Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.109544
Revised: May 29, 2025
Accepted: July 7, 2025
Published online: August 15, 2025
Processing time: 91 Days and 22.2 Hours
Pancreatic exocrine insufficiency (PEI) leads to fat malabsorption and maldi
To examine the cost-effectiveness of PERT for patients suffering from PEI in China.
A decision analytical Markov model was constructed to simulate the progress of patients with PEI in China. The population included in the analyses were patients suffering from PEI with advanced (non-resectable) pancreatic cancer, who have undergone surgery due to pancreatic cancer and who have undergone endoscopic treatment due to chronic pancreatitis. The cost-effectiveness analyses were undertaken from a Chinese societal perspective comparing PERT with no PERT. The incremental cost-effectiveness ratio in United States dollars per quality adjusted life year (QALY) gained is the main outcome. Input was informed by publicly available data supplemented with expert clinical advice.
The cost-effectiveness analyses estimated that PERT resulted in additional 0.45 to 2.93 QALYs at discounted costs of between 4315 dollars to 15193 dollars. This resulted in an incremental cost-effectiveness ratio of 5178 dollars to 9533 dollars per QALY. The one-way sensitivity analyses showed that the main drivers of the model were the cost of PERT and overall survival.
This study demonstrates that PERT is a cost-effective treatment for patients suffering from PEI in China.
Core Tip: Pancreatic enzyme replacement therapy (PERT) is a first-line treatment for pancreatic exocrine insufficiency (PEI). In China, PERT is not yet listed on the national essential medicine list, which is often a pre-requisite for patients in China to gain access to life-saving treatments. Chinese health policy has an increased emphasis on cost-effectiveness analysis as a prerequisite for drug reimbursement. This analysis undertakes a cost-effectiveness analysis on PERT for the treatment of PEI from a Chinese societal perspective, finding that PERT is cost-effective for the treatment of PEI in China.
- Citation: Kim H, Byrnes J, Jiang KR, Liao Z, Jones A, Kim K, Fragkogianni D, Roberts KJ. Cost-effectiveness analysis of pancreatic enzyme replacement therapy in patients with pancreatic exocrine insufficiency in China. World J Gastrointest Oncol 2025; 17(8): 109544
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/109544.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.109544
Pancreatic exocrine insufficiency (PEI) is a deficiency of pancreatic enzyme production or function, resulting in an inability to digest food properly[1]. The main clinical consequence of PEI is fat malabsorption and maldigestion, resulting in amongst others, malnutrition, weight loss, abdominal pain, flatulence, and steatorrhea[2,3].
PEI is associated with numerous conditions including but not limited to chronic pancreatitis, pancreatic cancer and pancreatic resection surgery[4]. It is estimated that PEI occurs in 36%-94% of patients with chronic pancreatitis[5,6]; 68%-92% of patients with unresectable pancreatic cancer[7,8] and 50%-74% of patients after pancreatic resection surgery[9,10].
Pancreatic enzyme replacement therapy (PERT) is the standard of care for first line treatment of PEI[11]. PERTs contain high-titer pancreatic enzymes such as amylase, lipase and protease, which have been extracted and purified from porcine pancreatic glands[12]. Thus, PERT aims to deliver sufficient enzymatic activity into upper gastrointestinal tract as simultaneously as possible with the meal in order to restore nutrient digestion and aid absorption[13]. The importance of PERT treatment is increasingly appreciated. For example, among patients with pancreatic cancer PERT is associated with increased survival. A recent meta-analysis demonstrated that unresectable pancreatic cancer PEI patients treated with PERT experienced an increase in overall survival of 3.8 months (95% confidence interval: 1.37 months to 10.54 months)[14].
It is well-documented that there is an under-utilisation of PERT in patients suffering from PEI[15,16] with one study reporting only 8.5% of PEI patients with chronic pancreatitis and 5.5% of PEI patients with pancreatic cancer receiving adequate PERT [defined as ≥ 120000 United States Pharmacopeia (USP) units of lipase daily][17].
Recently, Chinese drug policy has increased its emphasis on Health Technology Assessment with cost-effectiveness analysis set to become mandatory for economic assessment in Drug Policies[18,19]. In addition, establishing a national essential medicine system is another important measure of Chinese healthcare reform, which contains the national essential medicine list (NEML), aiming to ensure access to, and the safety and provision of, adequate amounts of essential medicines to patients. In reality, pancreatin, a Medicare part B drug (medical insurance), has been listed on the national reimbursement drug list but not yet on the NEML, therefore Chinese patients are less able to access treatment.
The aim of this study is to examine the cost-effectiveness of PERT for patients suffering PEI from a Chinese societal perspective.
A targeted literature review formed the basis for the evidence used as input in the model.
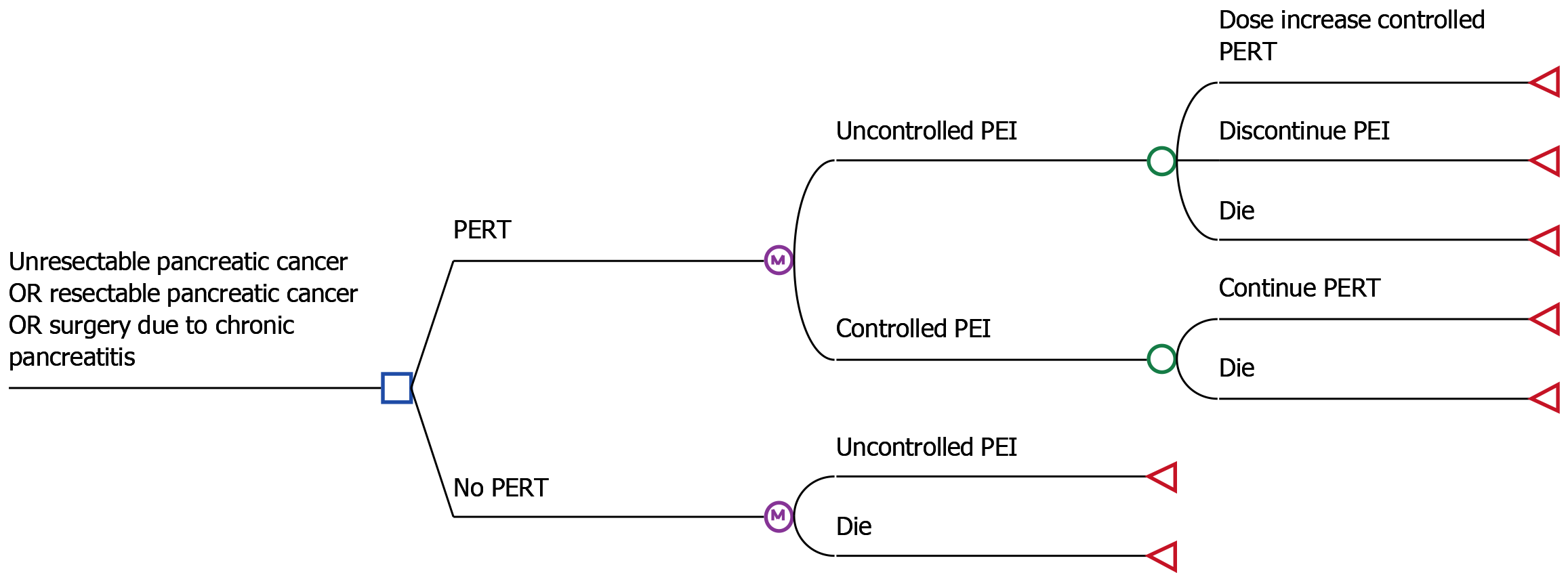
A decision analytical Markov model[20] was constructed to simulate the progress of patients with PEI in China (see Figure 1). Three separate scenarios were examined namely: Endoscopic treatment due to chronic pancreatitis.
The cost-effectiveness analyses were undertaken from a Chinese societal perspective. The cost-effectiveness of PERT compared to no PERT is reported as the incremental cost-effectiveness ratio (ICER) in United States dollars per life year (LY) saved and per quality adjusted LY (QALY) gained. In China, there is no standard ICER threshold and therefore the World Health Organization (WHO) threshold of 3 × gross domestic product (GDP) per capita is used instead[21]. China’s GDP per capita was 10435.78 dollars in 2020 according the World Bank[22] and thus a threshold of 31304.34 dollars (= 3 × 10435.78 dollars) was assumed in this study.
Time horizons of 5, 10, 15 years were chosen for scenario 1, 2 and 3 respectively in order to capture the costs and effectiveness of patients suffering from PEI. Cycle length was set to 1 month.
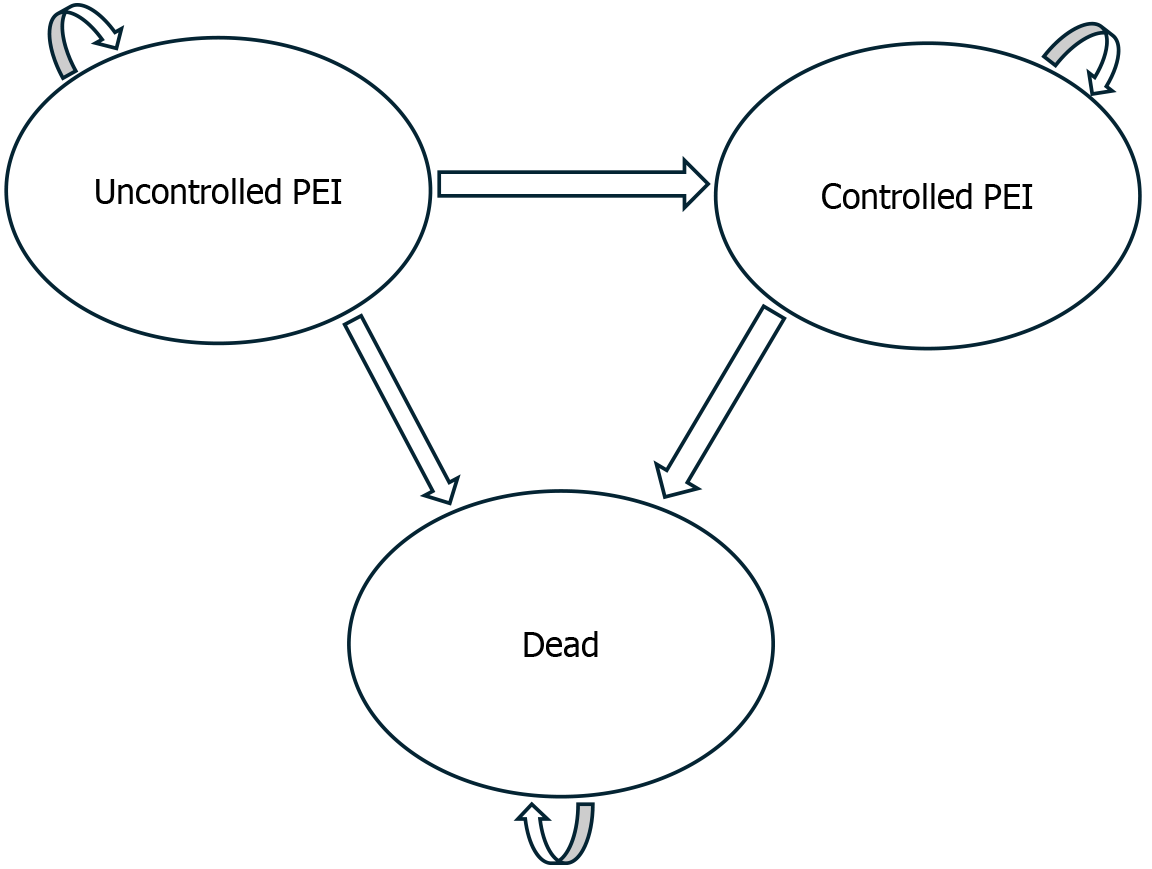
The model consisted of three health states: Uncontrolled PEI, controlled PEI and dead (see Figure 2).
The risk of death for each scenario was informed by published clinical trials[23-25]. Individual patient data was reconstructed from published Kaplan-Meier curves[26] and events beyond the follow-up period were extrapolated using parametric modelling with a log-normal distribution (see Supplementary Figure 1). The log-normal distribution was chosen as it provided the best fit for the overall survival data amongst standard parametric distributions per the Akaike Information Criterion.
The overall survival for PERT was then calculated by applying a hazard ratio (HR) for baseline overall survival. A recent systematic review by Iglesia et al[14] of advanced pancreatic cancer patients suffering from PEI estimated the median overall survival be 12.6 months for PERT vs 8.7 months for patients not treated with PERT (mean difference = 3.8 months, P < 0.05). It therefore assumed that the HR for PERT compared to no PERT was 1/(12.7/8.7) = 0.69 for advanced pancreatic cancer patients in scenario 1. A retrospective study of 469 patients suffering PEI following surgery due to pancreatic cancer by Roberts et al[27], estimated the HR of PERT vs no PERT to be 0.57 (95% confidence interval: 0.38-0.87, P = 0.009), which was used for the scenario 2. For scenario 3 the HR was retrieved from a study by Winny et al[23], where it was estimated to be 0.48 (P < 0.05).
The transition probability from uncontrolled PEI to controlled PEI for scenario 1 was sourced from Iglesia et al[14] and from Seiler et al[28] for scenarios 2 and 3. Controlled PEI was defined as a coefficient of fat absorption (CFA) greater than 80%. The probability of achieving this was estimated using the cumulative distribution function of the reported mean CFA from Iglesia et al[14], assuming the distributions of CFA are normal. For patients not receiving PERT the assumption was that they would remain in the uncontrolled PEI health state unless they passed away.
The targeted literature review did not identify any studies reporting health-related quality of life (HRQoL) utility estimates for patients suffering from PEI for the three scenarios. Therefore, a stepwise approach was used to obtain utility estimates for each scenario. First, utility estimates for patients with advanced pancreatic cancer, endoscopic treatment due to chronic pancreatitis, and surgery due to pancreatic cancer were obtained from the literature. Symptom-specific utility decrements for patients suffering from PEI were then taken from these utility values.
The baseline utility was estimated to be 0.78 for patients with advanced pancreatic cancer (scenario 1) receiving gemcitabine and/or bevacizumab[29]. For patients with resected pancreatic cancer (scenario 2), median utility was reported to be 0.786 (n = 178) and 0.771 (n = 176) at baseline for patients receiving gemcitabine or an experimental drug called S-1 respectively[30], resulting in a weighted average utility of 0.779[24,25] [178/(178 + 176) × 0.786 + 176/(178 + 176) × 0.771]. Patients who had received endoscopic treatment due to chronic pancreatitis were estimated to have a utility of 0.793 (SD = 0.21)[31].
Specific symptoms associated with PEI that majority of patients suffer from reported to be abdominal pain (84%) and diarrhoea (75%)[32]. The utility decrement of abdominal pain and diarrhoea were estimated to be -0.051 and -0.204 respectively for patients with metastatic pancreatic cancer by the United Kingdom National Institute for Health and Care Excellence[33].
Costs were applied for PERT treatment and to ongoing care of PEI patients. It was assumed that costs were the same for both resectable and unresectable pancreatic patients. Costs are reported in United States dollars using a 2022 foreign exchange spot rate of 1 United States dollar = 6.36 Chinese Yuan[34].
PERT was assumed to be given with 3 meals and 2 snacks. A review by Dominguez-Muñoz[35] in 2018 found that the dose of PERT in pancreatic cancer patients should be 73500 Ph U/USP with main meals and 43000 Ph U/USP with snacks. The estimated daily dose based on 3 main meals and 2 snacks would then be 306500 Ph U/USP. For patients with chronic pancreatitis a survey of local experts (data on file) found that patients on average would receive 30000 Ph U/USP with main meals and 15000 Ph U/USP with snacks leading to a daily dose of 120000 Ph U/USP.
PERT therapy is available in capsules consisting of 10000 Ph U/USP which costs 0.26 dollars each. Daily cost of PERT treatment is estimated to be 3.12 dollars [= 0.26 dollars × (120000/10000)] for PEI patients with pancreatitis and 7.97 dollars [= 0.26 dollars × (306500/10000)] for PEI patients with pancreatic cancer.
Cost of ongoing care was informed by a survey of clinical experts (data on file). It was assumed that all patients see a dietician every 4 weeks (89.66 dollars/visit), including transportation costs.
For both resectable and unresectable cancer patients, ongoing care is assumed to consist of a visit to an oncologist every 6 months (3.69 dollars/visit), a computed tomography (CT) scan every 6 months (320.18 dollars/CT scan), and a blood test for cancer makers every 6 months (40.88 dollars/blood test). The yearly cost of ongoing care for pancreatic cancer patients is therefore estimated to be 1895.08 dollars.
Ongoing care for patients suffering from pancreatitis included 5 specialist visits per year (5.90 dollars/visit), a scan every 6 months (78.62 dollars/scan) and a blood test for cancer and biochemical markers every 6 months (147.33 dollars/test). The yearly cost of ongoing care for patients with pancreatitis is therefore estimated to be 1646.98 dollars.
As most of the input variables are based on single sources one way sensitivity analyses was performed by varying the model input by ± 25% except for cycle length, discount rate, and time horizon. Probabilistic sensitivity analysis was performed by assigning probability distributions to key model parameters (see Table 1 for details) and resampling the inputs 5000 times. The model was built in Microsoft Excel and the probabilistic sensitivity analyses were performed in @Risk version 8 on a windows platform.
| Value (SD) | One way sensitivity analysis (%) | PSA distribution | Ref. | |
| Survival (HR) | ||||
| Scenario 1 | 0.69 (0.14) | +/- 25 | Lognormal | Iglesia et al[14] |
| Scenario 2 | 0.57 (0.06) | +/- 25 | Lognormal | Roberts et al[27] |
| Scenario 3 | 0.48 (0.20) | +/- 25 | Lognormal | Winny et al[23] |
| Clinical efficacy | ||||
| Scenario 1 | 0.53 (0.05) | +/- 25 | Beta | Iglesia et al[14] |
| Scenario 2 | 0.58 (0.06) | +/- 25 | Beta | Seiler et al[28] |
| Scenario 3 | 0.81 (0.08) | +/- 25 | Beta | Seiler et al[28] |
| Utilities | ||||
| Scenario 1 | 0.78 (0.13) | +/- 25 | Beta | Romanus et al[29] |
| Scenario 2 | 0.78 (NR) | +/- 25 | Beta | Hagiwara et al[30] |
| Scenario 3 | 0.79 (0.052) | +/- 25 | Beta | Laramée et al[31] |
| Disutilities | ||||
| Abdominal pain | -0.051 (NR) | +/- 25 | Beta | NICE 2017[33] |
| Diarrhoea/decreased appetite/fatigue | -0.204 (NR) | +/- 25 | Beta | NICE 2017[33] |
| Costs | ||||
| PERT | ||||
| Scenario 1 | 7.93 dollars/day (NA) | +/- 25 | Gamma | Dominguez-Muñoz[35] |
| Scenario 2 | 7.93 dollars/day (NA) | +/- 25 | Gamma | Clinical expert survey (data on file) |
| Scenario 3 | 3.10 dollars/day (NA) | +/- 25 | Gamma | Clinical expert survey (data on file) |
| Ongoing care | ||||
| Scenario 1 | 4503.22 dollars/year (NA) | +/- 25 | Gamma | Clinical expert survey (data on file) |
| Scenario 2 | 4503.22 dollars/year (NA) | +/- 25 | Gamma | Clinical expert survey (data on file) |
| Scenario 3 | 2248 dollars/year (NA) | +/- 25 | Gamma | Clinical expert survey (data on file) |
| Other inputs | ||||
| Cycle length | 1 month | Not varied | Not varied | |
| Discounting | 5% | Not varied | Not varied | |
| Time horizon | ||||
| Scenario 1 | 5 years | Not varied | Not varied | |
| Scenario 2 | 10 years | Not varied | Not varied | |
| Scenario 3 | 15 years | Not varied | Not varied |
PERT treated patients with advanced pancreatic cancer (scenario 1) are estimated to live an average of 1.33 LYs (0.94 QALYs) at a discounted cost of 4960 dollars. Patients not treated with PERT were predicted to live 0.94 LYs (0.49 QALYs) at a discounted cost of 645 dollars, resulting in ICERs of 10996 dollars/LY and 9533 dollars/QALY.
In scenario 2, patients treated with PERT are estimated to live 1.03 (= 4.41 - 3.12) years longer than patients not receiving PERT treatment. Moreover, they gained 1.58 (= 3.22 - 1.64) QALYs over non-PERT treated patients. This was achieved at an incremental cost of 13935 dollars (= 15677 dollars - 1743 dollars) resulting in ICERs of 10763 dollars/LY and 8813 dollars/QALY.
For patients who had endoscopic treat due to chronic pancreatitis, the ICER was estimated to be 14829 dollars per LY saved and 5178 dollars per QALY gained (see Table 2).
