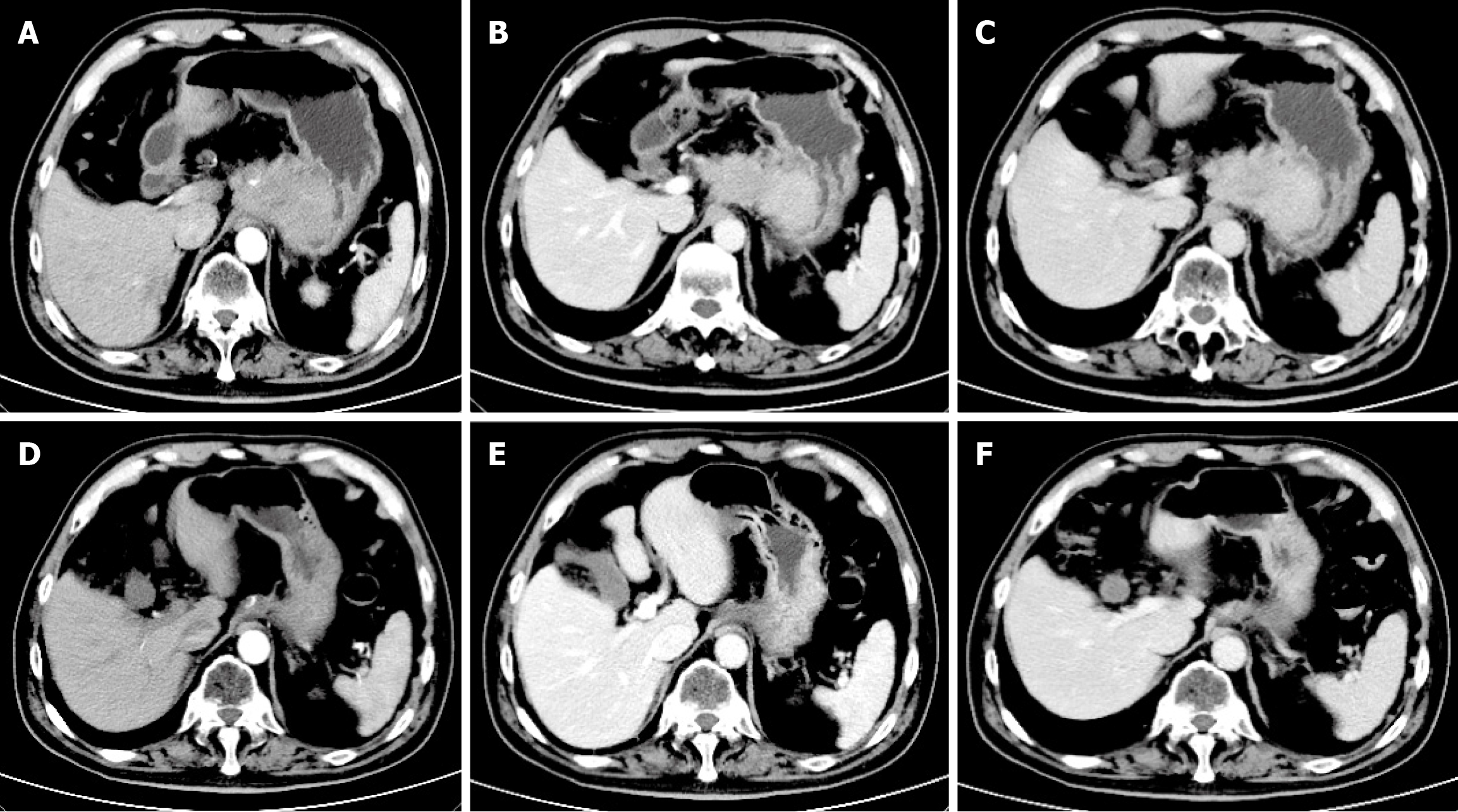
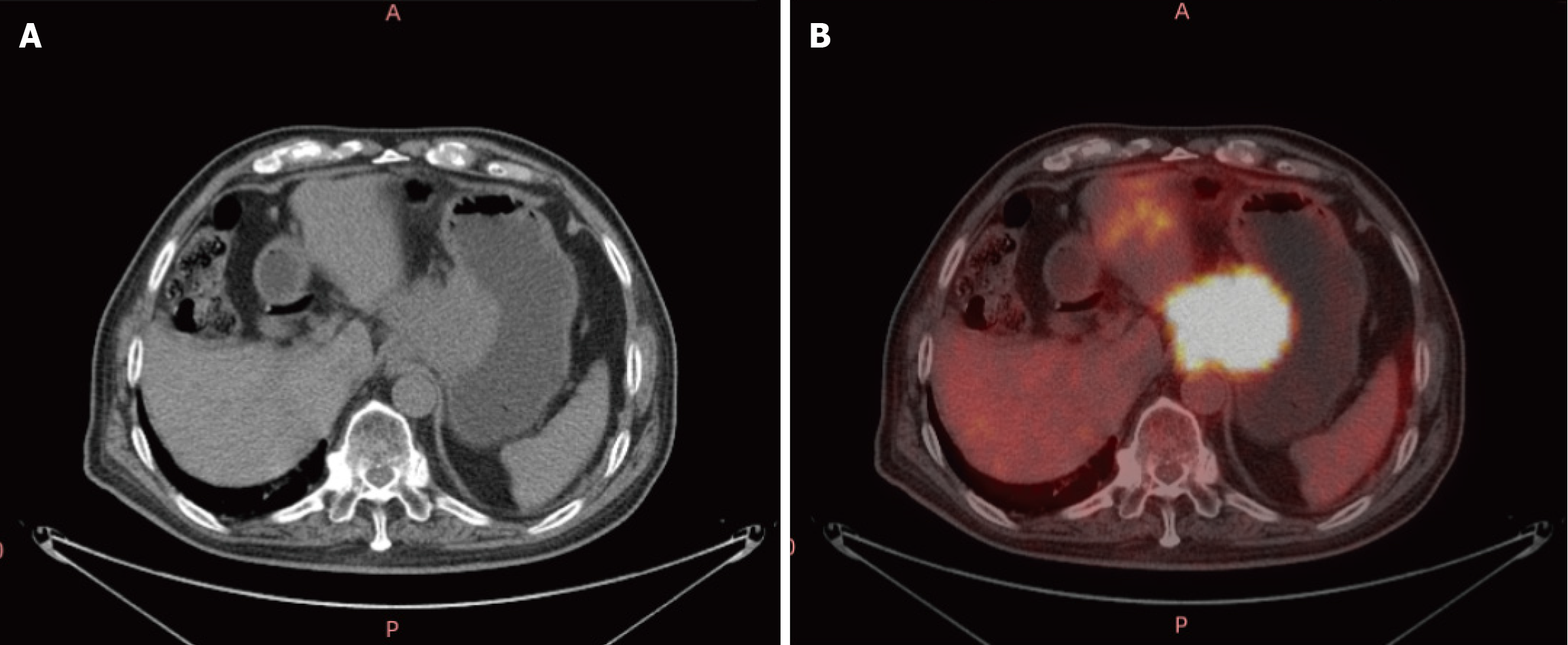
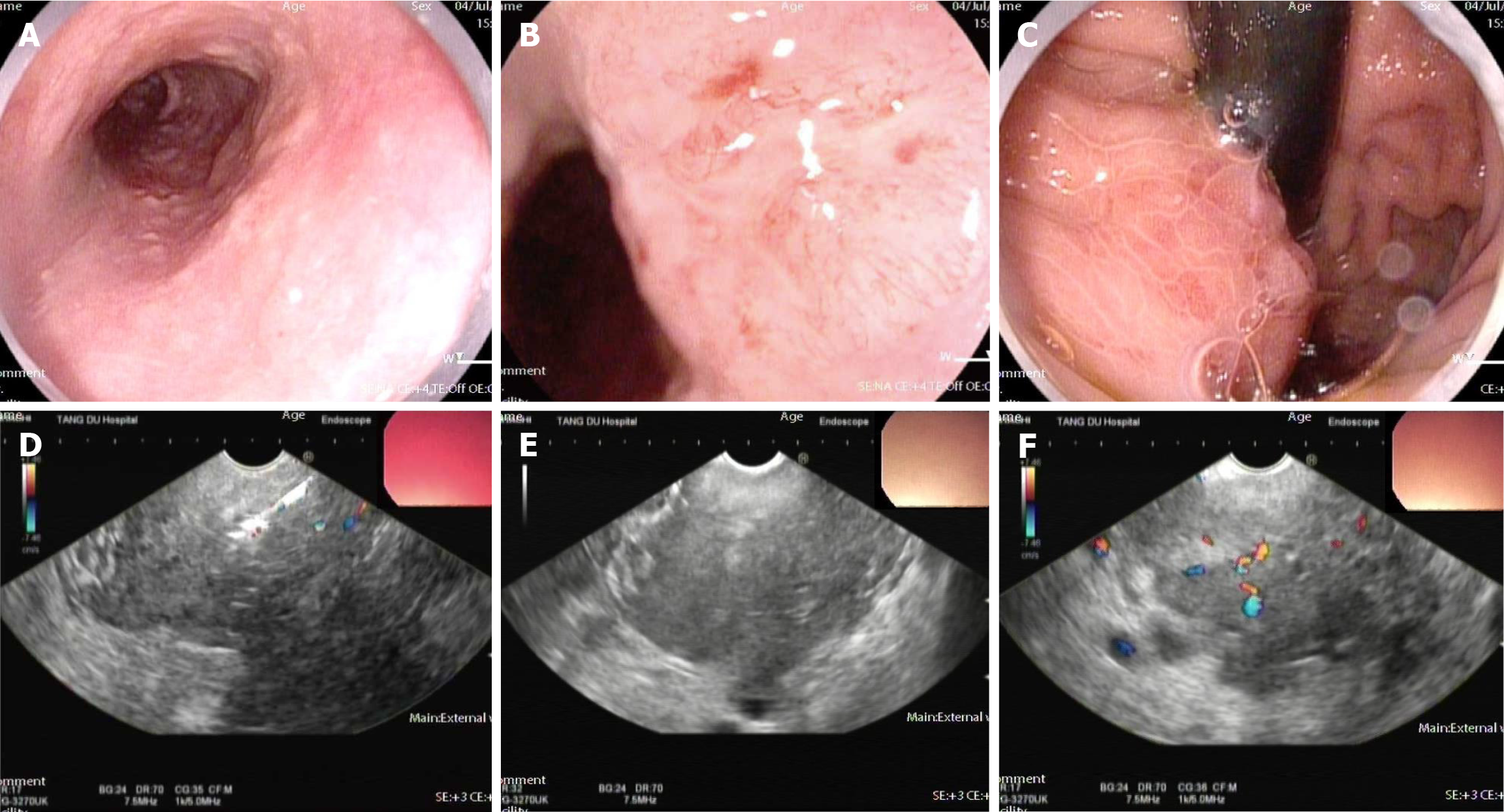
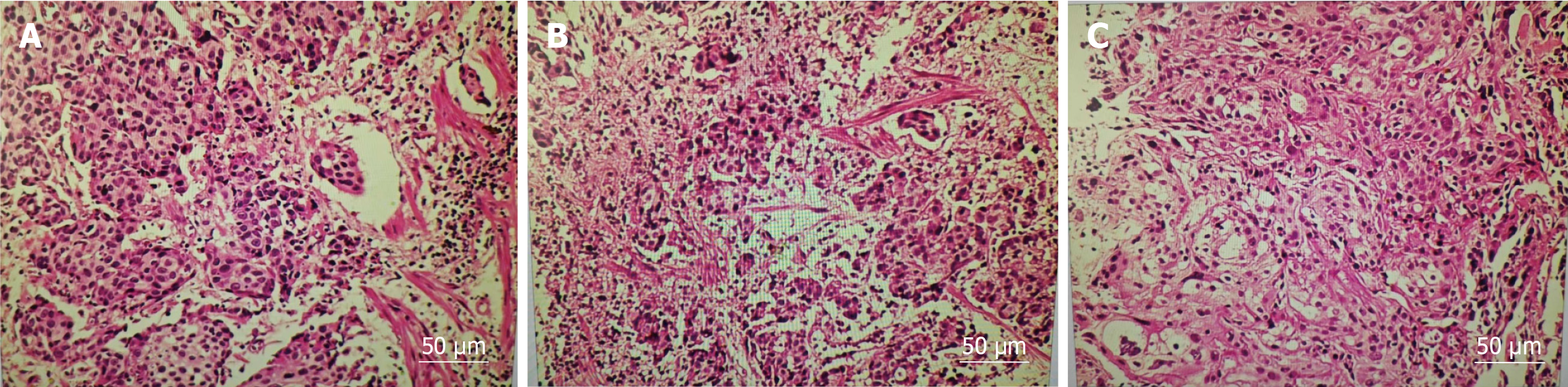
Case 1: Enhanced CT (Figure 1) conducted at our hospital revealed that the lesion size had increased to 5.8 cm × 7.7 cm × 6.0 cm and it surrounded the celiac trunk. Furthermore, multiple enlarged lymph nodes were observed in both abdominal and retroperitoneal regions. A PET-CT scan indicated a malignant lesion in the cardia-fundus of the stomach, while no significant malignant lesions were observed in other organs (Figure 2). Gastro-endoscopy showed a superficial, flat, slightly rough, and red esophageal lesion located 34-37 cm from the incisor teeth, with the widest part accounting for half of the esophageal lumen, as well as a submucosal lesion in the cardia-fundus (Figure 3). Endoscopic biopsy and EUS-FNA were performed to obtain pathological tissues, which indicated squamous cell carcinoma in both the esophagus and cardia-fundus (Figure 4). Pathological examinations confirmed that the initial submucosal lesion in the cardia-fundus was not a GIST as initially suspected, but submucosal metastasis originating from ESCC.
Case 2: Gastro-endoscopy revealed a patchy mucosal roughness in the esophagus 30-32 cm from the incisors and a submucosal lesion in the gastric fundus with smooth surface mucosa. Enhanced CT indicated the presence of a space-occupying lesion along the lesser curvature of the stomach, but no lesion in the esophagus. Pathological biopsy by gastro-endoscopy indicated esophageal cancer, while no evidence of malignancy was detected in the gastric lesion. EUS showed that the esophageal lesion was confined to the mucosal layer, while the mass in the gastric fundus was a hypoechoic lesion originating from the muscularis propria. PET-CT demonstrated abnormally increased glucose metabolism in the esophageal wall and gastric lesser curvature. Based on the above examinations, the two lesions were considered T1-stage ESCC and a GIST, respectively.
The patient was ultimately diagnosed with ESCC (T1bNxM1) accompanied by IGM that was characterized by exophytic growth of the gastric wall and encirclement of the celiac trunk.
Postoperative pathology confirmed submucosal ESCC grade II-III. The gastric lesion was also confirmed to be squamous cell carcinoma, infiltrating the surrounding adipose tissues. No lymph node metastases were observed. The patient was diagnosed with ESCC (T1bN0M1) and IGM.
Due to the absence of surgical indication, the patient began chemotherapy and immunotherapy in June 2024 with the administration of sintilimab at a dose of 200 mg in combination with cisplatin on day 1, in addition to albumin-bound paclitaxel on days 1 and 8. At the time of submission, the patient had received 7 cycles of combination treatment. A follow-up CT scan on December 26, 2024 (Figure 1) revealed no significant lesions in the esophagus.
The patient underwent a partial esophagectomy and gastrectomy.
A follow-up CT scan on December 26, 2024 (Figure 1) revealed no significant lesions in the esophagus. Additionally, the gastric submucosal metastasis reduced to 1.7 cm × 3.4 cm × 2.2 cm in size. The metastatic lymph nodes in the abdominal cavity and retroperitoneum had also shrunk. The decrease in lesions exceeded 30% in volume, indicating partial remission. The patient continues to receive ongoing treatment.
Adjuvant chemotherapy was recommended postoperatively. However, the patient was lost to follow-up subsequent to the procedure, and long-term prognosis is unknown.
Metastasis plays an important role in the progression of ESCC and is closely associated with tumor prognosis. The incidence of metastases in newly diagnosed ESCC is approximately 35%-40%[8]. The most common metastatic patterns are lymphatic metastasis, hemorrhagic metastasis to distant organs, and direct invasion of adjacent tissues in ESCC[9]. IM is another metastasis pathway. The incidence of IGM is higher in advanced-stage disease than that in early-stage disease[5,10]. In our study, we present two noteworthy cases in which gastric submucosal lesions were initially discovered and thought to be GISTs. During the following diagnostic process, primary T1-stage ESCC was confirmed, and the submu
IM is characterized by a discontinuous spread of the tumor, resulting in metastatic lesions in distant locations such as the esophagus, stomach, or intestines[5]. Research indicates that IM of ESCC predominantly exhibit the following characteristics: (1) Metastases are more frequently observed in the esophagus or stomach compared to the intestine; (2) These foci are often located a considerable distance from the primary tumor; and (3) Metastatic SMTs typically lack intraepithelial components and are sometimes associated with erosive alterations[11]. The occurrence of IM in patients with stage I ESCC is approximately 11%, while IGM is observed in only 1% to 4.58% of individuals undergoing surgical resection for esophageal cancer[8]. Another investigation involving 1259 post-surgical patients with ESCC revealed that 93 individuals (7.4%) developed IM, with 13 cases (1%) specifically exhibiting IGM[5]. ESCC usually metastasizes to the lesser curvature of the upper third of the gastric wall, and IGM is most frequently located in the cardia and fundus of the stomach. This distribution is attributed to the submucosal lymphatic drainage from the lower and middle esophagus connected to the cardia and fundus[10,12,13]. IGM predominantly presents as solitary lesions rather than multiple lesions[14]. The most common symptoms of gastric metastases include upper gastrointestinal bleeding, anemia, and epigastric distension and discomfort. However, some patients may present with nonspecific symptoms or remain asymptomatic[14]. Endoscopic examination of IGM reveals multiple features such as multiple nodules, ulceration, polypoid tumors, diffuse infiltration, and SMT-like lesions with exophytic morphology, which require to be differentiated from primary gastric cancers or other SMTs, such as GISTs. In addition, the primary esophageal cancer can be very small, compared with gastric metastatic lesions[8,12]. As the blood supply to the stomach is more abundant than that to the esophagus, this is conducive to the rapid growth of IGMs. In our cases, gastric SMT-like lesions were initially discovered and this was the main reason the patients sought medical treatment. Based on clinical experience, the space-occupying lesions were initially thought to be GISTs. However, ESCCs in situ and gastric submucosal metastases were pathologically diagnosed by endoscopic biopsy and EUS-FNA. Due to the findings above, we suggest that when a gastric SMT is encountered in clinical practice, uncommon lesions also need to be taken into consideration. Therefore, further examinations, such as gastro-endoscopy, EUS, contrast-enhanced CT/magnetic resonance imaging, PET-CT, and so on, are recommended to assist in the diagnosis and prevent misdiagnosis and missed diagnosis.
The mechanism of IGM in ESCC, involving multiple aspects, such as the characteristics of lymphatic drainage, the anatomical structure of the esophagus and stomach, and the biological behavior of tumor cells, is a complex biological process. IGM may be attributed to the presence of diminutive lymphatic vessels contained in the mucosa lamina propria or submucosa of the esophagus and stomach[15]. The longitudinal lymphatic channels within the mucosal layers are not directly interconnected, but their submucosal lymphatic vessels maintain a direct connection[16]. Therefore, esophageal cancer cells could travel along the lymphatic system in the submucosa to the gastric wall and eventually form IGM. Primary esophageal carcinomas located in the lower portion of the esophagus are more prone to IGM. As the tumor is adjacent to the stomach, after penetrating the muscularis propria, it can directly spread to the cardia, and invade the gastric wall by breaking through the anatomical barrier at the esophagogastric junction. From molecular mechanism studies, it is known that cancer-associated fibroblasts (CAFs), M2 macrophages, and regulatory T cells facilitate IM of esophageal cancer through their modulatory effects on the tumor microenvironment by forming an immune barrier against the CD8+ T cell-mediated anti-tumor immune response[17]. CAFs play a significant role in the establishment of the premetastatic microenvironment and contribute to metastasis[18]. CAFs facilitate the proliferation, invasion, and metastasis of esophageal cancer cells through the secretion of various cytokines, chemokines, and exosomes[18]. It has been shown that secretion of wingless-type MMTV integration site family member 2 by CAFs suppresses anti-tumor T cell responses through the suppressor of cytokine signaling 3/phosphorylated Janus kinase 2/phosphorylated signal transducer and activator of transcription 3 pathway[9]. Transforming growth factor-β and matrix metalloproteinases secreted by CAFs inhibit the functionality of regulatory T cells[9]. CAFs also exert a negative regulatory effect on CD8+ T cell responses by facilitating the recruitment of myeloid-derived suppressor cells or by secreting immunosuppressive factors, including interleukin-6 and tumor necrosis factor-α[19]. M2 macrophages, a subset of tumor-associated macrophages, exhibit an enhanced capacity to secrete interleukin-10[20], which enhances the proliferation, invasion, migration, and resistance to apoptosis of esophageal cancer cells by attenuating the anti-tumor activity of CD8+ T cells[20]. It was demonstrated that exosomes secreted by M2 macrophages facilitate the migration of ESCC via the long-noncoding RNA actin filament associated protein 1 antisense RNA 1/microRNA-26a/activating transcription factor 2 signaling pathway[21]. These mechanisms collectively contribute to the immune evasion of esophageal cancer cells, which is the final step in metastasis. In addition, metabolic reprogramming-mediated alterations in tumor behavior also have a crucial effect on metastasis. Glucose, amino acid, and fatty acid metabolism within the gastric microenvironment could support the Warburg effect of tumor cells and promote the proliferation and progression of metastatic foci[22-24].
IGM is generally regarded as an indicator of advanced disease with poor patient prognosis[10,13]. The five-year survival rate for ESCC concomitant with IGM is typically lower than that observed for standard ESCC[25]. Surgery, radiotherapy, and chemotherapy are the standard treatment options for patients with ESCC complicated with IGM[26]. The management is contingent upon the histopathological characteristics of the primary tumor, the dimensions of the metastatic lesions, the invasion of adjacent tissues, and the patient’s general conditions[13]. Adjuvant chemotherapy comprising 5-fluorouracil and cisplatin represents the standard therapeutic approach for patients with locally advanced (stage II or III) ESCC[26]. A comparative study evaluating the efficacy of cisplatin/fluorouracil, cisplatin/fluorouracil combined with radiotherapy, and cisplatin/fluorouracil in conjunction with docetaxel in the treatment of ESCC revealed three-year survival rates of 62.6%, 68.3%, and 72.1%, respectively[26]. These findings indicate that the combination of platinum-based chemotherapy with albumin-bound paclitaxel is associated with a more favorable prognosis for ESCC. In recent years, the role of immune checkpoint inhibitors in the treatment of ESCC has garnered heightened attention. The Chinese Society of Clinical Oncology (2024) endorses the combination of cisplatin and albumin-bound paclitaxel with sintilimab as a first-line therapeutic strategy for advanced, unresectable ESCC. In the present study of case 1, the submucosal metastasis had already surrounded the celiac trunk at the time of diagnosis, and the opportunity for surgery was lost. Therefore, the patient received treatment with cisplatin/albumin-bound paclitaxel in combination with sintilimab. After 7 cycles of treatment, gastric metastasis foci achieved partial remission. In case 2, the patient underwent surgery and postoperative adjuvant chemotherapy was recommended. Regrettably, the patient was subsequently lost to follow-up; thus, we were unable to observe the long-term prognosis of this patient.
This case report offers a detailed description of two cases which were initially misdiagnosed as GISTs but finally as IGM of primary T1-stage ESCC. In clinical practice, it is necessary to be acutely aware of this uncommon condition and to take it into account in the differential diagnosis of gastric SMTs to ensure an accurate diagnosis and optimal patient management.
| 1. | Deboever N, Jones CM, Yamashita K, Ajani JA, Hofstetter WL. Advances in diagnosis and management of cancer of the esophagus. BMJ. 2024;385:e074962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 2. | An L, Li M, Jia Q. Mechanisms of radiotherapy resistance and radiosensitization strategies for esophageal squamous cell carcinoma. Mol Cancer. 2023;22:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 3. | Puhr HC, Prager GW, Ilhan-Mutlu A. How we treat esophageal squamous cell carcinoma. ESMO Open. 2023;8:100789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Reichenbach ZW, Murray MG, Saxena R, Farkas D, Karassik EG, Klochkova A, Patel K, Tice C, Hall TM, Gang J, Parkman HP, Ward SJ, Tétreault MP, Whelan KA. Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res. 2019;144:95-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 5. | Ebihara Y, Hosokawa M, Kondo S, Katoh H. Thirteen cases with intramural metastasis to the stomach in 1259 patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2004;26:1223-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 65.7] [Reference Citation Analysis (1)] |
| 8. | Yang MQ, Sun MJ, Zhang HJ. Mucosal esophageal carcinoma following endoscopic submucosal dissection with giant gastric metastasis: A case report and review of literature. World J Gastroenterol. 2023;29:5935-5944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Huang TX, Tan XY, Huang HS, Li YT, Liu BL, Liu KS, Chen X, Chen Z, Guan XY, Zou C, Fu L. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut. 2022;71:333-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 10. | Jagtap SV, Khoja S, Jagtap SS, Gudur R, Janugade H. Leptomeningeal Carcinomatosis Secondary to Esophageal Cancer Diagnosed on Cytology. J Neurosci Rural Pract. 2020;11:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Yoshida E, Kimura Y, Kyuno T, Kawagishi R, Sato K, Kono T, Chiba T, Kimura T, Yonezawa H, Funato O, Kobayashi M, Keira Y, Onuma K, Inoue H, Takagane A, Takemasa I. Gastric intramural metastasis caused by needle tract seeding after preoperative fine needle aspiration for pancreatic body cancer subsequently resected by total pancreatectomy: a case report and literature review. World J Surg Oncol. 2023;21:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Sun K, Lv H, Chen B, Nie C, Zhao J, Wang S, Wang J, Xu W, Chen X. Dawning precision treatment for gastric cancer: The latest biomarkers. J Transl Int Med. 2021;9:228-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Hirano K, Nomura K, Ochiai Y, Hayasaka J, Suzuki Y, Mitsunaga Y, Odagiri H, Masui A, Kikuchi D, Hoteya S. Metastatic Gastric Tumors: Clinical and Endoscopic Features. Cureus. 2024;16:e58678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kim MY, Jung HY, Choi KD, Song HJ, Lee JH, Kim DH, Choi KS, Kim SA, Lee GH, Kim JH. Solitary synchronous metastatic gastric cancer arising from t1b renal cell carcinoma: a case report and systematic review. Gut Liver. 2012;6:388-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Zhu L, Xia W, Wang F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res. 2018;10:6295-6303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Ping Q, Yan R, Cheng X, Wang W, Zhong Y, Hou Z, Shi Y, Wang C, Li R. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther. 2021;28:984-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 18. | Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1314] [Cited by in RCA: 1324] [Article Influence: 331.0] [Reference Citation Analysis (0)] |
| 19. | Mi X, Xu R, Hong S, Xu T, Zhang W, Liu M. M2 Macrophage-Derived Exosomal lncRNA AFAP1-AS1 and MicroRNA-26a Affect Cell Migration and Metastasis in Esophageal Cancer. Mol Ther Nucleic Acids. 2020;22:779-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | Huang S, Guo Y, Li Z, Zhang Y, Zhou T, You W, Pan K, Li W. A systematic review of metabolomic profiling of gastric cancer and esophageal cancer. Cancer Biol Med. 2020;17:181-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Zhu X, Wang K, Liu G, Wang Y, Xu J, Liu L, Li M, Shi J, Aa J, Yu L. Metabolic Perturbation and Potential Markers in Patients with Esophageal Cancer. Gastroenterol Res Pract. 2017;2017:5469597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Zhang H, Zhao C, Liu Q, Zhang Y, Luo K, Pu Y, Yin L. Dysregulation of fatty acid metabolism associated with esophageal inflammation of ICR mice induced by nitrosamines exposure. Environ Pollut. 2022;297:118680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Watanabe M, Kuwano H, Araki K, Kawaguchi H, Saeki H, Kitamura K, Ohno S, Sugimachi K. Prognostic factors in patients with submucosal carcinoma of the oesophagus. Br J Cancer. 2000;83:609-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Yang H, Wang F, Hallemeier CL, Lerut T, Fu J. Oesophageal cancer. Lancet. 2024;404:1991-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 25. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1051] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 26. | Hara H, Tahara M, Daiko H, Kato K, Igaki H, Kadowaki S, Tanaka Y, Hamamoto Y, Matsushita H, Nagase M, Hosoya Y. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |