Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.109579
Revised: June 5, 2025
Accepted: July 9, 2025
Published online: August 15, 2025
Processing time: 91 Days and 10 Hours
As the population of colorectal cancer (CRC) survivors continues to grow, the demand for effective, evidence-based post-treatment strategies becomes incr
To demonstrate that structured lifestyle interventions, specifically tailored dietary and PA programs, can significantly improve behavioral targets as well as disease-free and overall survival (OS).
We designed a 2 × 2 factorial phase II randomized controlled trial to compare the effects of dietary and PA interventions with standard care.
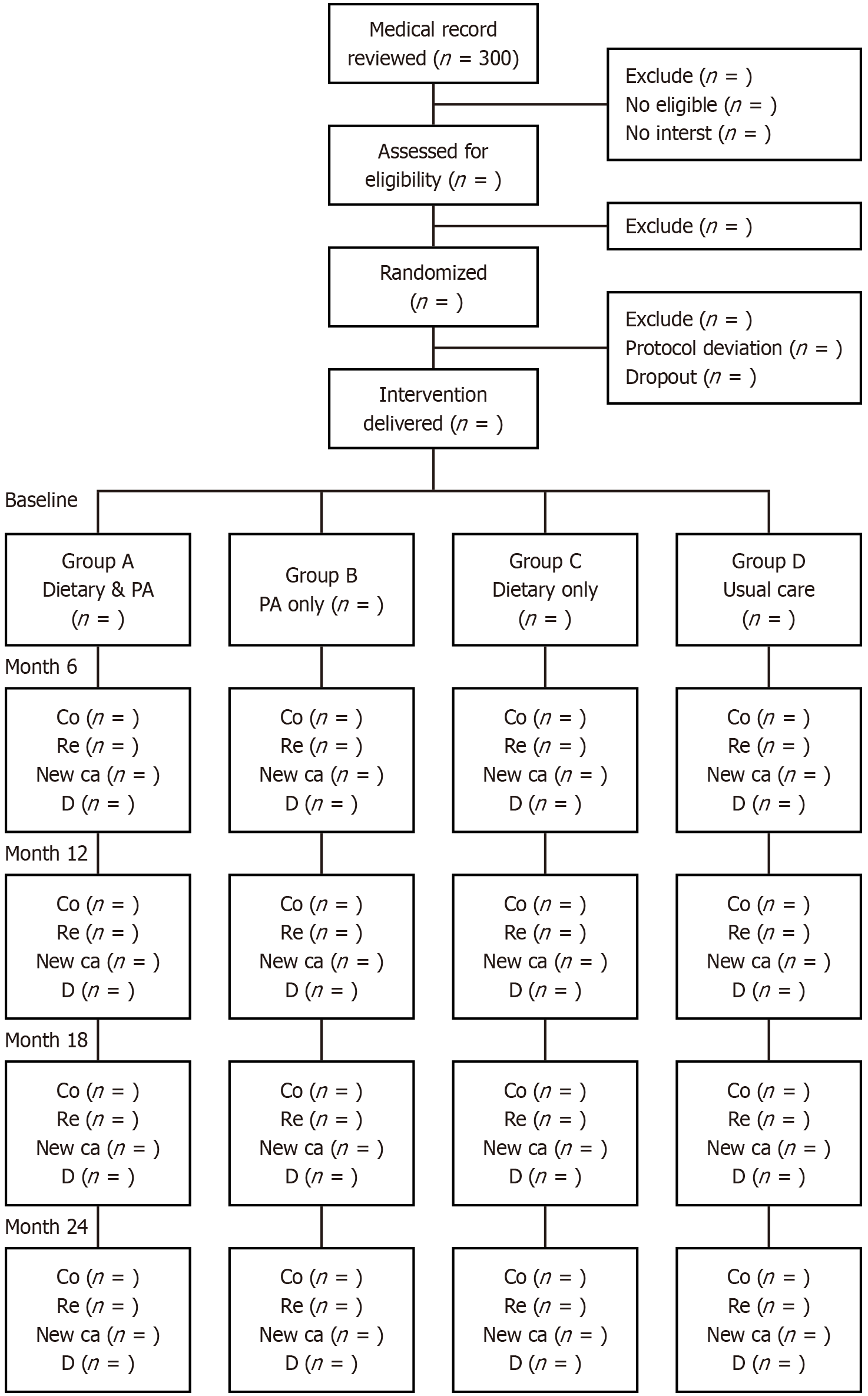
A total of 300 CRC survivors in complete remission will be recruited from oncology centers in Misurata and Zliten (Libya) and the Habib Bourguiba University Hospital (Tunisia). Participants will be randomized into four groups: Combined intervention, diet-only, PA-only, or usual care. They will be followed for 24 months, with outcomes including disease-free survival, OS, and quality of life. Ethical approval has been obtained (Sfax ID: 61/24; Misurata ID: 04/2023), and the trial is registered at ClinicalTrials.gov (NCT06194786).
This study will provide crucial region-specific evidence on the feasibility and effectiveness of lifestyle interventions in CRC survivorship care. By evaluating the role of a high-fiber, low-red meat diet and structured PA, we aim to demonstrate the potential of these behaviors to improve survival outcomes and support their integration into future clinical practice guidelines.
Core Tip: The number of colorectal cancer (CRC) survivors is steadily rising, creating an urgent demand for effective and sustainable post-treatment care strategies. Although lifestyle factors such as diet and physical activity (PA) are known to influence cancer outcomes, there is a notable lack of evidence-based, structured programs to guide survivors in adopting and maintaining these changes—especially in African and resource-limited settings. This study evaluates the impact of two targeted behavioral interventions—increasing PA and reducing red and processed meat intake—on long-term outcomes in CRC survivors. It is among the first of randomized controlled trials in Africa to assess the combined and independent effects of these lifestyle changes on survival and quality of life. Our findings could transform survivorship care by providing region-specific evidence to support the integration of lifestyle counseling into routine oncology practice. This program may serve as a model for preventive care that improves both disease-free and overall survival while influencing future clinical guidelines and public health policy.
- Citation: Aboturkia AM, Ben Kridis W, Aqoup MO, Abdoalmola MO, Ben Ali S, Khanfir A. Diet and physical activity in colorectal cancer patients: A research protocol of a randomized controlled study. World J Gastrointest Oncol 2025; 17(8): 109579
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/109579.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.109579
According to the Global Cancer Observatory[1], colorectal cancer (CRC) is the primary disease of the digestive system and the third most frequent cancer globally. The risk of CRC is increased by a diet low in fiber and high in fat and red and processed meat (RPM). That may influence both CRC prognosis and the effectiveness of lifestyle interventions. A higher prevalence of metabolic syndrome (MetS) and associated insulin resistance in North African populations could influence the metabolic response to dietary changes[2-4]. Additionally, cultural dietary patterns, religious fasting practices, and family-centered decision-making structures may affect compliance. Conversely, CRC can be avoided with regular activity and a diet rich in fruits, vegetables and dietary fiber[2]. The prognosis of CRC has improved recently due to advan
This study will be initiated in the center of tumors from Misurata and Zilatin in the state of Libya and the department of medical oncology at Habib Bourguiba University Hospital in Sfax, Tunisia, over 24 months. Aboturkia AM and Ben Kridis W will obtain patients' consent. Aqoup MO will generate the allocation sequence.
The trial was registered at ClinicalTrials.gov with the NCT06194786 number. Patients with CRC who are over 18 years old with complete remission and who give their informed consent will be enrolled. Those with psychiatric disorders, genetic CRC syndromes, recurrent or chronic CRC at the time of recruitment, and people who were unfit for PA were also excluded from this study. To achieve our goal, we have decided to use a 2 × 2 factorial design. We will compare PA and dietary interventions with usual care in CRC patients. To our knowledge, this is the first study in Africa to employ a 2 × 2 factorial randomized controlled design to evaluate behavioral interventions among CRC survivors. This design enables us to assess the independent and combined effects of PA and diet on survival outcomes. Before being randomly assigned, each patient will receive an Arabic-language pamphlet about maintaining a healthy diet and getting enough exercise. The patients will be instructed to walk for thirty minutes five days a week, to practice walking for no more than thirty minutes each day, to consume no more than 450 mg of RPM per week (that is, three times a week), and to consume no more than two servings of dairy products with a reduction in sugar and an increase in fiber consumption to 450 mg per day. Then patients will be divided into four groups using a double-blind randomization (Figure 1).
Participants will be randomized to study arms using a computer-generated randomization sequence prepared by an independent statistician.
In order to ensure confidentiality, we have avoided using names and have assigned a number to each patient. Patients in groups A and B receiving the dietary intervention will attend one in-person meeting at the start of the study and one phone meeting each month for the next twelve months. Survivors of CRC who are part of the PA (groups A and C) will attend one initial face-to-face meeting and then one monthly telephone meeting for a period of one year. Also, over the 12-month period, they will receive pamphlets every three months.
Our objectives were to raise PA levels, lower RPM consumption, and enhance cancer outcome [disease-free survival (DFS) and OS]. Thirty minutes a day, five days a week, of moderate-to-vigorous intensity PA is the recommended PA level. A healthy diet is one that has 450 mg of fiber per day and no more than 450 mg of RPM each week. Dietary interventions were designed based on established international guidelines. Red meat intake was limited to ≤ 450 g/week, consistent with recommendations from the World Cancer Research Fund and the American Institute for Cancer Research, which advise consuming no more than three portions (approximately 350-500 g cooked weight) per week to reduce cancer risk. The fiber intake target was corrected to ≥ 30 g/day, in accordance with the recommendations of the European Food Safety Authority and other national dietary guidelines[7-9]. The judgment criteria will include the following: Dietary-associated anemia, chorionic embryonic antigen, Food Frequency Questionnaire (FFQ), function assessment of cancer therapy, OS, DFS, and computed tomography (Table 1). DFS was defined as the time from randomization to the first documented disease recurrence (local, regional, or distant), second primary cancer, or death from any cause. OS was defined as the time from randomization to death from any cause.
| Measures | When (month) | |
| Primary outcome | ||
| PA target-cancer outcome | Accelerometer or stopwatch or phone timer | 0, 6, 12, 18, 24 |
| Dietary target-red/processed meat | FFQ | 0, 6, 12, 18, 24 |
| Secondary outcome | ||
| Overall survival | Time between randomization and date of last news | 12-24 |
| Disease free survival | Computed tomography and CEA | 12-24 |
| Magnitude of PA change | Accelerometer or stopwatch or phone timer | 12-24 |
| Magnitude of dietary change | FFQ | 12-24 |
| Quality of life | FACT-G | 12-24 |
Tumor recurrence and progression were assessed according to Response Evaluation Criteria in Solid Tumors version 1.1. Imaging assessments (contrast-enhanced computed tomography or magnetic resonance imaging) and tumor marker evaluations were performed every 6 months during the first 2 years and annually thereafter up to 5 years, in accordance with standard follow-up protocols. This schedule was chosen to ensure timely detection of recurrence while minimizing unnecessary imaging and associated risks, such as radiation exposure and overdiagnosis.
PA adherence will be monitored through a combination of methods tailored to participant circumstances. Where possible, wearable accelerometers will be provided to objectively measure activity duration and intensity over 7-day periods. For participants without access to accelerometers, self-monitoring using stopwatches or phone timers will be encouraged to record the duration and frequency of exercise sessions. Dietary adherence will be evaluated using dietary recalls, supplemented by a semi-quantitative FFQ adapted to regional dietary habits. This multimodal strategy aims to ensure accurate and feasible monitoring of lifestyle adherence. FFQs are a practical tool for assessing habitual dietary intake in large-scale studies but can face challenges in accuracy, especially across diverse cultural contexts. In African populations, FFQs require careful adaptation to reflect local food items, preparation methods, and portion sizes to improve their validity. Previous studies have shown that culturally tailored FFQs can achieve moderate to good validity when compared against dietary recalls. Nevertheless, self-reported dietary data are susceptible to recall bias and social desirability bias, which may affect reliability. To address these limitations, our study combines recalls conducted by trained interviewers with the FFQ and, where possible, includes objective biomarkers to validate intake. This multimethod approach enhances the accuracy of dietary assessment and minimizes potential measurement errors.
To better characterize the heterogeneity within the African population, we conducted exploratory subgroup analyses based on clinically relevant variables, including age, body mass index (BMI; normal vs overweight/obese), diabetes status, tumor stage at diagnosis, and tumor site. These factors were selected due to their known influence on cancer outcomes and their variability in African populations.
Based on prior studies evaluating lifestyle interventions in CRC populations, we anticipate that our combined dietary and PA intervention will result in a 10%-20% relative improvement in DFS and OS. This range reflects clinically meaningful effect sizes consistent with observational data linking metabolic health improvements to better cancer outcomes. We believe that an improvement of 10%-20% in the intervention arm compared to normal care would be taken into consideration. We need to include 55 patients per group, or a total of 220 patients, in order to provide 80% power with a false positive error rate of no more than 5%. A 10% dropout rate is what we anticipate, and 244 individuals will need to be recruited for the trial. Our study will include 300 patients. To minimize the impact of confounding factors, multivariable regression analyses will be used, adjusting for baseline variables such as age (< 60 vs ≥ 60 years), BMI (normal vs overweight/obese), diabetes status, tumor stage at diagnosis and tumor site.
This trial will provide region-specific evidence on the effectiveness of lifestyle interventions in CRC survivors from North Africa, a population largely absent from the current literature. By incorporating culturally adapted educational materials and measuring behavioral adherence, our study not only tests the efficacy of known interventions but also explores their implementation in a real-world, resource-constrained setting. The findings of this study have the potential to inform clinical guidelines and public health strategies targeting cancer survivorship in African settings. By providing region-specific evidence on the impact of dietary and lifestyle interventions on CRC outcomes, the study can contribute to the development of context-appropriate recommendations that address the unique metabolic, cultural, and resource-related factors influencing patient care in Africa. Additionally, the results may support integration of structured nutritional counseling and PA promotion into routine oncology care and survivorship planning. At the policy level, these insights could guide national cancer control programs in incorporating lifestyle interventions as cost-effective, non-pharmacologic strategies to reduce recurrence and improve quality of life for cancer survivors across the continent.
To date, no consensus exists on incorporating structured lifestyle counseling into standard care for CRC survivors, particularly in African healthcare settings. Our program not only addresses this void but also tests the feasibility and impact of embedding behavioral interventions into survivorship protocols[10]. To the best of our knowledge, this is the first research on African CRC survivors' adherence to a particular PA and dietary regimen. It's interesting to note that there isn't enough information in the literature regarding how best to support lifestyle modifications in CRC survivors. This gap in understanding underscores the significance of our research. Over the duration of the 24-month research, we anticipated a decrease in RPM intake without leading to a deficiency or anemia, an increase in fiber intake (fruit and vegetables), and an improvement in PA levels with a higher quality of life, OS, and DFS.
Our findings must be interpreted within the context of the African population, which is characterized by unique gene-environment interactions. For example, specific polymorphisms affecting folate metabolism, insulin resistance, or inflammatory pathways may mediate the relationship between dietary fiber or red meat intake and CRC recurrence risk. Moreover, cultural norms regarding body image, gender roles in meal preparation, and trust in medical advice may influence adherence to lifestyle changes. Future genomic and ethnographic studies could further refine our understanding of these dynamics and improve the personalization of survivorship care in Africa. The African population differs significantly from European and North American populations in baseline risk factors for CRC, including dietary structure, PA, and metabolic profiles. Traditional African diets, once high in fiber and plant-based foods, are increasingly being replaced by energy-dense, processed foods rich in saturated fats and sugars due to rapid urbanization and economic transition[10,11]. Physical inactivity is also rising, especially in urban settings. These changes are contributing to increased rates of obesity, insulin resistance, and MetS in North Africa[5]. These region-specific characteristics could influence both the uptake and physiological effects of lifestyle interventions, thereby justifying the need for localized evidence.
Energy balance is a key factor in how food and PA affect CRC outcomes[10]. A sedentary lifestyle combined with a Western-style diet throws off the energy balance, resulting in hyperinsulinemia, elevated levels of insulin-like growth factor 1 (IGF-1), and insulin resistance[11,12]. These conditions also block the death of micrometastases[13], which promotes their growth and increases the risk of cancer recurrence and death[10].
MetS, a cluster of conditions including central obesity, hypertension, dyslipidemia, and insulin resistance, is increasingly recognized as a significant factor in cancer prognosis. In CRC, MetS may promote tumor recurrence through multiple interrelated mechanisms. Insulin resistance, a hallmark of MetS, leads to chronic hyperinsulinemia, which can enhance tumor cell proliferation via the insulin/IGF-1 axis. Elevated insulin and IGF-1 Levels stimulate mitogenic and anti-apoptotic pathways (e.g., PI3K/Akt, MAPK), potentially accelerating tumor growth and resistance to therapy. Furthermore, the pro-inflammatory state associated with MetS, characterized by increased levels of cytokines such as IL-6 and TNF-α, contributes to a tumor-permissive microenvironment, facilitating angiogenesis and metastasis[14].
In North African populations, the burden of MetS is notably high due to rising rates of obesity, physical inactivity, and dietary shifts toward Westernized eating patterns. These factors may compound CRC recurrence risk in this population, especially in the absence of targeted metabolic interventions. Moreover, certain genetic polymorphisms associated with insulin signaling and lipid metabolism, prevalent in North African cohorts, might further influence susceptibility to tumor progression and recurrence. Therefore, understanding and addressing MetS and insulin resistance in this demographic may offer important opportunities for secondary prevention and improved post-treatment outcomes in CRC[14].
Another potential mechanism linked to carcinogenesis in CRC is the impact of carcinogens originating from a diet high in meat and low in fiber. Local CRC recurrence may decrease as a result of the carcinogen's diminished effect[10]. Additionally, one of the most well-established dietary groups linked to an increased risk of CRC is a diet heavy in meat and poor in fiber.
Using validated FFQs, Cross et al[15] prospectively assessed the relationship between red meat consumption and cancer incidence through the National Institutes of Health-American Association for Retired Persons Diet and Health Study. Those with the greatest quantiles of red or processed meat consumption had a significantly higher risk of CRC cancer (HR = 1.24 with 95%CI: 1.12-1.36 and HR = 1.20 with 95%CI: 1.09-1.32, respectively) compared to those in the lowest quantiles. Compared to colon cancer, there was a stronger correlation between the risk and the likelihood of rectal cancer. The European Prospective Investigation into Cancer and Nutrition research, which followed almost half a million participants across 10 European nations, revealed higher CRC risk with red meat consumption[16]. The impact of nutrition on CRC cancer survival and recurrence has only been studied in a few numbers of studies[17], the results of which were constrained by small sample sizes and varied patient populations. The Cancer and Leukemia Group B 89803 study found that patients with stage III CRC cancer who had a diet high in red meat had a lower DFS compared to those in the lowest quantile (HR = 3.25; 95%CI: 2.04-5.19). Smart nutrition, on the other hand, was not linked to CRC death or recurrence. Another prospective cohort study of patients with stage I-III CRC cancer was published by Fung et al[18], and it found no significant correlation between increased consumption of red meat and survival.
Several prospective studies[19] have consistently found a correlation between lower rates of cancer mortality and higher levels of PA performed both before and after a CRC diagnosis. A meta-analysis[20] of seven prospective studies of CRC cancer survivors found that higher levels of PA after diagnosis were linked to a 42% (HR = 0.58) decrease in cancer mortality. Another prospective analysis has also been conducted to examine the impact of alterations in PA levels prior to and following diagnosis on survival outcomes. A significant reduction in CRC mortality was observed in an Australian cohort of CRC survivors[21] who disclosed increasing their PA by over two hours per week (HR = 0.64 with 95%CI: 0.44-0.93).
Understanding the behavioral factors driving adherence in this population will allow us to refine educational interventions for long-term effectiveness, guided by behavioral science frameworks such as the Theory of Planned Behavior. The educational intervention is based on key constructs of the Theory of Planned Behavior, specifically focusing on attitude toward the behavior and perceived behavioral control. To address attitudes, participants will receive evidence-based information on the relationship between dietary intake, PA, and CRC outcomes. Perceived behavioral control will be targeted through the provision of structured tools such as goal-setting worksheets, personalized dietary advice, and guidance for overcoming common barriers to lifestyle change. These components are designed to enhance behavioral intentions and facilitate the adoption of recommended practices[22].
Adherence to dietary and PA guidelines may be influenced by cultural norms and practices prevalent in African populations[23]. In many contexts, traditional diets are high in carbohydrates and starchy foods, with less emphasis on fiber-rich vegetables or lean proteins, which may affect the acceptability of dietary recommendations emphasizing high fiber and reduced red or processed meat intake. Social and familial eating patterns, as well as perceptions of body image and health, can also shape food choices. Similarly, PA may not be widely prioritized as a structured health behavior, particularly among older adults or women, where sociocultural expectations and safety concerns may act as barriers. Recognizing these factors, our intervention will incorporate culturally adapted messaging and practical strategies that align with local food availability, cooking practices, and daily routines to enhance feasibility and relevance.
This trial will provide region-specific evidence on the effectiveness of lifestyle interventions in CRC survivors from North Africa, a population largely absent from the current literature. By incorporating culturally adapted educational materials and measuring behavioral adherence, our study not only tests the efficacy of known interventions but also explores their implementation in a real-world, resource-constrained setting. The findings of this study have the potential to inform clinical guidelines and public health strategies targeting cancer survivorship in African settings. By providing region-specific evidence on the impact of dietary and lifestyle interventions on CRC outcomes, the study can contribute to the development of context-appropriate recommendations that address the unique metabolic, cultural, and resource-related factors influencing patient care in Africa. Additionally, the results may support integration of structured nutritional counseling and PA promotion into routine oncology care and survivorship planning. At the policy level, these insights could guide national cancer control programs in incorporating lifestyle interventions as cost-effective, non-pharmacologic strategies to reduce recurrence and improve quality of life for cancer survivors across the continent.
We acknowledge the support of the Faculty of Medicine, University of Sfax, Sfax, Tunisia, and the Faculty of Human Medicine, Azzaytuna University, Tarhuna, Libya.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Hartman AM, Jacobs DR Jr, Kato I, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Pietinen P, Rohan TE, Schatzkin A, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Smith-Warner SA. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294:2849-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527-3534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 587] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 4. | Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, Cerin E, Chan WY, Leung IP, Lam SH, Taylor AJ, Cheng KK. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 553] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 5. | Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, Shorthouse A, Saxton J. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Arch Phys Med Rehabil. 2011;92:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Lee CF, Ho JWC, Fong DYT, Macfarlane DJ, Cerin E, Lee AM, Leung S, Chan WYY, Leung IPF, Lam SHS, Chu N, Taylor AJ, Cheng KK. Dietary and Physical Activity Interventions for Colorectal Cancer Survivors: A Randomized Controlled Trial. Sci Rep. 2018;8:5731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4:1933-1947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010;8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA. 2017;317:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 752] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 10. | Meyerhardt JA. Beyond standard adjuvant therapy for colon cancer: role of nonstandard interventions. Semin Oncol. 2011;38:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Ho JW, Lee AM, Macfarlane DJ, Fong DY, Leung S, Cerin E, Chan WY, Leung IP, Lam SH, Taylor AJ, Cheng KK. Study protocol for "Moving Bright, Eating Smart"- A phase 2 clinical trial on the acceptability and feasibility of a diet and physical activity intervention to prevent recurrence in colorectal cancer survivors. BMC Public Health. 2013;13:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 358] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, Hallmans G, Kaaks R. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003;107:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Winn M, Karra P, Haaland B, Doherty JA, Summers SA, Litchman ML, Gunter MJ, Playdon MC, Hardikar S. Metabolic dysfunction and obesity-related cancer: Results from the cross-sectional National Health and Nutrition Examination Survey. Cancer Med. 2023;12:606-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, Overvad K, Olsen A, Tjønneland A, Clavel F, Boutron-Ruault MC, Kesse E, Boeing H, Bergmann MM, Nieters A, Linseisen J, Trichopoulou A, Trichopoulos D, Tountas Y, Berrino F, Palli D, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, Peeters PH, Engeset D, Lund E, Skeie G, Ardanaz E, González C, Navarro C, Quirós JR, Sanchez MJ, Berglund G, Mattisson I, Hallmans G, Palmqvist R, Day NE, Khaw KT, Key TJ, San Joaquin M, Hémon B, Saracci R, Kaaks R, Riboli E. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97:906-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 508] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 17. | Dray X, Boutron-Ruault MC, Bertrais S, Sapinho D, Benhamiche-Bouvier AM, Faivre J. Influence of dietary factors on colorectal cancer survival. Gut. 2003;52:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, Giovannucci E, Ogino S, Hu FB, Meyerhardt JA. Post diagnosis diet quality and colorectal cancer survival in women. PLoS One. 2014;9:e115377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 21. | Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20:1410-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C; English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2012;61:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 23. | Steyn NP, McHiza ZJ. Obesity and the nutrition transition in Sub-Saharan Africa. Ann N Y Acad Sci. 2014;1311:88-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |