Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.108484
Revised: May 17, 2025
Accepted: July 14, 2025
Published online: August 15, 2025
Processing time: 115 Days and 0.3 Hours
Esophageal cancer (EC), primarily esophageal squamous cell carcinoma in China, has a poor prognosis with a 5-year survival rate of approximately 25% after surgery alone. Neoadjuvant chemoradiotherapy combined with surgery is the standard treatment for locally advanced EC, with a 47% 5-year survival rate, although adverse events are common. Immunotherapy, particularly PD-1 inhibitors, has shown promise in treating advanced EC, and neoadjuvant che
To evaluate the efficacy, prognostic factors, and safety of adjuvant immunotherapy with anti-PD-1 inhibitors following radical surgery for EC.
A retrospective analysis was conducted on EC patients who received adjuvant immunotherapy after radical treatment at the 900th Hospital of the China Joint Logistics Force between January 2018 and October 2024. Demographic, treatment and laboratory data were collected. Progression-free survival (PFS) was assessed using the Kaplan-Meier method, and independent prognostic factors were identified using Cox regression. Optimal cutoff values for continuous variables, including body mass index (BMI) difference and neutrophil-to-lymphocyte ratio (NLR), were determined using the maxstat package in R.
A total of 44 patients were included, with a 2-year PFS rate of 68.6% [95% confidence interval (CI): 53%-88.7%]. Univariate analysis identified several factors significantly associated with prognosis, including the interval between surgery and immunotherapy, BMI difference between before surgery and first immunotherapy, pre
Adjuvant immunotherapy for EC shows good efficacy and safety. A BMI difference < 3.86 is a protective factor for PFS, highlighting the importance of monitoring nutrition and inflammation for personalized treatment.
Core Tip: This single-center, retrospective real-world study investigated the efficacy of adjuvant PD-1 immunotherapy following radical surgery for esophageal cancer and identifying related prognostic factors. A literature review published in the past 5 years on PubMed revealed no existing studies on this topic, making this research innovative. Additionally, the study found that the difference in body mass index (BMI) between the preoperative period and initiation of the first immunotherapy session serves as an independent prognostic factor. Specifically, a BMI difference < 3.86 was associated with better prognosis, providing valuable insights for guiding personalized follow-up treatment.
- Citation: Li LY, Zhang MQ, Ying WM, Zhang WZ, Jiang WJ, Xiong TJ, Wang FM, Fu ZC. Postoperative adjuvant PD-1 immunotherapy survival and body-mass-index dynamics in esophageal cancer: A real-world retrospective study. World J Gastrointest Oncol 2025; 17(8): 108484
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/108484.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.108484
Esophageal cancer (EC) is a malignancy with high morbidity and mortality worldwide, ranking as the 11th most common cancer and the seventh leading cause of cancer-related death[1]. In China, the epidemiology of EC is particularly well documented, with > 90% of cases being esophageal squamous cell carcinoma (ESCC)[2]. Despite significant advances in diagnostic and therapeutic approaches in recent years, the prognosis for patients with locally advanced EC remains poor. Following surgical treatment alone, the 5-year survival rate is approximately 25%, underscoring the limitations of current treatment strategies[3].
Currently, neoadjuvant chemoradiotherapy (CRT) combined with surgery is considered the standard treatment for resectable locally advanced EC or gastroesophageal junction cancer, with a 5-year overall survival (OS) rate of 47%. However, the incidence of adverse events (AEs) associated with this approach is high, ranging from 70% to 90%. Among these, the incidence of serious adverse reactions (grade ≥ 3) is 20%-40%[4]. A retrospective study on postoperative adjuvant CRT for squamous EC found that the 3-year disease-free survival (DFS) rate in the postoperative adjuvant CRT group was 58.2%, compared to 42.5% in the surgery-only group. Postoperative adjuvant CRT significantly reduced the local recurrence rate (20% vs 35%), although 10%-15% of patients discontinued treatment due to serious adverse effects[5]. In China, many surgeons still opt for surgical treatment alone or postoperative combined radiotherapy, as they are reluctant to use neoadjuvant radiotherapy combined with surgery. However, these alternative strategies have insufficient supporting data and demonstrate limited efficacy, preventing them from receiving high-level recommendations in clinical guidelines. There is an urgent need to explore more effective adjuvant treatment options and to develop more efficient, less toxic strategies for Chinese patients with ESCC.
In recent years, immunotherapy has shown remarkable clinical efficacy across various solid tumors, particularly in the treatment of advanced or metastatic cancers, where significant breakthroughs have been made. Immune checkpoint inhibitors (ICIs), such as PD-1/PD-L1 inhibitors, can inhibit tumor growth and metastasis by activating the patient’s own immune system[6]. Notably, in EC, the combination of chemotherapy with anti-PD-1 inhibitors has become the standard first-line treatment, and preoperative neoadjuvant chemotherapy combined with immunotherapy has also shown promising efficacy. However, studies examining the efficacy and safety of anti-PD-1 inhibitors in postoperative EC remain limited. In a Phase 3 multicenter trial, Checkmate577[7], the postoperative adjuvant regimen of nivolumab (240 mg every 2 weeks for 16 weeks, followed by 480 mg every 4 weeks for 1 year) demonstrated a median DFS of 22.4 months vs 11 months for the placebo group (2:1), with a hazard ratio (HR) of 0.69 [96.4% confidence interval (CI): 0.56-0.86; P = 0.0003], showing a significant advantage in DFS compared to the control group. However, this study included patients who underwent preoperative radiotherapy, and its findings may not be applicable to patients who had surgery alone. As such, the efficacy and safety of postoperative adjuvant immunotherapy for EC remain unclear, and the prognostic factors influencing the effectiveness of this approach have yet to be fully established, which hinders its broader application in clinical practice.
Building on the background outlined above, the aim of this study was to evaluate the efficacy of anti-PD-1 inhibitor adjuvant immunotherapy (hereafter referred to as immunotherapy) following radical surgery for EC and its impact on patient prognosis through a retrospective analysis. Additionally, this study examined clinical factors that may influence therapeutic efficacy, including the interval between surgery and immunotherapy, baseline immune status [e.g., lymphocyte counts, neutrophil-to-lymphocyte ratio (NLR)], and changes in body mass index (BMI), among others. The ultimate goal is to provide a theoretical foundation and clinical insights for optimizing postoperative adjuvant immunotherapy strategies in EC.
This retrospective study aimed to analyze the data of EC patients who received adjuvant PD-1 inhibitor immunotherapy following radical treatment between January 2018 and October 2024 at the 900th Hospital of the China Joint Logistics Force. The primary endpoint of the study was progression-free survival (PFS), defined as the time from the initiation of the first immunotherapy to the occurrence of objective tumor progression or death from any cause. The secondary endpoint was toxic AEs. All patients were subjected to clinical and telephone follow-up, and AEs related to immunotherapy were recorded as AEs. AEs were assessed using the Criteria for Adverse Event Evaluation (CTCAE version 5.0). The study was reviewed and approved by the Ethics Committee of the 900th Hospital of the China Joint Logistics Force.
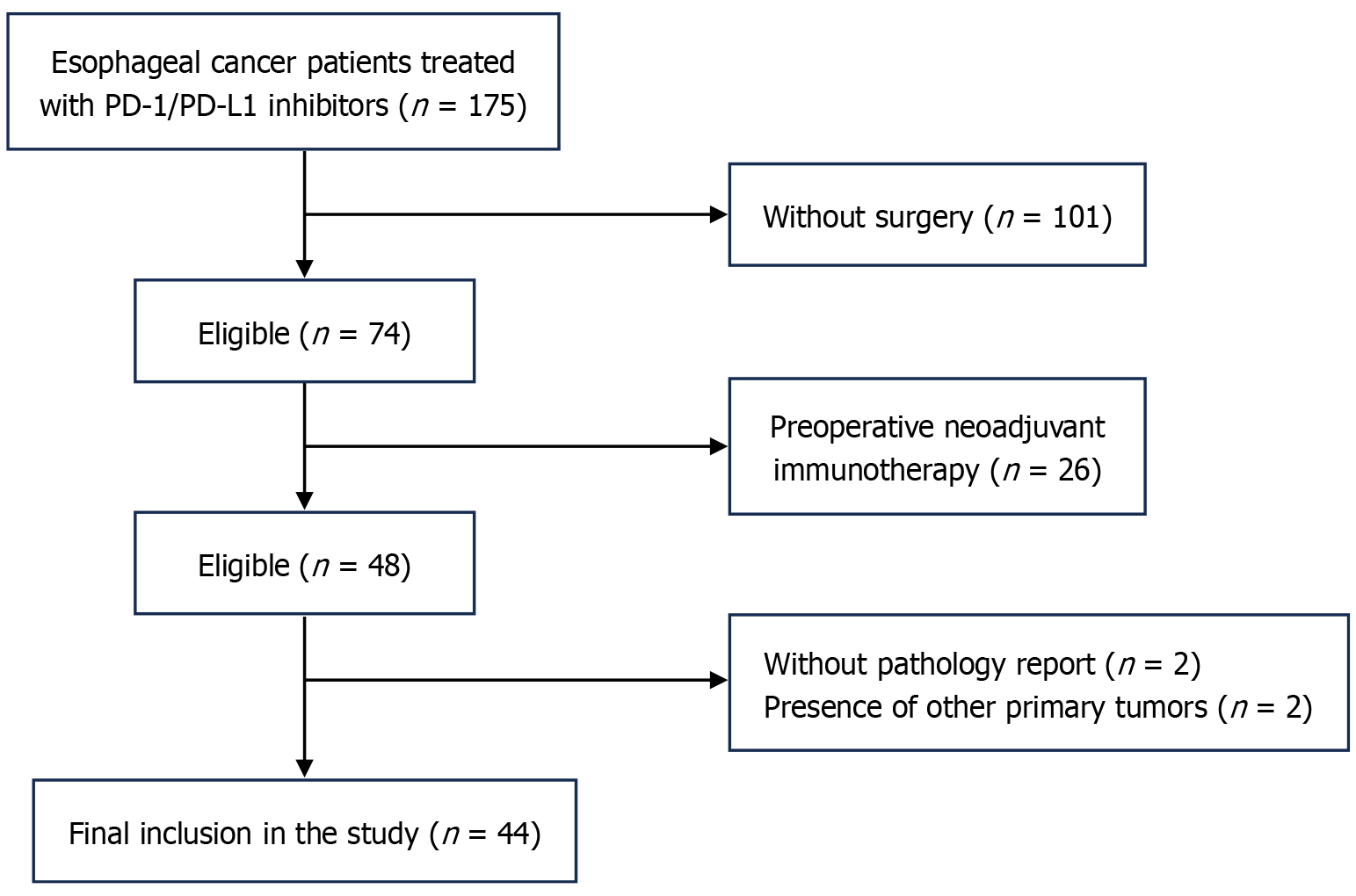
The data of EC patients who received adjuvant PD-1 inhibitor immunotherapy following radical surgery at the 900th Hospital of the China United Logistics Force between January 2018 and October 2024 were retrospectively analyzed. A total of 175 patients who had received ICIs were initially screened.
The inclusion criteria: (1) Age > 18 years; (2) No neoadjuvant chemotherapy, radiotherapy, or immunotherapy prior to radical surgery; (3) Availability of preoperative and pre-first immunotherapy blood data; and (4) All postoperative immunotherapy was administered as adjuvant therapy.
The exclusion criteria: (1) Any tumor-related treatment prior to surgery; (2) Tumor progression before initiation of postoperative immunotherapy; (3) Presence of any active infectious or autoimmune disease; and (4) Missing dosimetric or follow-up data.
All patients were staged based on postoperative histologic pathology according to the 8th edition of the American Joint Committee on Cancer staging criteria. Following these criteria, 131 patients were excluded: Two due to incomplete baseline data and two to the presence of other malignancies. Consequently, 44 patients were included in the final analysis (Figure 1).
All patients underwent thoracolaparoscopic radical cervicothoracic abdominal esophagectomy (triple incision), with R0 resection achieved in all cases. The PD-1 inhibitors used were sindilizumab, tirilizumab, teraplizumab, karelizumab, pembrolizumab and navulizumab, while the PD-L1 inhibitor used was envolizumab. The dosage regimen for all immunotherapies was 200 mg intravenously every 3 weeks (Q3W). Chemotherapy consisted of a combination of platinum and paclitaxel, administered Q3W. Radiotherapy was delivered using intensity-modulated radiotherapy, with prescribed doses commonly ranging from 50.4 Gy to 60 Gy, completed over 28-30 sessions. No changes in treatment regimens or dose reductions due to toxicities were recorded. All patients completed at least two cycles of immunotherapy.
Univariate and multivariate analyses were conducted using Cox proportional hazards regression models to evaluate the association between potential prognostic factors and clinical outcomes. Variables with a P < 0.05 in the univariate analysis were included in the multivariate model. Verify the normality of the variables using the Kolmogorov-Smirnov (KS) test. PFS was assessed using the Kaplan-Meier method. All statistical analyses were performed using R version 4.4.2 and SPSS version 26.0, with optimal cutoff values determined using the maxstat package in R. All statistical tests were two-sided, and P < 0.05 was considered significant.
From January 2018 to October 2024, a total of 175 patients with EC who received immunotherapy with PD-1 inhibitors were screened, and 44 patients were finally eligible for inclusion in the analysis, including 38 (86.4%) men and 6 (13.6%) women, with a median age of 63 years. Of 40 patients (90.9%) had squamous carcinoma. The most used immunological agent was tirilizumab (40.9%). By the time of follow-up, 9 patients (20.5%) had progressed, of whom, 7 patients (15.9%) had distant metastases, 3 patients liver metastases, 3 patients bone metastases, and 1 patient lung metastasis. The remaining baseline information is available at Table 1.
| Characteristic | Total | Characteristic | Total |
| Gender | Classification | ||
| Male | 38 (86.36) | Nonsquamous | 4 (9.09) |
| Female | 6 (13.64) | Squamous | 40 (90.91) |
| Median age, year | 64.0 (59.09-68.0) | Immunotherapy | |
| Age, year | Sindilizumab | 11 (25.0) | |
| ≤ 60 | 12 (27.27) | Karelizumab | 1 (2.27) |
| > 60 | 32 (72.73) | Tirelizumab | 18 (40.91) |
| T stage | Pabolizumab | 1 (2.27) | |
| 1b | 6 (13.64) | Teraplizumab | 10 (22.73) |
| 2 | 7 (15.91) | Others | 3 (6.82) |
| 3 | 31 (70.45) | Joint plan | |
| N stage | No | 3 (6.82) | |
| 0 | 11 (25.0) | CT | 29 (65.91) |
| 1 | 16 (36.36) | RT | 2 (4.55) |
| 2 | 9 (20.45) | RCT | 10 (22.73) |
| 3 | 8 (18.18) | Recurrence | |
| Differentiation | No | 35 (79.55) | |
| High | 9 (20.45) | Original | 2 (4.55) |
| Median | 21 (47.73) | Distant | 7 (15.91) |
| Low | 14 (31.82) |
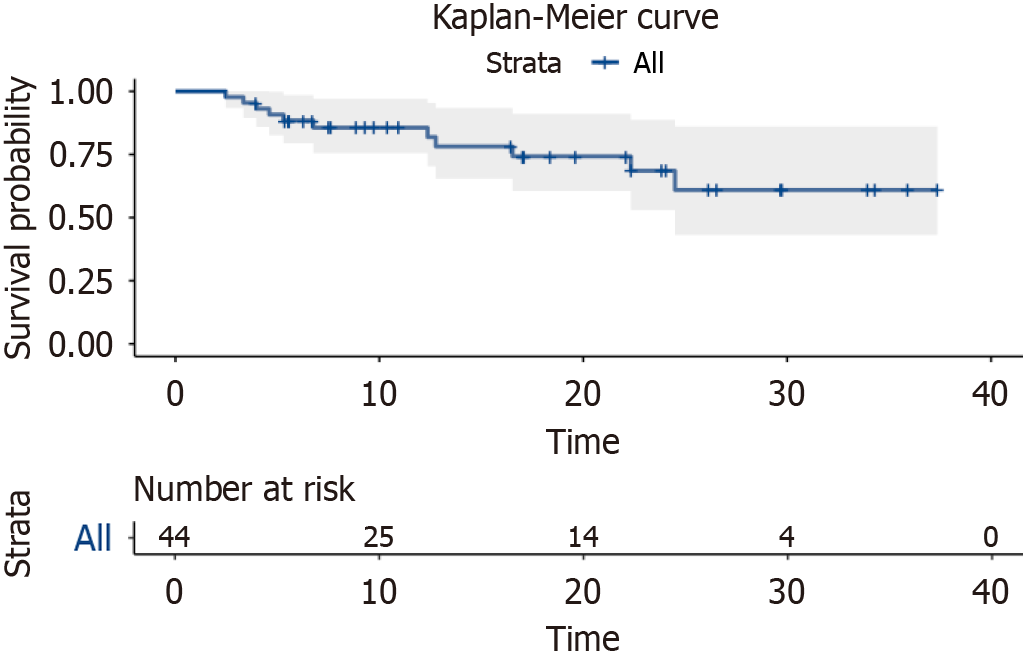
As of the time of follow-up, median PFS had not yet been reached. The 1-year PFS rate was 85.6% (95%CI: 75.5%-97%), and the 2-year PFS rate was 68.6% (95%CI: 53%-88.7%). The survival curves are shown in Figure 2.
To further investigate the factors influencing the prognosis of adjuvant PD-1 inhibitor immunotherapy after surgery, univariate analysis was performed, considering T-stage, N-stage, radiotherapy use, Combined positive score, BMI, and lymphocyte count. The Systemic Immune-Inflammation Index was calculated as platelet count × neutrophil count/Lymphocyte count. P < 0.05 was considered significant. The univariate analysis identified several factors associated with prognosis, including BMI (P = 0.046, HR = 0.74), lymphocyte count (P = 0.019, HR = 0.23), NLR (P = 0.014, HR = 1.18), and time since surgery (P = 0.027, HR = 1.04). These four factors were analyzed in a multivariate analysis. The difference between presurgical BMI and BMI at the time of first immunotherapy (P = 0.04, HR = 0.69, 95%CI: 0.49-0.98) was identified as an independent risk factor for prognosis (Table 2). No strong evidence was found to support an association between radiotherapy and PFS.
| Subgroup | Univariate analysis | Multivariate analysis | ||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| Age, year | ||||
| < 60 | 1.00 | 1.00 | ||
| ≥ 60 | 0.368 | 0.57 (0.17-1.95) | ||
| T stage | ||||
| ≤ 2 | 1.00 | 1.00 | ||
| > 2 | 0.494 | 0.65 (0.19-2.23) | ||
| N stage | ||||
| pN0 | 1.00 | 1.00 | ||
| pN ≥ 1 | 0.509 | 0.64 (0.17-2.43) | ||
| G | ||||
| G1 | 1.00 | 1.00 | ||
| G2 | 0.623 | 0.06 (0.13-3.47) | ||
| G3 | 0.947 | 1.06 (0.19-5.58) | ||
| TNM | ||||
| ≤ II | 1.00 | 1.00 | ||
| > II | 0.974 | 0.98 (0.26-3.7) | ||
| CPS | ||||
| < 10 | 1.00 | 1.00 | ||
| ≥ 10 | 0.139 | 0.31 (0.07-1.46) | ||
| Joint plan | ||||
| CT | 0.24 | 0.26 (0.03-2.44) | ||
| RT | 0.998 | 0 | ||
| RCT | 0.653 | 0.59 (0.06-5.8) | ||
| BMI | ||||
| BMI1 | 0.113 | 1.22 (0.95-1.57) | ||
| BMI2 | 0.932 | 0.99 (0.99-1.26) | ||
| BMIdv | 0.046 | 0.74 (0.55-1) | 0.04 | 0.69 (0.49-0.98) |
| L | ||||
| L1 | 0.019 | 0.23 (0.07-0.79) | 0.196 | 0.31 (0.05-1.84) |
| L2 | 0.437 | 0.63 (0.2-2.01) | ||
| N | ||||
| N1 | 0.199 | 1.36 (0.85-2.17) | ||
| N2 | 0.655 | 0.92 (0.62-1.35) | ||
| NLR | ||||
| NLR1 | 0.014 | 1.18 (1.03-1.34) | 0.688 | 1.13 (0.63-2.04) |
| NLR2 | 0.378 | 1.12 (0.87-1.43) | ||
| NLRdv | 0.100 | 0.87 (0.73-1.03) | ||
| Hb | ||||
| Hb1 | 0.741 | 0.99 (0.95-1.03) | ||
| Hb2 | 0.578 | 1.01 (0.97-1.06) | ||
| Hbdv | 0.440 | 1.02 (0.98-1.06) | ||
| WBC | ||||
| WBC1 | 0.937 | 1.02 (0.67-1.55) | ||
| WBC2 | 0.517 | 0.89 (0.63-1.27) | ||
| WBCdv | 0.440 | 0.87 (0.62-1.23) | ||
| SII | ||||
| SII1 | 0.105 | 1 (1-1) | ||
| SII2 | 0.937 | 1 (1-1) | ||
| SIIdv | 0.246 | 1 (1-1) | ||
| PLT | ||||
| PLT1 | 0.332 | 1 (0.99-1) | ||
| PLT2 | 0.446 | 1 (0.99-1) | ||
| Time | 0.027 | 1.04 (1-1.08) | 0.721 | 0.97 (0.84-1.12) |
Furthermore, a comprehensive analysis of the three BMI values revealed that the KS test confirmed their normal distribution.
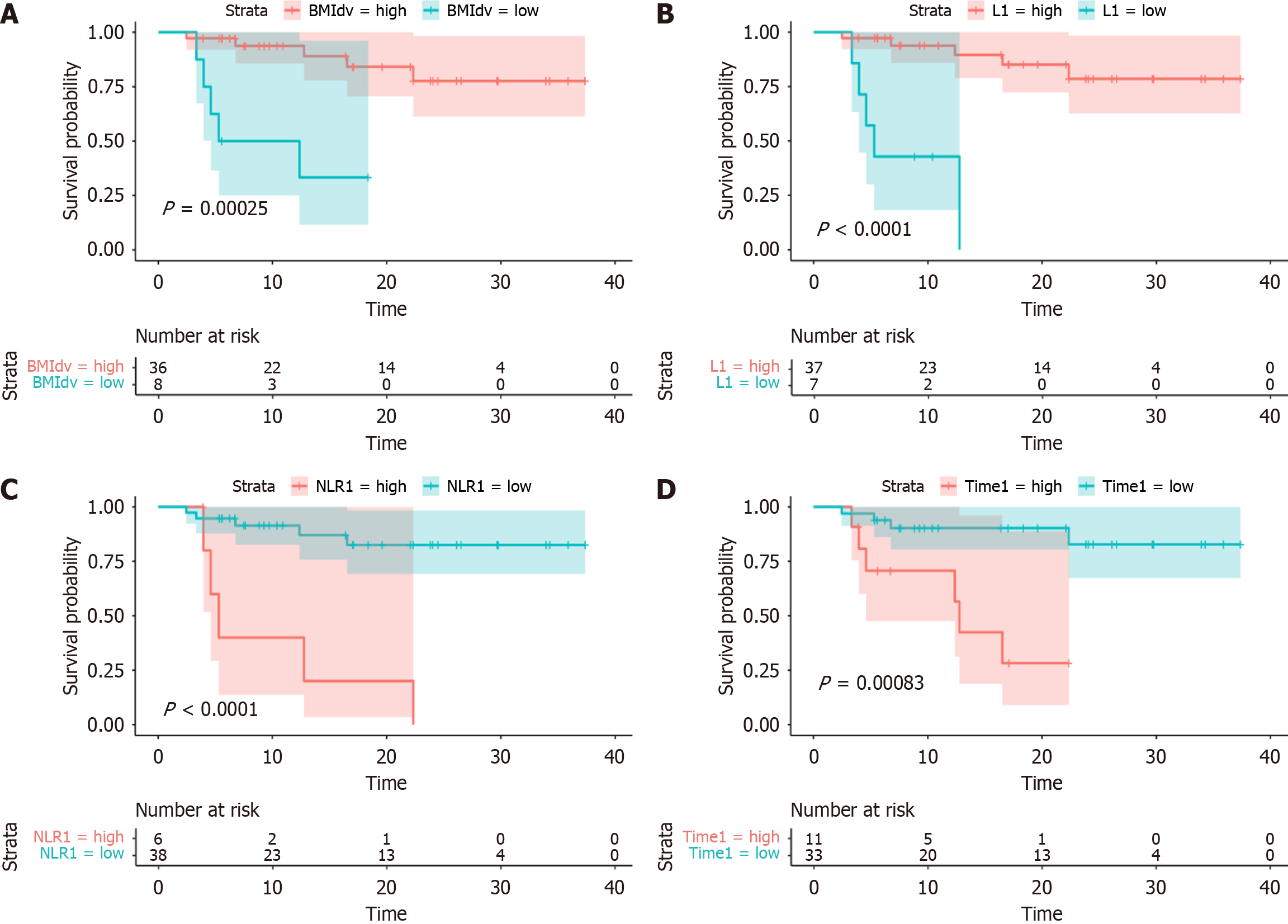
The four factors identified were further analyzed to determine the optimal cutoff values. The best cutoff value for the BMI difference was -3.86 (P = 0.00025). Patients with a BMI difference < 3.86 were categorized into the high group, while those with a difference > 3.86 were placed in the low group. At the time of follow-up, patients in the high group had not yet reached the median PFS, whereas median PFS in the low group was 8.83 mo. A BMI difference < 3.86 was an independent risk factor for prognosis. The best cutoff value for preoperative lymphocyte count (L1) was 1.22 × 109/L (P < 0.0001). The optimal cutoff value for preoperative NLR (NLR1) was 4.12 (normal range: 1-2) (P < 0.0001). The best cutoff value for the time difference between surgery and initiation of the first immunotherapy (Time) was 3.1 months (P = 0.00083). The survival analysis curves are presented in Figure 3.
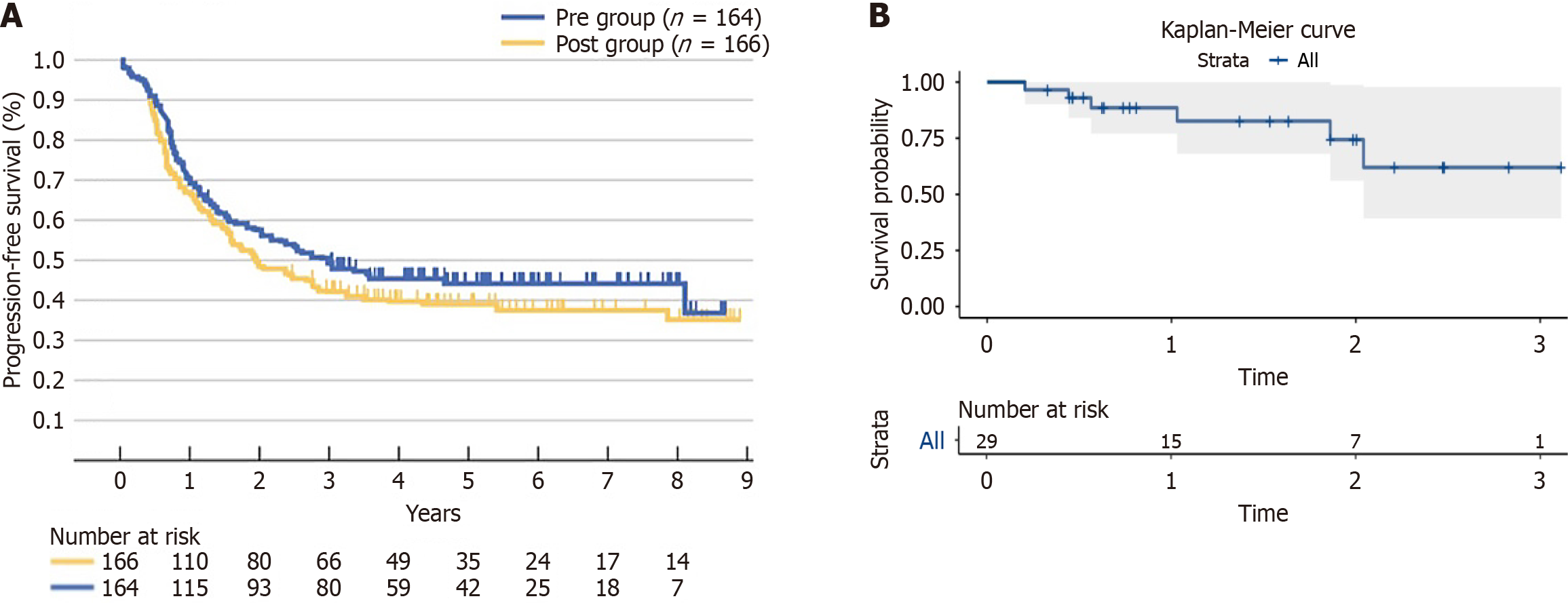
Twenty-nine of the enrolled patients received postoperative chemotherapy in combination with immunotherapy, resulting in a 1-year PFS rate of 88.5% (95%CI: 77%-100%), as shown in the Kaplan-Meier curves below. In comparison, the JCOG9907 trial reported a 1-year PFS rate of approximately 66.3% in the postoperative chemotherapy-only group. The Kaplan-Meier curves from the JCOG9907 trial are provided for comparison (Figure 4).
During adjuvant PD-1 inhibitor immunotherapy, 12 of 44 patients (27.3%) experienced treatment-related AEs. Of 2 patients (4.5%) reported grade ≥ 3 AEs; all of which were associated with decreased neutrophil counts. One immunotherapy-related death (2.3%) was recorded during follow-up, resulting from infectious shock (Table 3).
| AE | Total (n = 44), AE (n = 12, 27.3%) | |||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Tiredness | 3 (6.82) | 1 (2.27) | / | / |
| Nausea | / | 2 (4.55) | / | / |
| Hyperpigmentation | / | 1 (2.27) | / | / |
| Fever | / | 1 (2.27) | / | / |
| Hypothyroidism | / | 1 (2.27) | / | / |
| Neutropenia | / | 1 (2.27) | / | 2 (4.55) |
Numerous clinical studies have demonstrated that surgery alone is not sufficient for the treatment of locally advanced resectable ESCC. The JCOG9907 trial compared preoperative chemotherapy combined with esophagectomy to esophagectomy followed by postoperative chemotherapy in patients with clinical stage II/III ESCC. The trial revealed that OS in the preoperative chemotherapy group was significantly better than in the postoperative chemotherapy group, thereby supporting the use of neoadjuvant CF chemotherapy as a recommended approach for stage II/III ESCC in Japan[8]. Similarly, the CROSS trial compared surgery alone with 40 Gy radiotherapy combined with TP-synchronized neoadjuvant CRT followed by surgery. The results indicated that preoperative CRT is the standard of care for patients with locally advanced resectable ESCC[9]. However, in clinical practice, many patients undergo radical surgery without neoadjuvant therapy due to concerns about the potential impact of neoadjuvant toxicities on surgical safety. Consequently, it remains unclear which treatment strategies are optimal for these patients.
Currently, postoperative adjuvant therapies include postoperative radiotherapy and chemotherapy. For instance, adjuvant radiotherapy may be considered for high-risk postoperative patients, such as those with lymph node-positive or margin-positive disease. In patients with postoperative lymph node positivity, adjuvant CRT has been shown to improve 5-year OS from 30%-40% with surgery alone to 40%-50%. However, 10%-15% of patients may discontinue treatment due to severe AEs[8,10]. In contrast, a multicenter retrospective study from China on adjuvant chemotherapy demonstrated a 3-year OS of 65% in the adjuvant chemotherapy group compared to 52% in the surgery-only group (HR = 0.69, P = 0.008), with the overall incidence of AEs ranging from 60% to 80%. Serious AEs (grade ≥ 3) occurred in 15%-25% of cases[11]. Consequently, the efficacy and safety of immunotherapy in postoperative adjuvant therapy for EC has become an area of active research. Real-world studies on adjuvant immunotherapy have shown that most patients were unable to complete the treatment due to disease progression or AEs, and no significant benefit was observed on 1-year survival[12]. A review of 175 patients at our center between January 2018 and October 2024 revealed that only 44 patients received postoperative adjuvant immunotherapy, highlighting the currently low clinical application of adjuvant immunotherapy in EC. This finding also suggests significant challenges regarding the tolerance and efficacy of immunotherapy regimens, with its clinical use still in the exploratory stage and in urgent need of further evidence-based support.
To date, there have been no large, multicenter studies specifically investigating the prognosis of adjuvant PD-1 inhibitor immunotherapy following radical surgery without neoadjuvant therapy for EC. However, in lung cancer, another thoracic malignancy, extensive studies such as the KEYNOTE-91/PEARLs trial and the IMpower010 trial have provided valuable insights. Previous research[6,13] has demonstrated significant benefits of anti-PD-1 immunotherapy in patients with non-small cell lung cancer following complete resection. Given the similarity in the biological mechanisms between these two cancers, the present study aimed to explore the potential role of anti-PD-1 inhibitors as adjuvant therapy after radical surgery for EC, providing a novel approach to address this clinical challenge. The preliminary results of this study revealed that the median PFS had not been reached at the time of follow-up, with a 1-year PFS rate of 85.6% (95%CI: 75.5%-97%) and a 2-year PFS rate of 68.6% (95%CI: 53%-88.7%). These findings suggest promising efficacy and safety of anti-PD-1 inhibitors in the adjuvant setting for EC. In comparison, the reported 1-year PFS rates for postoperative adjuvant radiotherapy (65%-70%) and chemotherapy (55%-60%) in the literature are lower, indicating a potential advantage of immunotherapy. Some studies have shown that the combination of sindacil and triptolide can significantly improve PFS in postoperative EC patients, supporting the potential of immunotherapy in this context. Moving forward, identifying the patient population most likely to benefit from postoperative immunotherapy will be crucial in guiding clinical decision-making and optimizing treatment strategies.
Traditionally, high BMI has been associated with an increased risk of surgical complications[14,15]. However, emerging evidence suggests that overweight and obese patients may experience superior survival outcomes when treated with PD-L1 inhibitors[16-19]. The biological mechanisms underlying the relationship between BMI and immunotherapy remain largely exploratory, but it is accepted that they are primarily related to leptin. A previous study[20] found that elevated leptin levels could enhance the response to ICI therapy by activating T cells and increasing PD-1 expression on these cells. Additionally, BMI may influence ICI efficacy by altering free fatty acid metabolism[21] and modifying the composition of the gut microbiome[22]. No study has systematically evaluated the prognostic impact of the change in BMI from preoperative to immunotherapy initiation. In this study, we demonstrated that a BMI difference < 3.86 between preoperative and immunotherapy initiation was significantly associated with prolonged PFS (P < 0.05). The BMI difference reflected the patients' nutritional status. Therefore, improving the nutritional status of patients after radical surgery for EC may enhance the efficacy of immunotherapy, and the BMI difference could serve as a predictor of the effectiveness of postoperative immunosuppressive therapy. Further large-scale prospective studies are needed to elucidate the relationship between BMI difference and the prognosis of immunotherapy following surgery. This finding offers new directions for individualized treatment. Moreover, we explore potential strategies for optimizing measurement based on BMI difference and whether the dose of immunosuppressive agents should be adjusted to balance efficacy and toxicity in patients with significant BMI differences.
The prognostic value of preoperative NLR has been confirmed in several studies. Sakin et al[23] identified an optimal cutoff value of 2.8 for NLR in patients with EC undergoing surgery, with a high preoperative NLR (≥ 2.8) negatively impacting DFS and OS. Similarly, Duan et al[24] found that among ESCC patients undergoing curative surgery, those with high preoperative NLR (> 3.0) had significantly lower tumor-specific and recurrence-free survival compared to those with a low preoperative NLR. This suggests that NLR is an important prognostic factor, particularly in patients with stage IIIA disease. Xu et al[25] reported an optimal cutoff value of 2.99 for NLR in ESCC patients, concluding that preoperative NLR was significantly associated with long-term prognosis, especially in patients with lymph node metastases and stage II/III disease. In the present study, the optimal cutoff value for preoperative NLR was 4.12, and the prognosis in the low NLR group was significantly better than that in the high NLR group (P < 0.0001), consistent with previous studies. However, the substantial difference between the optimal cutoff value for NLR in this study and those in prior studies may be attributed to the smaller sample size of this study. While this study primarily focused on compound inflammatory markers (e.g., NLR and PLR), the prognostic value of lymphocyte count alone is influenced by individual variations. Thus, positive results based on lymphocyte count may be due to chance and warrant validation in larger, more robust cohorts.
To date, there has been no large-scale, multicenter clinical trial demonstrating the optimal timing for initiating postoperative adjuvant therapy. Rhodin et al[26] retrospectively analyzed 1634 patients who received adjuvant chemotherapy after radical esophagectomy for EC. Their findings indicated that the timing of adjuvant chemotherapy initiation following EC resection was not associated with 5-year OS (P = 0.86). The National Comprehensive Cancer Network guidelines remain unclear on this matter[27]. Considering the individual variability among patients after radical esophagectomy for EC, the initiation of adjuvant immunotherapy should take into account both the patient's postoperative recovery status and the indications for adjuvant therapy. The present study demonstrates that immunotherapy initiated approximately 3 months after postoperative recovery provides a significant survival benefit.
A subgroup analysis was conducted for 29 patients who received postoperative chemotherapy combined with immunotherapy, yielding a 1-year PFS rate of 88.5% (95%CI: 77%-100%). In comparison, the JCOG9907 trial reported a 1-year PFS rate of approximately 66.3%[28]. These findings suggest that, in the postoperative setting, patients receiving combined immunotherapy and chemotherapy achieve better short-term prognosis than those treated with chemotherapy alone. This improvement indicates a potential additive benefit of immunotherapy over conventional chemotherapy regimens in the adjuvant setting. The wide CI (77%-100%) in this cohort may reflect the limited sample size, underscoring the need for further validation in larger prospective studies.
Regarding treatment safety, the ATTRACT-3 phase 3 study[29] reported grade 3/4 treatment-related AEs in 19% of patients receiving nivolumab monotherapy, with a treatment-related mortality rate of 1%. The incidence of grade ≥ 3 AEs in combination chemotherapy regimens has been reported to range from 41% to 54%[30,31]. In contrast, the current study observed a proportion of grade 3 AEs of 4.5% and a mortality rate of 2.3%, suggesting that postoperative immunotherapy has a manageable safety profile.
This study had several limitations. First, it was a single-center, retrospective analysis with a small sample size (n = 44) and lacked a randomized control group, which may have introduced selection bias. Second, although strict screening criteria were applied to the included cases (all of whom had not received adjuvant therapy after radical surgery), confounding factors, such as differences in molecular subtypes, may still have influenced the interpretation of the results. Further multicenter, prospective studies are required to validate the generalizability of the BMI difference and NLR cutoff value, as well as to perform additional stratified analyses based on molecular subtypes.
Adjuvant immunotherapy has demonstrated a significant impact on survival in patients with resectable EC in clinical trial settings[7]. This study, utilizing real-world data, confirms that adjuvant immunotherapy following radical surgery offers better efficacy and safety. A preoperative-to-first-immunotherapy BMI difference < 3.86 was identified as a key protective factor for PFS, suggesting its potential as a prognostic marker. Based on survival curve analysis and dynamic monitoring of laboratory indicators, it is recommended that inflammatory markers, such as lymphocyte count and NLR, be systematically assessed throughout treatment to enable dynamic adjustments in the treatment regimen. Although this study highlights the clinical value of dynamic BMI monitoring, its predictive efficacy warrants further validation through multicenter cohort studies to clarify its role in guiding individualized treatment decisions.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8198] [Article Influence: 8198.0] [Reference Citation Analysis (2)] |
| 2. | Herskovic A, Russell W, Liptay M, Fidler MJ, Al-Sarraf M. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol. 2012;23:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, Willett C, Stiles B, Sharma P, Tang L, Wijnhoven BPL, Hofstetter WL. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:2677-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 4. | Zhang R, Wang Z, Kang X, Wang X, Zhang B, Ng HL, Xue L, Yang W, Shi L, Wang H, Wang L, Li Y; Esophageal Cancer Quality Control Expert Committee of the National Cancer Center. Quality control indices for standardized diagnosis and treatment of esophageal cancer in China (2022 edition). J Natl Cancer Cent. 2023;3:167-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Mao Y, Gao S, Li Y, Chen C, Hao A, Wang Q, Tan L, Ma J, Xiao G, Fu X, Fang W, Li Z, Han Y, Chen K, Zhang R, Li X, Rong T, Fu J, Liu Y, Mao W, Xu M, Liu S, Yu Z, Zhang Z, Fang Y, Fu D, Wei X, Yuan L, Muhammad S, He J. Minimally invasive versus open esophagectomy for resectable thoracic esophageal cancer (NST 1502): a multicenter prospective cohort study. J Natl Cancer Cent. 2023;3:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, Chen YM, Chella A, Sugawara S, Voong D, Wu F, Yi J, Deng Y, McCleland M, Bennett E, Gitlitz B, Wakelee H; IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 900] [Article Influence: 225.0] [Reference Citation Analysis (0)] |
| 7. | Kelly R, Ajani J, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zhang J, Zhu L, Lei M, Kondo K, Cleary J, Moehler M. LBA9_PR Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): First results of the CheckMate 577 study. Ann Oncol. 2020;31:S1193-S1194. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1053] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 9. | Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, Klump B, Budach W, Teichmann R, Schmitt M, Schmitt G, Franke C, Wilke H. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 930] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 10. | Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J; AME Thoracic Surgery Collaborative Group. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 696] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 11. | Li C, Wang X, Wang L, Chen J, Zhang W, Pang Q, Zhao Y, Sun X, Zhang K, Li G, Li L, Qiao X, Liu M, Wang Y, Deng L, Wang W, Bi N, Zhang T, Deng W, Ni W, Chang X, Han W, Zhou Z, Liang J, Feng Q, Wang L, Chen D, Lv J, Zhu S, Han C, Xiao Z. Clinical practice and outcome of radiotherapy for advanced esophageal squamous cell carcinoma between 2002 and 2018 in China: the multi-center 3JECROG Survey. Acta Oncol. 2021;60:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Kwak HV, Banks KC, Hung YY, Alcasid NJ, Susai CJ, Patel A, Ashiku S, Velotta JB. Adjuvant Immunotherapy in Curative Intent Esophageal Cancer Resection Patients: Real-World Experience within an Integrated Health System. Cancers (Basel). 2023;15:5317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, Esteban E, Isla D, Martinez-Marti A, Faehling M, Tsuboi M, Lee JS, Nakagawa K, Yang J, Samkari A, Keller SM, Mauer M, Jha N, Stahel R, Besse B, Peters S; EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 Investigators. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23:1274-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 424] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 14. | Fujitani K, Ajani JA, Crane CH, Feig BW, Pisters PW, Janjan N, Walsh GL, Swisher SG, Vaporciyan AA, Rice D, Welch A, Baker J, Faust J, Mansfield PF. Impact of induction chemotherapy and preoperative chemoradiotherapy on operative morbidity and mortality in patients with locoregional adenocarcinoma of the stomach or gastroesophageal junction. Ann Surg Oncol. 2007;14:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Healy LA, Ryan AM, Gopinath B, Rowley S, Byrne PJ, Reynolds JV. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg. 2007;134:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, Tinari N, De Tursi M, Zoratto F, Veltri E, Marconcini R, Malorgio F, Russano M, Anesi C, Zeppola T, Filetti M, Marchetti P, Botticelli A, Antonini Cappellini GC, De Galitiis F, Vitale MG, Rastelli F, Pergolesi F, Berardi R, Rinaldi S, Tudini M, Silva RR, Pireddu A, Atzori F, Chiari R, Ricciuti B, De Giglio A, Iacono D, Gelibter A, Occhipinti MA, Parisi A, Porzio G, Fargnoli MC, Ascierto PA, Ficorella C, Natoli C. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 17. | Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020;6:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, Price KA, Molina JR, Pagliaro LC, Halfdanarson TR, Grothey A, Markovic SN, Nowakowski GS, Ansell SM, Wang ML. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5:1008-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 595] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 19. | Wei T, Ti W, Song Q, Cheng Y. Study of PD-1 Inhibitors in Combination with Chemoradiotherapy/Chemotherapy in Patients with Esophageal Squamous Carcinoma. Curr Oncol. 2022;29:2920-2927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O'Connor DJ, Méndez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 631] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 21. | Rassy EE, Ghosn M, Rassy NA, Assi T, Robert C. Do immune checkpoint inhibitors perform identically in patients with weight extremes? Immunotherapy. 2018;10:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science. 2018;359:1366-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 519] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 23. | Sakin A, Alay M, Sahin S, Aydemir O, Aldemir MN, Sakin A, Kotan C. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. North Clin Istanb. 2021;8:435-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, Ma QL, Lin P. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:5591-5597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Xu GW, Wu HR, Xiong R, Li CW, Liu CQ, Xu MQ, Xie MR. Value of the preoperative neutrophil-to-lymphocyte ratio as a prognostic factor for long-term survival in postoperative esophageal squamous cell carcinoma patients. Thorac Cancer. 2018;9:1707-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Rhodin KE, Raman V, Jawitz OK, Tong BC, Harpole DH, D'Amico TA. The Effect of Timing of Adjuvant Therapy on Survival After Esophagectomy. Ann Thorac Surg. 2020;110:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Ajani JA, D'Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, Gibson M, Grierson P, Hofstetter WL, Ilson DH, Jalal S, Keswani RN, Kim S, Kleinberg LR, Klempner S, Lacy J, Licciardi F, Ly QP, Matkowskyj KA, McNamara M, Miller A, Mukherjee S, Mulcahy MF, Outlaw D, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:393-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 234] [Reference Citation Analysis (1)] |
| 28. | Yokota T, Ando N, Igaki H, Shinoda M, Kato K, Mizusawa J, Katayama H, Nakamura K, Fukuda H, Kitagawa Y. Prognostic Factors in Patients Receiving Neoadjuvant 5-Fluorouracil plus Cisplatin for Advanced Esophageal Cancer (JCOG9907). Oncology. 2015;89:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 799] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 30. | Qiu MZ, Oh DY, Kato K, Arkenau T, Tabernero J, Correa MC, Zimina AV, Bai Y, Shi J, Lee KW, Wang J, Poddubskaya E, Pan H, Rha SY, Zhang R, Hirano H, Spigel D, Yamaguchi K, Chao Y, Wyrwicz L, Disel U, Cid RP, Fornaro L, Evesque L, Wang H, Xu Y, Li J, Sheng T, Yang S, Li L, Moehler M, Xu RH; RATIONALE-305 Investigators. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ. 2024;385:e078876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 56] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 31. | Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, Wu X, Yao Y, Shen L, Kojima T, Gotovkin E, Ishihara R, Wyrwicz L, Van Cutsem E, Jimenez-Fonseca P, Lin CY, Wang L, Shi J, Li L, Yoon HH. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 128] [Article Influence: 64.0] [Reference Citation Analysis (0)] |