| Scenario 1 (unresectable pancreatic cancer) | Scenario 2 (pancreatic cancer post-surgery) | Scenario 3 (chronic pancreatitis after endoscopic treatment) | ||||
| PERT | No PERT | PERT | No PERT | PERT | No PERT | |
| LYs | 1.33 | 0.94 | 4.41 | 3.12 | 9.79 | 8.76 |
| QALYs | 0.94 | 0.49 | 3.22 | 1.64 | 7.65 | 4.72 |
| Costs (dollar) | 4960 | 645 | 15677 | 1743 | 17846 | 2653 |
| ICER (LY/dollar) | 10996 | 10763 | 14829 | |||
| ICER (QALY/dollar) | 9533 | 8813 | 5178 | |||
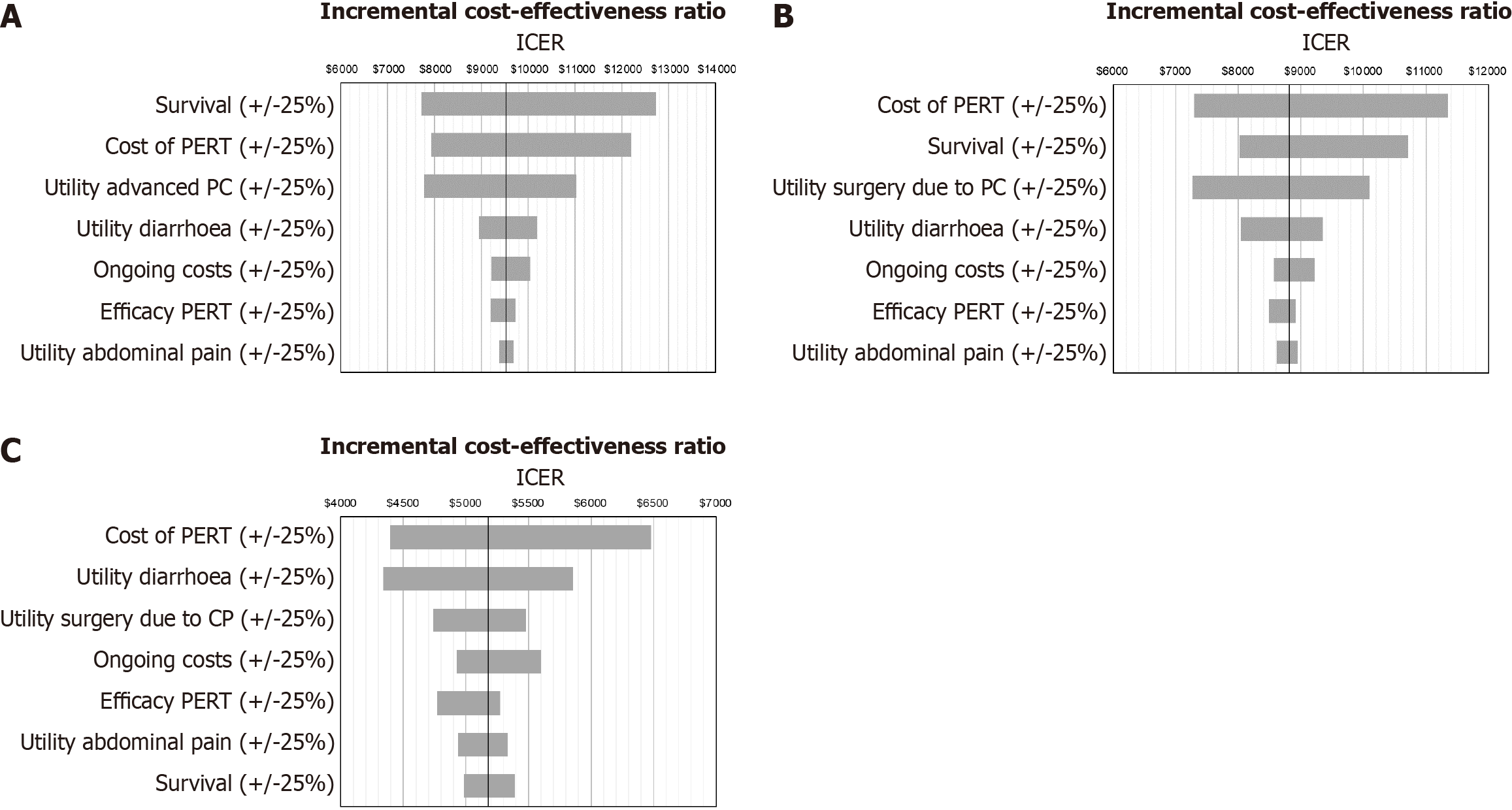
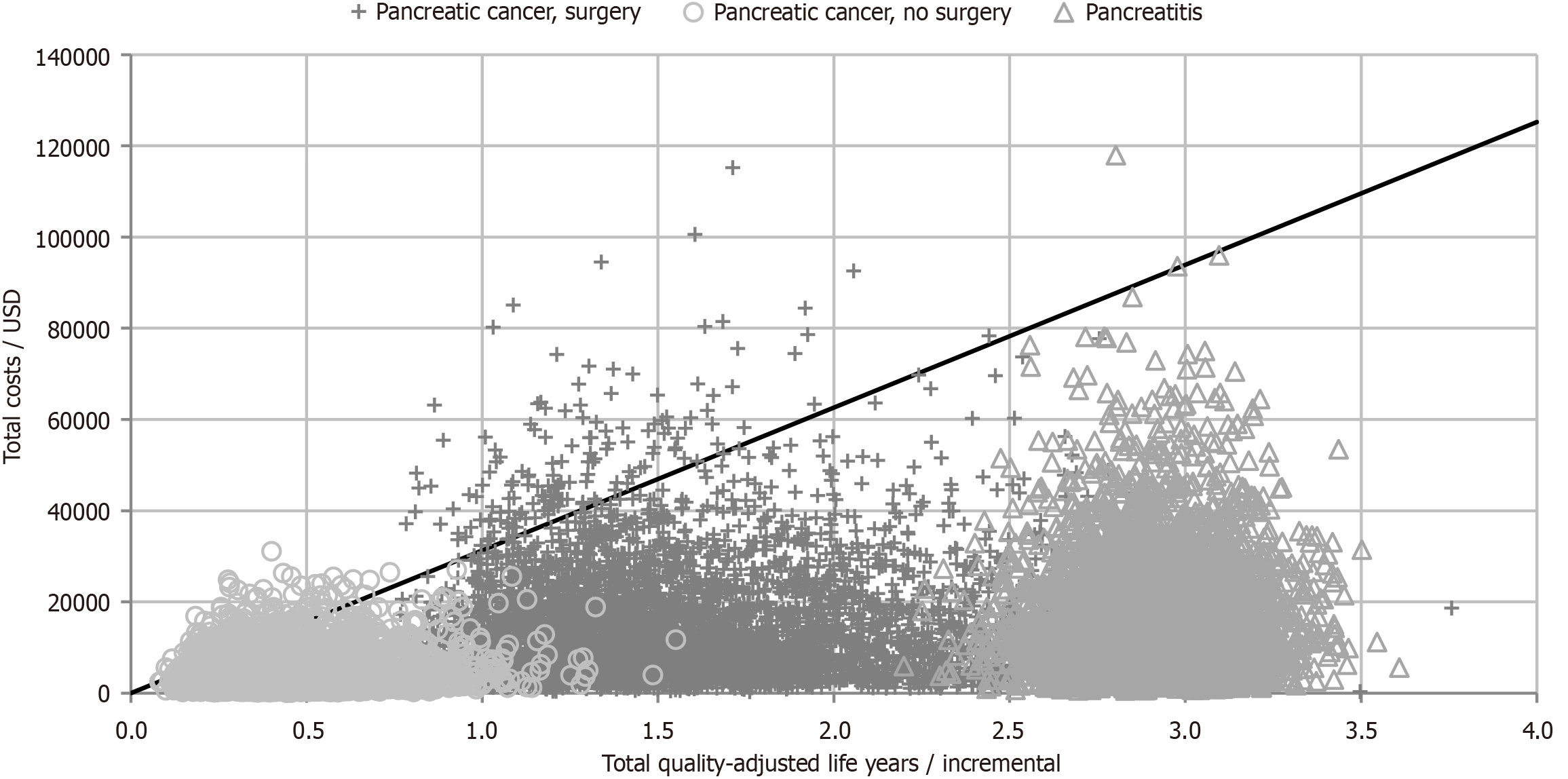
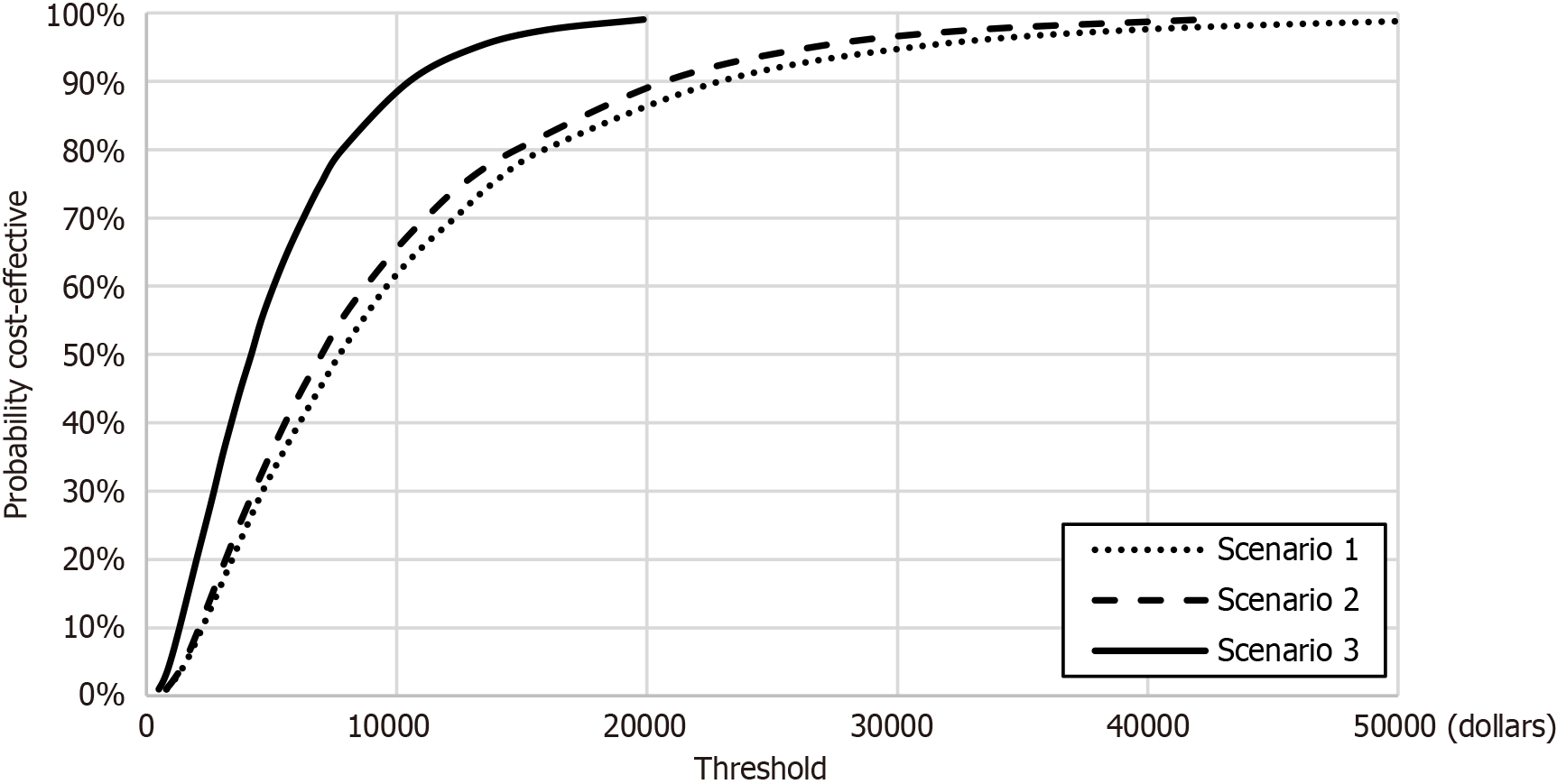
The results of the one-way sensitivity analysis showed that model was sensitive to cost of PERT, survival and utility values (see Figure 3). The scatter plots for the Monte Carlo simulations are displayed in Figure 4. PERT is cost-effective across 95.3% (scenario 1), 96.1% (scenario 2) and 99.9% (scenario 3) simulations at a willingness-to-pay threshold of 31304 dollars (see Figure 5 for the acceptability curves).
All the ICERs generated in the analysis are significantly below the WHO prescribed threshold of 3 × GDP and would therefore be classified as cost-effective[21]. Probabilistic sensitivity analysis found that PERT was below this threshold between 95.5%-99.9% of the time, depending on the patient sub-group.
In the one-way sensitivity analyses, increasing the cost of PERT by 25% led to an increase in the ICER of all scenarios from 6500 dollars to 12700 dollars. However, this was still well-below the stated threshold. Other significant drivers of the cost-effectiveness according to the one-way sensitivity analyses were the overall survival for pancreatic cancer (both unresectable and post-surgery), and quality of life utilities. This is not surprising as data from multiple sources were used due to the fact that there is no data available on mortality and health related quality of life in Chinese PEI patients.
Pancreatic cancer is notorious for its poor prognosis. Whilst aggressive cancer biology is largely attributed as the cause, it is clear that PEI affects most patients, and is progressive after diagnosis[8]. Given the daily requirements for nutrition, it is perhaps unsurprising to see that PEI is associated with worse survival[14,27]. Furthermore, treatment with PERT has been associated with an improvement in median survival which is equivalent to the best anticancer therapies that are currently available in both unresectable[14] and resectable[36] pancreatic cancer, when analyses are performed to control for key variables. Whilst anticancer therapies are associated with an improvement in survival, it is often at the expense of quality-of-life, where surgery and/or chemotherapy are associated with pain, gastrointestinal symptoms, reduced mobility, and more. In this study, it is observed that PERT is associated with both improved survival and improved quality-of-life. Thus, not only is PERT associated with improved survival, equivalent to established anti-cancer therapy, but also improves QoL by addressing the symptoms of PEI. This explains why PERT therapy is highly cost effective in these scenarios.
One of the main limitations of this study is the lack of direct evidence available on the HRQoL of the health states utilised in the model. Our stepwise approach gives us a proxy for the true value, but given that we identify these utility values as a driver of the model results, this appears to warrant further research. Another limitation is that our HR for scenario two is obtained from a study which, among patients with resectable cancer, included patients undergoing surgery for periampullary tumours[27]. Whilst those with pancreatic cancer were the dominant subgroup, it was not possible to distinguish pancreatic from non-pancreatic cancers. However, in that study a clear relationship was seen between an increasing pancreatic duct width and increasing difference in survival between non-PERT and PERT treated patients. Given that pancreatic cancer is almost always associated with pancreatic duct dilation, it is considered reliable to use the data as presented.
In practice, different formulations of PERT vary in effectiveness according to patient characteristics. For example, lipase is inactivated if potential of hydrogen falls below 4, so some enzymes may be enteric coated to protect against this. Conversely, in achlorhydric patients uncoated enzymes would fare better. The variation in outcomes resulting from this parameter uncertainty highlights the need for further research on how cost-effectiveness could vary across clinically defined sub-groups.
One other cost-effectiveness analysis of PERT in PEI patients was published in 2012. In that study, Morawski et al[37] found that PERT was cost-effective from a Polish payer perspective with an ICER (€6312 per QALY) below the acceptable willingness to pay threshold of 3 × GDP per capita. While there are several methodological differences between that study and the present study, the results are consistent with our findings.
The treatment of PEI in patients with unresectable pancreatic cancer, pancreatic cancer patients who have undergone pancreatic surgery, and patients with chronic pancreatitis who have received endoscopic treatment is cost-effective from a Chinese societal perspective with an ICER below 3 × GDP per capita.
| 1. | Alkaade S, Vareedayah AA. A primer on exocrine pancreatic insufficiency, fat malabsorption, and fatty acid abnormalities. Am J Manag Care. 2017;23:S203-S209. [PubMed] |
| 2. | Lindkvist B, Domínguez-Muñoz JE, Luaces-Regueira M, Castiñeiras-Alvariño M, Nieto-Garcia L, Iglesias-Garcia J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology. 2012;12:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Rasmussen HH, Irtun O, Olesen SS, Drewes AM, Holst M. Nutrition in chronic pancreatitis. World J Gastroenterol. 2013;19:7267-7275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 4. | Toouli J, Biankin AV, Oliver MR, Pearce CB, Wilson JS, Wray NH; Australasian Pancreatic Club. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust. 2010;193:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Lévy P, Domínguez-Muñoz E, Imrie C, Löhr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014;2:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Dumasy V, Delhaye M, Cotton F, Deviere J. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol. 2004;99:1350-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Matsumoto J, Traverso LW. Exocrine function following the whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Sikkens EC, Cahen DL, de Wit J, Looman CW, van Eijck C, Bruno MJ. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014;48:e43-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Forssmann K, Schirr K, Schmid M, Schwall G, Silbernik D, Singer MV, Trede M. [Postoperative follow-up in patients with partial Whipple duodenopancreatectomy for chronic pancreatitis]. Z Gastroenterol. 1997;35:1071-1080. [PubMed] |
| 10. | Keck T, Wellner UF, Riediger H, Adam U, Sick O, Hopt UT, Makowiec F. Long-term outcome after 92 duodenum-preserving pancreatic head resections for chronic pancreatitis: comparison of Beger and Frey procedures. J Gastrointest Surg. 2010;14:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Ru N, Zou WB, Wu H, Hu LH, Li XB, Liu GF, Li ZS, Liao Z; Chronic Pancreatitis Group of Chinese Medical Doctor Association. Chinese guidelines for the diagnosis and treatment of pancreatic exocrine insufficiency (2018 edition). J Dig Dis. 2019;20:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran's Gastrointestinal and Liver Disease 9th ed. W.B. Saunders: Philadelphia, 2010. |
| 13. | Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol. 2013;19:7258-7266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (6)] |
| 14. | Iglesia D, Avci B, Kiriukova M, Panic N, Bozhychko M, Sandru V, de-Madaria E, Capurso G. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. United European Gastroenterol J. 2020;8:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Forsmark CE, Tang G, Xu H, Tuft M, Hughes SJ, Yadav D. The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment Pharmacol Ther. 2020;51:958-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Landers A, Muircroft W, Brown H. Pancreatic enzyme replacement therapy (PERT) for malabsorption in patients with metastatic pancreatic cancer. BMJ Support Palliat Care. 2016;6:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Kharbat M, Craig PI. Editorial: management of exocrine pancreatic insufficiency remains a challenge-can we do better? Aliment Pharmacol Ther. 2020;51:1206-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Li H, Sun R, Liu J, Ming J, Zhao X, Liu Y, Xie Y. PDG56 Application of Health Technology Assessment in China National Reimbursement Drug List Price Negotiations: The Evolving Trend and Implications. Value Health. 2020;23:S139. [DOI] [Full Text] |
| 19. | Huang C, Ung COL, Wushouer H, Bai L, Huang T, Li X, Guan X, Shi L. Health technology assessment-informed pricing negotiation in China: higher negotiated price for more effective targeted anticancer medicines? Health Res Policy Syst. 2022;20:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Ademi Z, Kim H, Zomer E, Reid CM, Hollingsworth B, Liew D. Overview of pharmacoeconomic modelling methods. Br J Clin Pharmacol. 2013;75:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Daroudi R, Akbari Sari A, Nahvijou A, Faramarzi A. Cost per DALY averted in low, middle- and high-income countries: evidence from the global burden of disease study to estimate the cost-effectiveness thresholds. Cost Eff Resour Alloc. 2021;19:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | World Bank Group. GDP per capita (current US$) - China. [cited July 2, 2025]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=CN. |
| 23. | Winny M, Paroglou V, Bektas H, Kaltenborn A, Reichert B, Zachau L, Kleine M, Klempnauer J, Schrem H. Insulin dependence and pancreatic enzyme replacement therapy are independent prognostic factors for long-term survival after operation for chronic pancreatitis. Surgery. 2014;155:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 787] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 25. | Shin S, Park CM, Kwon H, Lee KH. Erlotinib plus gemcitabine versus gemcitabine for pancreatic cancer: real-world analysis of Korean national database. BMC Cancer. 2016;16:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 1720] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 27. | Roberts KJ, Schrem H, Hodson J, Angelico R, Dasari BVM, Coldham CA, Marudanayagam R, Sutcliffe RP, Muiesan P, Isaac J, Mirza DF. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB (Oxford). 2017;19:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Seiler CM, Izbicki J, Varga-Szabó L, Czakó L, Fiók J, Sperti C, Lerch MM, Pezzilli R, Vasileva G, Pap A, Varga M, Friess H. Randomised clinical trial: a 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharmacol Ther. 2013;37:691-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Romanus D, Kindler HL, Archer L, Basch E, Niedzwiecki D, Weeks J, Schrag D; Cancer and Leukemia Group B. Does health-related quality of life improve for advanced pancreatic cancer patients who respond to gemcitabine? Analysis of a randomized phase III trial of the cancer and leukemia group B (CALGB 80303). J Pain Symptom Manage. 2012;43:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Hagiwara Y, Ohashi Y, Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T; JASPAC 01 Study Group. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: Results from a randomised phase III trial (JASPAC 01). Eur J Cancer. 2018;93:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Laramée P, Wonderling D, Cahen DL, Dijkgraaf MG, Gouma DJ, Bruno MJ, Pereira SP. Trial-based cost-effectiveness analysis comparing surgical and endoscopic drainage in patients with obstructive chronic pancreatitis. BMJ Open. 2013;3:e003676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Johnson CD, Arbuckle R, Bonner N, Connett G, Dominguez-Munoz E, Levy P, Staab D, Williamson N, Lerch MM. Qualitative Assessment of the Symptoms and Impact of Pancreatic Exocrine Insufficiency (PEI) to Inform the Development of a Patient-Reported Outcome (PRO) Instrument. Patient. 2017;10:615-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | NICE. Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer. [cited July 2, 2025]. Available from: https://www.nice.org.uk/guidance/ta476. |
| 34. | Oanda. Oanda currency converter. [cited July 2, 2025]. Available from: https://www.oanda.com. |
| 35. | Dominguez-Muñoz JE. Diagnosis and treatment of pancreatic exocrine insufficiency. Curr Opin Gastroenterol. 2018;34:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Roberts KJ, Bannister CA, Schrem H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology. 2019;19:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Morawski JH, Prüfert A, van Engen A, Foerster D, Sander-Struckmeier S, Małecka-Panas E, Pezzilli R. Cost-effectiveness analysis of pancreatin minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. J Med Econ. 2012;15 Suppl 1:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |